Abstract
Desorption electrospray ionization mass spectrometry (DESI-MS) imaging of biological samples allows untargeted analysis and structural characterization of lipids ionized from the near-surface region of a sample under ambient conditions. DESI is a powerful and sensitive MS ionization method for 2D and 3D imaging of lipids from direct and unmodified complex biological samples. This review describes the strengths and limitations of DESI-MS for lipid characterization and imaging together with the technical workflow and a survey of applications. Included are discussions of lipid mapping and biomarker discovery as well as a perspective on the future of DESI imaging.
Keywords: ambient mass spectrometry, imaging, lipidomics, disease diagnosis, desorption electrospray ionization, lipid characterization
1. Introduction
DESI is one of a recently developed group of ambient ionization techniques in mass spectrometry in which samples are examined in the ambient environment with minimal pretreatment [1–3]. DESI has been increasingly used for direct lipid analysis from biological samples, especially in the medical field.[4– 6] These characteristics combined with its ease of execution make DESI a technique of some significance in the field of imaging mass spectrometry [7]. The DESI-MS imaging experiment is carried out by directly scanning the unmodified sample in the x and y directions through an impinging spray of charged droplets; the chemical information obtained can then be plotted as two-dimensional images recording the abundance of specific ions [8]. Many of the promising results for the analysis of biological samples, are based on differences in lipid composition [9, 10]. Thus far, DESI-MS imaging has been used for the analysis of multiple types of human tissue encompassing diseased tissue, adjacent normal tissue and non-diseased tissue. The measurements are highly reproducible although not strictly quantitative. In this review we cover the principles of DESI-MS imaging, its use for lipid analysis and characterization, describe the lipid classes detected and discuss the technique.
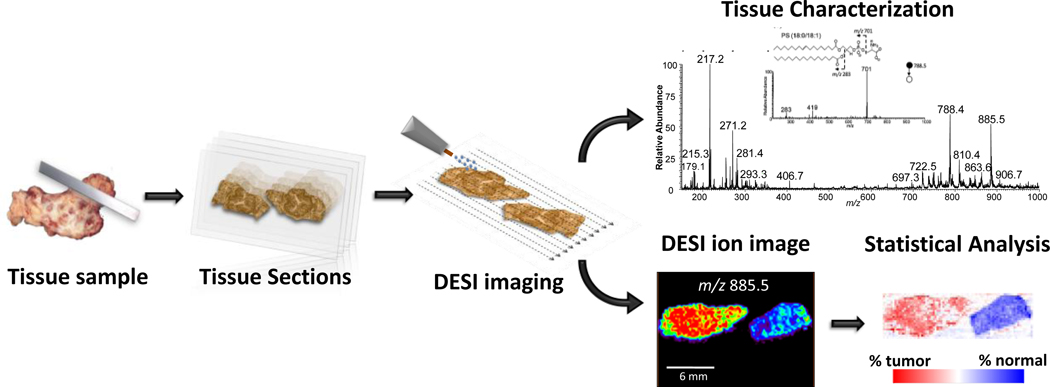
High performance liquid chromatography (HPLC), gas chromatography (GC) and thin-layer chromatography (TLC) are traditionally used for lipid separation and can be followed by MS analysis [11, 12]. However, these are lengthy methods and do not provide information on spatial distribution as is achieved by MS imaging techniques. Imaging mass spectrometry allows direct analysis of the spatial distribution of a variety of compounds without the need for fluorescent or radioactive labeling normally used in histochemical protocols [13, 14]. Most imaging MS is currently done using the desorption ionization methods of secondary ion mass spectrometry (SIMS)[15, 16] and matrix assisted laser desorption ionization (MALDI)[17]. Both ionization methods are vacuum techniques which require sophisticated instrumentation. As an electrospray based ionization method, DESI imaging brings different and complementary capabilities, although MALDI remains the main imaging MS technique for biological samples analysis. MALDI has been traditionally used to image larger biomolecules such as proteins and peptides in biological tissues[18], even though it is also effective for the analysis of smaller molecules such as lipids [19]. One limitation for small molecule analysis by MALDI is the interference of matrix ions in the low m/z region of the mass spectrum [20]. In contrast, lipids are easily ionized and measured by DESI-MS. DESI-MS has been used for the direct analysis, characterization and imaging of many classes of lipids including fatty acyls (FA), glycerophospholipids (GP), glycerolipids (GL), sphingolipids (SP) and sterol lipids (ST). More recently, DESI-MS has been mainly applied for tissue section analysis in an attempt to enable disease diagnosis based on lipid profiles and the presence of specific lipid species as characterized by tandem MS experiments, complemented by the use of multivariate statistical analysis of the data (Fig. 1) [21].
Fig. 1.
Schematic of DESI-MS imaging of biological tissue. Tissue samples are cryosectioned and tissue sections thaw mounted onto glass slides, then directly analyzed by conventional or reactive DESI imaging. Tissue characterization is performed based on the lipid profiles detected and identification of particular lipid species by tandem MS analysis. For cancer diagnostics, statistical analysis of the entire image data is performed with the goal of generating classification rules. Figure adapted in part from reference 4.
Lipids comprise a group of diverse molecules with different structures and functionalities that play important roles in cellular processes [22]. The study of the lipid composition as means of characterizing biological samples is potentially important since understanding the role that lipids play in normal cells can lead to an understanding of how lipids function in disease states [9]. Extensive investigations have reported that the lipid composition of tissues can vary with disease state [23]. In the area of cancer research, a number of studies focus on the molecular changes that occur in cells, signaling the beginning of malignancy and providing a means for early detection and treatment intervention [24, 25]. GPs for example reflect cellular growth, maturation and differentiation as well as the histological cell type. Cell membranes have non-uniform distributions of GPs with particular species appearing specifically in the inner or outer membrane [26]. Alterations in this distribution are indicative of cell transformations, including malignancy [27]. In colon cancer, it has been shown that both primary and colon cancer liver metastasis exhibit abnormal GP distributions compared to normal tissue, indicating structural and functional modifications of the cell membrane [28, 29]. Changes in lipid tissue content also indicate tumor progression in human brain cancer, with significant alterations in sphingomyelin (SM), glycerophosphoserine (PS) and galactoceramides (GalCer) content during glioma development [30]. As building blocks for GPs, FAs are important to cell structure and function and therefore it is expected that the FA composition of cancerous cells would differ from that of normal cells. Many studies have sought to examine the changing profiles of FAs in order to understand the metabolic pathways in malignancy. For example, changes in FAs composition occur between intraepithelial cervical lesions, cervical cancer and normal cells showcasing the path toward carcinogenesis [31, 32]. FA patterns have also been found to differ between stomach cancer tissue and normal gastric mucosa, with overall FA content being increased in cancerous tissue [33]. Besides cancer, lipid composition alterations have been reported for many other diseases, such as in the neurodegenerative Alzheimer’s disease[34] and cardiovascular diseases including atherosclerosis[35]. These findings reported by an increasing number of research studies in lipidomics emphasize the importance of determining the composition of lipids in biological tissues for its prognostic value for determining and diagnosing disease.
2. DESI-MS Methodology for Lipid Analysis
In the DESI-MS experiment, a spray of charged droplets is directed towards the sample. When the spray impacts the sample, a thin layer of solvent is formed into which the analytes may dissolve. As other primary droplets arrive at the sample surface, they splash secondary microdroplets containing the dissolved analytes from the solvent film. This mechanism, “droplet pickup”[36], causes analyte containing droplets to be generated in the open air, and then delivered to the mass spectrometer through a heated extended capillary. Fundamental studies have shown that under typical DESI experimental conditions the average velocity of the primary droplets is about 120 m/s, with an average diameter of about 3 µm [37]. Simulations of the DESI process show the release of dozens of microdroplets in the range of 0.8 to 3.3 µm, from a single droplet-thin film collision event [36]. After the desorption process, ionization occurs via mechanisms that are similar to those of electrospray ionization. Tandem MS or high mass resolution experiments are normally used for detailed characterization of individual lipids.
2.1. Biological Sample Preparation
Biological samples analyzed by DESI-MS include tissue extracts [38], bacterial colonies [39], plant tissue [40, 41] and animal tissue sections [7]. Samples can be directly spotted or deposited in a solid surface for analysis while in the case of tissue sections, imaging analysis is done from a single piece of tissue normally 5–25 µm thick, mounted onto a glass slide and stored at −80°C freezer until analysis. While tissue analysis by DESI has so far been limited to fresh frozen tissue, expansion to formalin fixed paraffin embedded samples is expected. Surfaces of choice for DESI-MS include polytetrafluoroethylene printed glass which gives the most intense, stable and long-lasting signals for standard phospholipid samples applied directly to the surface [38]. Nevertheless, glass slides are the most commonly used substrates for lipid analysis[42] when done for tissue imaging. This is largely due to the fact that traditional tissue analysis by histochemical procedures is normally performed on sections mounted onto glass slides.
2.2. Image Quality
The sample stage used in DESI imaging is moved in the x direction while mass spectra are recorded continuously. A sequence of lines in the x direction is used to cover the entire sample surface being targeted [43]. Note that while rastering across the sample the direction of y-motion should be selected so as to avoid splashing sample onto areas not yet analyzed. After data acquisition is complete, the spatial distribution of particular ions detected from the sample can be plotted in a two-dimensional image. While the first 2D DESI imaging experiments used a semi-automatic lab-built moving stage, the expansion of DESI applications led to commercialization of DESI ion sources.
Several experimental parameters have been reported to affect the quality of DESI-MS data [44]. While for standard 1D DESI-MS experiments the size, shape and direction of the spray spot is not as significant, optimization of the spray spot for DESI-MS imaging experiments is needed in order to obtain high quality 2D images [8, 45]. The most important parameters are the quality of the DESI spray tip, the geometric parameters of the spray source (angles and distances between spray, sample and mass spectrometer inlet), and the spray parameters (gas and solvent flow rate) [46]. A small circular spray spot for tissue imaging can be obtained by precisely cutting the silica capillary so that the edges are not jagged [45]. Most of the reports using home-built DESI imaging sources used an inner fused silica capillary for solvent delivery of 50 µm i.d. and 150 µm o.d. and an outer fused silica capillary 250 µm i.d. and 350 µm o.d. for gas delivery, which yields spray spots of ~ 200 µm diameter or less for common aqueous solvent systems such as methanol:water (1:1) and ACN:water (1:1) [8]. While lower spatial resolution can be achieved[47], a trade-off between image quality and data acquisition time is involved in all microprobe experiments. The time of analysis increases quadratically with spatial resolution and for many but by no means all biological applications of ambient MS imaging, a resolution of 200 µm is sufficient to obtain the desired information (Fig. 2). In terms of geometrical parameters, several studies have reported optimal settings which have become quite standard for imaging purposes [43]. A tip-to-surface distance of 2–3 mm, incident angle of 50–54°, collection angle of 5–10°, and a spray-to-inlet distance of 4–8 mm are generally ideal parameters for DESI-MS imaging [44]. Overall, the spray parameters should be adjusted in accordance to the solvent system and the ease with which analytes are dissolved in and desorbed from the sample surface. The physical damage done by the spray depends on the choice of gas pressure and solvent flow rate. While a stronger spray normally yields a more abundant signal, the spatial resolution is decreased, affecting image quality [43]. Typical gas pressures vary between 130–180 psi and solvent flow rates between 1.5 µL/min and 5 µL/min for imaging experiments using aqueous solvents.
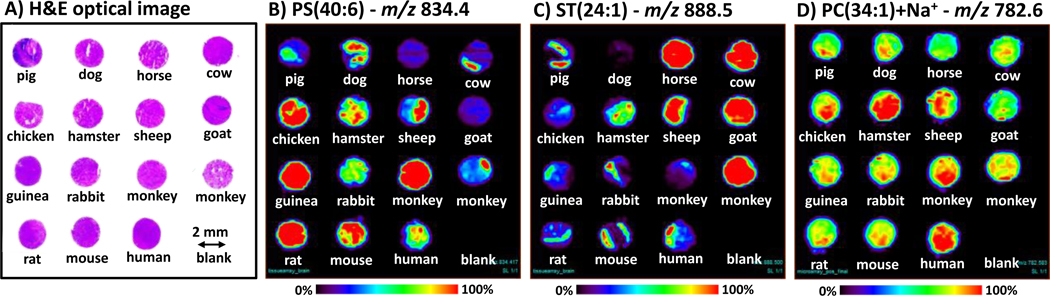
Fig. 2.
DESI-MS images recorded on a microarray of normal brain tissues of fourteen animal species. Three sequential frozen tissue arrays were obtained from US Biomax, Inc; one for negative ion mode analysis, one for positive ion mode, and one for hematoxylin and eosin (H&E) staining. Each tissue core is 2 mm in diameter and 5 µm thick. Samples were analyzed using methanol:water (1:1) at 1.5 µL/min. Total time of analysis was ca. 2 hours, viz. ~ 8 minutes/tissue core, with a spatial resolution of 250 µm. Optical image of the H&E stained array is shown in (A). DESI-MS ion images are shown for the main ions detected, characteristic of brain tissue[45], (B) m/z 834.4, PS (40:6); and (C) m/z 888.5, in the negative ion mode and (D) m/z 782.6, PC(34:1)+Na+ in the positive ion mode. While detailed comparison of the images is not intended, the ion images reveal that many of the same lipid species are present in the brains of the many different animal species analyzed. Differences in lipid distributions between species can be attributed largely to the presence of white and/or grey matter within the tissue section analyzed in the cases of the ions selected for display.
2.3. Solvent Systems
While geometric and spray parameters affect DESI-MS image quality, the chemical and physical properties of the solvent system chosen affect the molecular information obtained. Many studies have shown that the solvent composition significantly affects the extent of desorption and ionization of analytes during DESI [48, 49]. The ability to modify the spray components to enhance or promote the ionization of different species is a feature of DESI which greatly amplifies its application for lipid analysis. In conventional DESI-MS experiments, mixtures of water with methanol or acetonitrile (ACN), with or without an acidic modifier, are the most commonly used solvent systems for the analysis of lipids from biological samples [38]. Tissue with high lipid content, such as brain and spinal cord, are commonly analyzed without the need for the addition of an acid modifier, allowing abundant detection of different FAs, GP such as glycerophosphoethanolamines (PE), plasmenyl glycerophosphoetanolamines (plasm-PE), PS, glycerophosphoinositols (PI), glycerophosphoglycerols (PG) and SP such as sulfatides (ST) in the negative ion mode and detection of GPs such as PE and glycerophosphocholine (PC), and SP such as SM and Cer in the positive ion mode [45, 50]. Addition of a weak acid modifier such as acetic acid or formic acid to a methanol/water binary solvent system is also common for analysis of polar lipids by DESI-MS in the positive ion mode, as reported for rat brain [51], mouse pancreas [51], mouse lung extract [42], chicken heart [51] and human astherosclerotic plaque [52]. Conventional DESI-MS analysis of polar lipids from other tissue types such as canine bladder [53], human bladder [10], kidney [4], prostate [54] and testis [55] has also been successfully performed using a binary mixture of acetonitrile and water (1:1) without any acid modifier. The polarity of the solvent mixture being used greatly affects the information obtained from the tissue and it can be adjusted to enhance signals from other compound classes. As an example, we recently observed that distinctive lipid profiles can be obtained from a mouse brain section by using a mixture of dimethylformamide (DMF) with water on the one hand and methanol/water on the other. While a mixture of methanol:water (1:1) yields a mass spectra with high signals for GPs in the m/z range of 700–1000, as previously reported [45], a mixture of DMF:water (1:1) greatly enhances the signals of low molecular weight compounds, such as small metabolites, FAs and FA dimers (Fig. 3). Furthermore, the DMF:water solvent system is not as destructive, allowing sequential staining of the tissue section or even additional DESI-MS analysis. As another example, a binary solvent system of methanol and chloroform (3:1) has been found to significantly enhance the desorption of several gangliosides [56]. A recent study has shown that the surface tension of the DESI spray solution can be manipulated by adding surfactants to conventional solvent systems, such as methanol:water (1:1). An increase in desorption ionization signal of more than an order of magnitude was achieved for many analytes studied using the surfactant spray solutions [57]. While lipid-rich tissues can be analyzed easily using common solvents, tissue containing relatively high concentrations of protein can be more difficult. Lipid analysis of human lens tissue by DESI-MS imaging is not possible with common solvent systems but addition of 0.5% hydrochloric acid (HCl) to a methanol:water (4:1) solvent system allowed detection of many different lipids in the positive ion mode [58]. In this case, lipid detection was only possible when the DESI spray physically disrupted the tightly compacted structure of the tissue fiber cells. Spatial distribution within this tissue structure was in agreement with the results of MALDI imaging [59] as is generally the case when comparisons are possible.
Fig. 3.
Negative ion mode mass spectra of mouse brain tissue section (15 µm) mounted onto glass slide analyzed using (A) methanol:water (1:1) and (B) DMF:water (1:1) as solvent systems under the same experimental conditions. Methanol:water (1:1) spectra are very similar to those previously reported for mouse brain [45], while the DMF:water (1:1) solvent system enhances the signals from low m/z lipids and small metabolites.
2.4. Adduct Formation
One auxiliary method of enhancing the lipidomic information in conventional DESI-MS analysis is the addition of salts to promote adduct formation and so enhance ionization of particular lipid classes. This approach can be used to change polarity for the analysis of lipids of a particular class. For example, PC and SM, which are normally analyzed as protonated or sodiated ions in the positive ion mode due to the permanent positive charge located on the quaternary amine of the head groups, can be analyzed in the negative ion mode by forming adducts with anionic species, such as acetate and formate [12, 38]. There are two main advantages of increasing detectability of these classes of lipids in the negative ion mode; firstly, these adducts enable greater structural characterization during tandem MS analysis and secondly, a single DESI experiment can be carried out to analyze PCs and SMs together with other acidic lipids. Another approach is to use adduct formation to detect classes of lipid that are not well ionized in either polarity by conventional DESI-MS analysis. The detection of cholesteryl esters (CE), for example, can be greatly facilitated through the formation of ammonium adducts in the positive ion mode by doping a conventional methanol:water (3:1) solvent with 0.01% ammonium chloride (NH4Cl) [52]. A recent study has shown that the formation of silver adducts in the positive ion mode greatly enhances the detection of biologically relevant alkenes [60]. DESI-MS analysis of 26 unsaturated lipid standards using conventional solvent systems doped with 5.5 µg/mL AgNO3 revealed that silver adduct formation was highly favorable for FA, fatty acid esters and prostaglandins, although silver ion attachment is not applicable to many polar lipids. The detection limits for the silver adducts of FAs were typically at least an order of magnitude lower than those achieved for the deprotonated form in the negative ion mode. Most interestingly, silver adduct formation enabled the detection of triacylglycerols (TAG) directly from canine bladder tissue; this class of lipid not previously detected in conventional DESI-MS imaging experiments from complex biological samples [60].
2.5. Reactive DESI
It is an advantage of spray-based techniques that reagents can be added to the solvent spray such that specific compounds or functional groups present in the sample can be targeted to undergo chemical reactions. These reactive DESI experiments[61] can be used to improve sensitivity and selectivity for molecules which are not easily ionized by DESI-MS [62, 63]. The chemical reactions occur at the spot being sampled concurrently with acquisition of mass spectra, allowing imaging of the target compound to be achieved. Examples of reactions that have been used in reactive DESI include cyclization of phenylboronic acid with cis-diols [64], transacetalization of acylium ions [65], oxime formation involving the carbonyl group on steroids and hydroxylamine [62], hemiacetal salt formation between alcohol groups and betaine aldehyde (BA) [63], and hydrazine formation between hydrazines and carbonyl groups of ketosteroids [66].
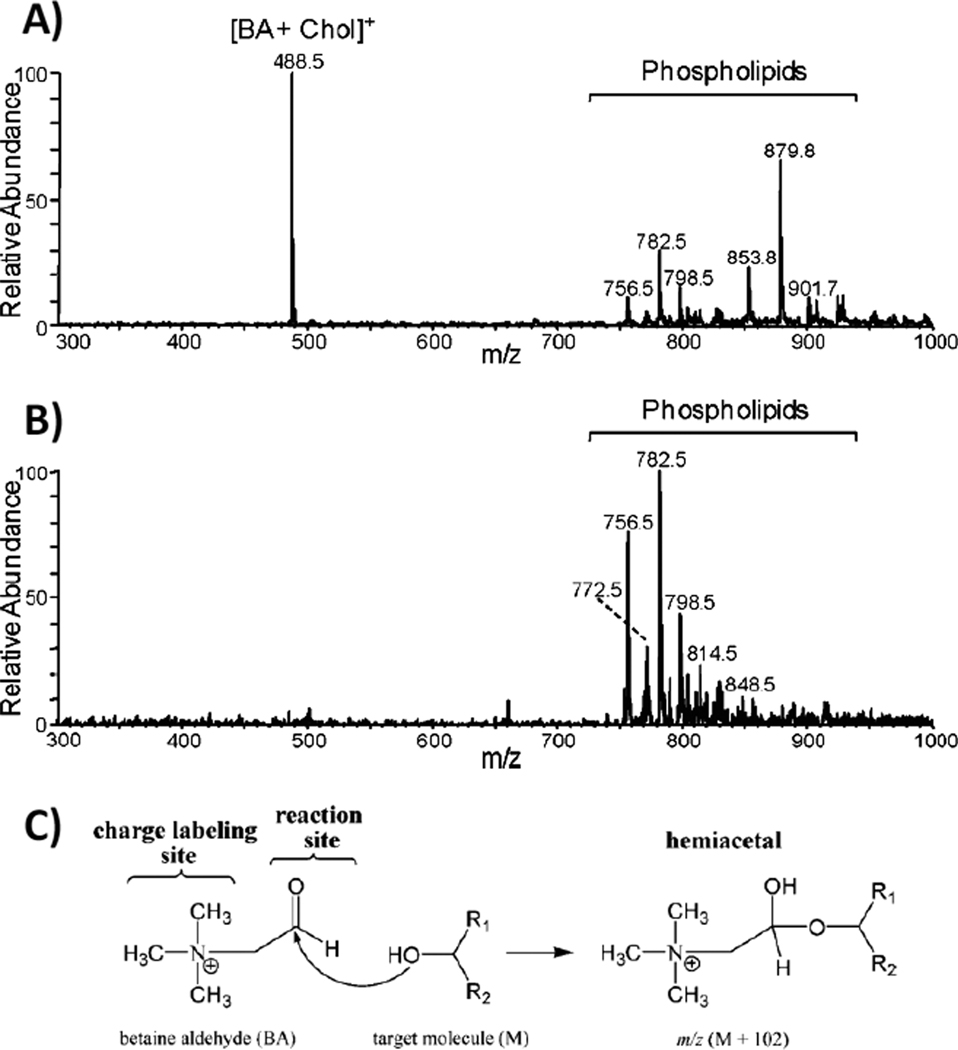
Reactive DESI lipid analysis has been performed to target anabolic steroids in raw urine samples by derivatizing steroid carbonyl groups with hydroxylamine which is added to DESI spray solution [62]. Protonated hydroxylamine ions, formed during electrospray, are carried in the primary microdroplets of solution towards the substrate. They react rapidly in the collapsing droplet with neutral steroids, improving the ionization efficiency of all seven steroids studied, including a glycosylated steroid. The main sterol synthesized by animals, cholesterol, has a low proton affinity and a low acidity, making its detection by techniques such as ESI and MALDI challenging [67, 68]. Reactive DESI is successfully used to perform direct and rapid analysis of cholesterol in dried serum samples, lipid extracts and animal tissue sections of several types. This is achieved by incorporating into the DESI solvent betaine aldehyde, a compound that selectively and rapidly reacts with the alcohol group of the cholesterol through nucleophilic addition, forming a charged hemiacetal easily detected by DESI-MS. Not only cholesterol but also other STs such as vitamin D2 and vitamin D3 can be detected in reactive DESI when using BA as the reagent. Quantitative analysis of cholesterol has been achieved with limits of detection of pure cholesterol in the range of 1 ng. The success of the reactive DESI experiment for cholesterol detection can be seen in the spectrum shown in Fig. 4. While a cholesterol signal is completely absent in the analysis of a rat brain section by conventional DESI imaging, the derivatized ion at m/z 488.5 is observed at high abundance directly from tissue. The conditions for this reactive DESI experiment were optimized and the solvent system ACN:H2O:DMF (8:3:1) doped with 65 ppm of BA was found to be ideal for imaging of cholesterol. Besides rat brain, reactive imaging of cholesterol has also been achieved in atheroma tissue and porcine adrenal gland [69]. In human atherosclerotic plaque tissue, the distribution of cholesterol was detected more abundantly in certain lipid rich regions of tissue, which is consistent with what is known about plaque formation in humans [52].
Fig. 4.
(A) Reactive DESI mass spectrum (background subtracted) of rat brain tissue using as solvent ACN:H2O:DMF (8:3:1) doped with 65 ppm BA, showing derivatized cholesterol (S/N ≥ 100) and various phospholipids. (B) Normal DESI mass spectrum (background subtracted) of rat brain tissue using ACN:H2O:DMF (8:3:1) as spray solvent without adding the BA reagent. Note that some of the additional peaks seen in the mass range m/z 850 to 1000 of the reactive DESI (A) compared to the normal DESI spectrum (B) are reaction products of PCs with BA and water. (C) Mechanism of in situ reaction between BA cation and the hydroxyl group of an alcohol. Figure adapted from reference 63.
3. DESI-MS applications
3.1. Lipid Characterization
Tandem MS
Linear ion traps (LIT) mass analyzers are commonly used for DESI experiments and therefore, tandem mass spectrometry has been the main method for lipid characterization and identification. Since DESI gives mass spectra that resemble those given by ESI, confirmation of the lipid assignments by DESI can be achieved by spectral comparison of tandem MS data to existing ESI mass spectral literature or to MS/MS data for authentic lipid standards [70–74]. Several studies have been performed with the goal of characterizing lipids detected directly from tissue or from lipid extracts by conventional DESI-MS, with emphasis on the fragmentation pathways of lipid classes such as PCs, SMs, PSs, PEs, STs, PIs, PGs and gangliosides [12, 38, 50, 56, 58]. Identification and characterization of FA, DG, LPG and LST in the negative ion mode has been achieved through tandem MS measurements augmented by accurate mass measurements [66]. Fragmentation of lipid adducts can be informative of the adduct structure. For example, the main fragment of PC acetate adducts in the negative ion mode corresponds to the neutral loss of methyl acetate arising from an intermolecular SN2 reaction between the choline head group and the acetate [38]. A sequential product ion (MS3) spectrum selecting the main fragment ion for further dissociation, shows peaks corresponding to the acyl chains, confirming the identity of the FAs attached to the glycerol backbone. Similarly, the main fragment ion of PC and SM chlorine adducts correspond to the loss of neutral methyl chloride from the choline head group [52]. When reactive DESI is used as means of analysis, it is important to understand the products of the reaction being used in order to predict the fragment ions that will be formed on its dissociation. For example, the reaction between BA and other secondary alcohols of molecular weight M are detected as ions of m/z (M+102), where 102 is the molecular weight of BA [63]. Imaging by DESI-MS can also be performed in the tandem MS mode, by which the distribution of a particular compound of known fragmentation is recorded [75]. The distribution of the drug clozapine, in different tissue sections has been reported by DESI-MS imaging in the MS/MS mode, by mapping the distribution of its main fragment ion at m/z 270 [76]. This highly specific method of imaging is useful when high chemical noise is present or to map the different distributions of two compounds with the same nominal mass.
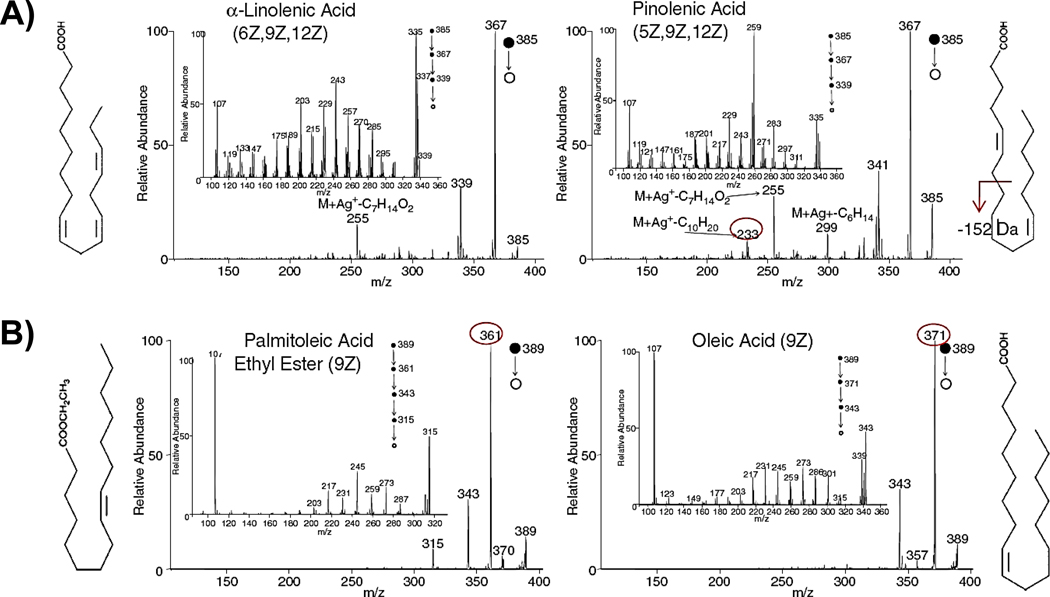
The distinction between isomeric and isobaric lipids is a particularly important one, given the large number of naturally occurring lipids and their close relationship to each other. A successful example of FA isomer distinction by DESI-MS analysis was recently demonstrated through silver adduct formation [60]. Three sets of isomers were evaluated and were distinguished based on tandem MS analysis of the Ag+-adducts formed from these FAs. For a pair of FA isomers varying in olefin location, such as α-linolenic acid (6Z, 9Z, 12Z–C18H30O2) and pinolenic acid (5Z, 9Z, 12Z–C18H30O2), data up to MS5 was used to elucidate olefin bond location (Fig. 5A). Distinction between ethyl ester derivatives and the higher homologous free FAs, such as that between the isomer pair palmitoleic acid ethyl ester (9Z) and oleic acid (9Z) (Fig. 5B), was readily achieved using simple MS/MS, since the ethyl ester yielded a dominant ethylene loss fragmentation ion while the FA spectrum was dominated by water elimination. The ability to achieve isomer differentiation using the silver adduct could be incorporated into DESI imaging using a reaction monitoring scan. This would provide a means of simultaneously acquiring 2D images and obtaining structural confirmation of FAs.
Fig. 5.
Isomeric FAs can be successfully distinguished by tandem MS after silver attachment in DESI analysis. (A) FA isomers varying olefin location, α-linolenic acid (6Z, 9Z, 12Z–C18H30O2) and pinolenic acid (5Z, 9Z, 12Z–C18H30O2) and (B) Ethyl ester derivatives and their free FA isomers, palmitoleic acid ethyl ester (9Z) and oleic acid (9Z). Figure adapted from reference 60.
Exact mass measurement
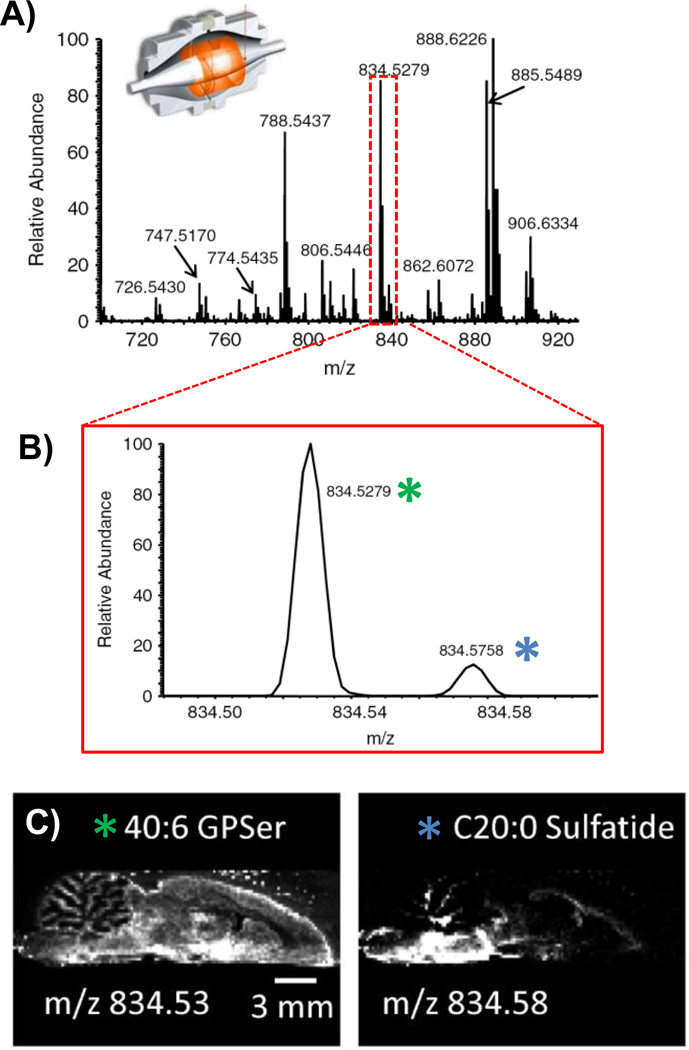
An alternative route to lipid characterization is to perform high-resolution measurements using such mass analyzers as the FT-ICR or orbitrap. For example, high-resolution imaging of rat brain tissue has been performed by DESI-MS using an orbitrap mass spectrometer (Fig. 6) [77]. Selecting 30K mass resolution for imaging experiments, it was observed that while the lipid profile obtained from a rat brain tissue was similar to what previously seen at low resolution, a number of new peaks were identifiable. Several pairs of peaks differed by less than 0.1 m/z units. Interestingly, this study showed that the ion m/z 834 previously identified as PS (40:6) using a linear ion trap consists of two different molecular species, PS(40:6) and C20 ST, with distinctive spatial distribution in the rat brain, highlighting the relevance of high mass accuracy in DESI imaging experiments. Nevertheless, the higher abundance of PS(40:6) when compared to C20 ST justifies the previous assignment of the m/z 834 ion as PS(40:6); this ion yielded the main fragments in the MS/MS experiment.
Fig. 6.
DESI-MS imaging can be performed using high-resolution measurements, increasing its capability for lipid analysis and identification. Data for a sagittal rat brain section imaged by negative ion mode DESI-MS using an orbitrap mass spectrometer at 30K mass resolution.[77] (A) Mass spectrum (B) Peaks at m/z 834 differing by less than 0.1 m/z units, identified as PS (40:6), m/z 834.5279, and C20 sulfatide, m/z 834.5758. (C) Distinctive distributions within the rat brain section for these species, highlighting the usefulness of high mass-resolution analysis by DESI imaging. Figure adapted from reference 77.
DESI-MS after TLC/HPTLC separation
While one would like to detect as many species as possible in a single rapid analysis, matrix effects in complex biological samples impact the ease of detection of certain species, including some of those at relatively low concentrations. One solution is to perform a fast and relatively crude separation step, such as thin layer chromatography (TLC), followed by direct analysis by DESI-MS. TLC provides a rapid, robust, and inexpensive means of separation of lipids from solutions containing complex mixtures [78]. HPTLC followed by direct interrogation of the components on the TLC plates by DESI-MS has been used for the analysis of porcine brain lipids [12]. Direct analysis of a porcine brain extract was also performed by DESI without prior HPTLC separation, and this allowed the more abundant lipid species PS(36:1), PS(40:6), PE(34:2), PI(38:4) and ST(24:1) to be detected. Peak overlaps of isobaric compounds, 13C isotopic contributions, differences in ionization efficiencies and low absolute concentrations made identification of the other lipids present difficult. However, HPTLC separation followed by DESI-MS imaging enabled eight lipid class-specific spots to be imaged in the negative ion mode and more than 50 lipids to be identified.
Besides its use for analyzing lipid extracts, HPTLC has been recently coupled to DESI-MS for the analysis of intact tissue sections. Lipids were eluted from a 16 µm thick rat brain tissue slice directly mounted onto an HPTLC plate, followed by DESI-MS analysis of the lipid species in the negative ion mode [56]. Detection of multiple PI, PS, plasm-PE, PE, hydroxylated sulfatide (hST), ST, as well as gangliosides was achieved. The gangliosides detected included, GQ1, GT1, GD1a/b, and GM1. These results showed good qualitative agreement with HPLC/DESI-MS analysis of rat brain tissue. The coupling of HPTLC to DESI-MS is a simple and rapid method for the analysis of gangliosides and other lipids directly from thin tissue sections.
3.2. DESI-MS imaging of Biological Tissue
Tissue Characterization
DESI-MS profiling of intact biological tissue was first performed in 2005 in the positive ion mode using normal mouse pancreas tissue, normal rat brain tissue and normal adipose tissue surrounding a chicken heart [51]. The biomolecule information achieved from tissue analysis by DESI-MS imaging is concentrated in different regions of the positive and negative ion mass spectra. In the negative ion mode, the low m/z region (100–400) is dominated by monomers of fatty acids and metabolites, the central region (m/z 500–700) is dominated by dimers of fatty acids, diacylglycerols and lyso phospholipids, and the high m/z region (700–1100) is dominated by complex lipids such as glycerophospholipids and sphingolipids. In the spectra recorded from the pancreatic mouse and rat brain tissue the most abundant species were protonated and sodiated PCs and SMs, with the particular species and relative ion abundances varying between the two tissue types [51]. The adipose tissue from the chicken heart was analyzed without sectioning the tissue and free FAs were observed in the negative ion mode simply by directing the DESI spray at the tissue surface. Normal rat brain tissue was analyzed using DESI-MS imaging in the negative ion mode and a 2D ion image was created showing the spatial distribution of particular lipids throughout the tissue section [7]. In the recorded spectra, the lipid species seen most abundantly were deprotonated free FAs, PS, PI and ST. The individual ion images demonstrate the varying distribution of these species throughout the white and gray matter of the brain. DESI-MS imaging applications have since expanded rapidly. Of note is the construction of a 3D molecular image using sequential coronal sections of a mouse brain [45]. As was seen in the initial experiments conducted on rat brain, two distinctive MS peak patterns were observed in the negative ion mode for the brain sections analyzed, corresponding to differences associated with the lipid compositions of white and gray brain matter [45]. Because PS(40:6) is the most abundant lipid found in the gray matter and ST(24:1) is the most abundant lipid found in white matter [45] the relative distribution of just these two compounds provides a rough indication of the grey matter/white matter distribution in 3D. More detail is revealed by including ion images of different lipid species detected within the 36 2D mouse brain sections analyzed, resulting in 3D mouse brain models in which several anatomical structures, such as the lateral corpus callosum, could be identified. These models provide comprehensive information on the 3D molecular distribution of lipids within the brain, and correlation between these molecules and brain substructures (Fig. 7).
Fig. 7.
From among the many different lipid species detected in a DESI mass spectrum (A), specific ions can be followed in an imaging experiment to construct 2D ion images (B). In this example, the ion images of PS(40:6), m/z 834.4 (green) and ST(24:1), m/z 888.8 (red) are overlaid. 3D models (C) can be constructed from a suitable set of 2D data. They illustrate the spatial distributions of specific biomolecules in tissue substructures. By adding the dimension of depth to the image a better visualization of the object and the relative positions of its constituent structures in space is achieved. Figure adapted from reference 45.
Lipids are the most common biomolecules in spinal cord tissue, making up 50% of its dry weight [50]. Normal rat spinal cord tissue sections have been analyzed and imaged by DESI-MS to determine its lipid composition and spatial distribution (Fig. 8) [50]. Like the brain, the spinal cord consists of white matter, containing sensor and myelinated axons, surrounding the gray matter, composed primarily of neuronal cell bodies. Rat spinal cord cross sections were imaged in both the negative and positive ion modes to show the correlation of chemical information with morphological features. The resulting ion images show localization of particular species in either the white matter or gray matter The most commonly observed species in the negative ion mode mass spectra were deprotonated free FAs, PC, PE, plasm-PE, PG, PS, SM and ST, while PC, PE and SM were observed in positive ion spectra. In the negative ion mode, the ion image of m/z 750.6, plasm-PE(38:4) shows an approximately homogenous distribution within the white matter, but much lower signal intensity within the gray matter [50]. In the positive ion mode, the ion images for PC(24:1) and SM(42:1) show the same localization of these lipids within the white matter region of the spinal cord cross section. The “butterfly” shape of the gray matter was resolved in the ion images, with DESI achieving a spatial resolution of less than 200 µm.
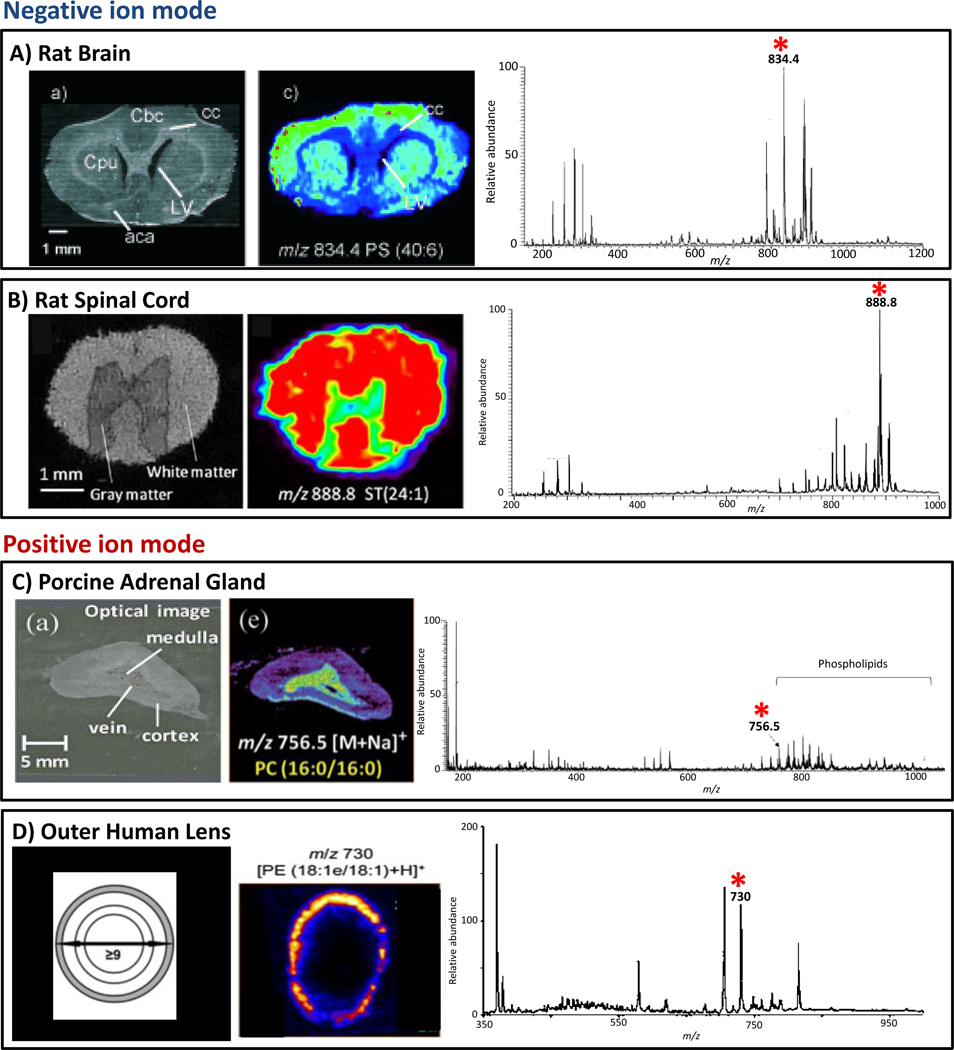
Fig. 8.
Tissue characterization by DESI-MS imaging. (A) Optical image of rat brain; DESI-MS ion image of PS(40:6), m/z 834.4, acquired at unit resolution in negative ion mode; representative negative ion mode DESI-MS mass spectrum from rat brain with the peak corresponding to PS(40:6) starred. (B) Optical image of rat spinal cord; DESI-MS ion image of ST(24:1), m/z 888.8, acquired at unit resolution in negative ion mode; representative negative ion mode DESI-MS mass spectrum from rat spinal cord with the peak corresponding to ST(24:1) starred. (C) Optical image of porcine adrenal gland; DESI-MS ion image of PC(16:0/16:0), m/z 756.6, acquired at unit resolution in positive ion mode; representative positive ion mode DESI-MS mass spectrum from porcine adrenal gland with the peak corresponding to PC(16:0/16:0) starred. (D) Schematic showing the delineations used to select regions of the human lens, the outer region is marked in grey; DESI-MS ion image of PE(18:1e/18:1), m/z 730 acquired at unit resolution in the positive ion mode; representative positive ion mode DESI-MS mass spectrum from the outer layer of human lens with the peak corresponding to PE(18:1e/18:1) starred. Adapted from references 7, 50, 69 and 58.
DESI-MS imaging was used to characterize the time-dependent alterations in lipid profiles in spinal cord tissue following spinal cord injury (SCI) and to correlate these changes to the biochemical events associated with the injury [66]. Comparisons with adjacent normal tissue, with a focus on the lipid species detected in the negative ion mode, were features of the study [66]. Increased relative intensities, of FAs, diacylglycerols (DAG), lysolipids (between +120% and +240%) as well as a small decrease in intensities of lipids (−30%) in the lesion epicenter and adjacent areas were seen after spinal cord injury [66]. This data indicates that hydrolysis of lipids occurs during the demyelination process due to activation of phospholipase A2 enzyme. Increased abundances of prostaglandin and hydroxyeicosatetraenoic acid detected within the injured signal cord tissue are indicative of oxidative degradation following injury. Furthermore, malondialdehyde, a product of lipid peroxidation and a marker of oxidative stress, was detected by reactive DESI imaging, which revealed increased relative abundances of malondialdehyde in the dorsal area of the injured spinal cord tissue when compared to the normal sample, as a consequence of the peroxidation biochemical events triggered by SCI. DESI-MS imaging can provide chemical and spatial information contributing to increased understanding of the lipid biochemistry occurring during spinal cord injury.
Porcine and rabbit adrenal gland tissues were imaged using DESI-MS in order to investigate the spatial distribution of multiple lipid species as well as that of small molecules such as epinephrine and norepinephrine (Fig. 8) [69]. The adrenal gland is separated into two distinct structures, the medulla and the cortex, with veins in the medulla. The particular GP species found to be localized within the medulla are, PC(36:0), PC(44:4), PC(30:1), PC(32:1) and PC(34:0) [69]. Those localized within the cortex include, PC(36:4), PC(36:1) and PC(38:4). In the negative ion mode, species such as arachidonic acid, oleic acid, eicosatrienoic acid, DHCer(34:0), PE(38:4), PI(36:4) and PI(38:4) are observed localized to the cortex of the adrenal gland. In comparison, ascorbic acid, plasm-PE(38:4), plasm-PE(40:4) and PS(40:4) are found within the medulla. Cholesterol was also mapped in porcine adrenal gland using reactive DESI experiments. The resulting DESI-MS ion images show that the signal is less intense in the area close to the vein within the medulla as compared to the cortex. While similar distributions of lipids were observed for the rabbit adrenal gland, the distribution and relative proportion of epinephrine and norepinephrine, which are known to be species specific, were found to be different to that observed for porcine adrenal gland. DESI-MS ion images of epinephrine showed that its signal was detected at greater abundance at the top edge of the porcine adrenal medulla while being uniformly distributed in the medulla of rabbit adrenal gland. DESI-MS imaging clearly shows great potential for the analysis of endogenous small molecules directly from the tissue of different animal species.
The lipid composition of the human lens was also analyzed and imaged by DESI-MS (Fig. 8). Human lens is high in dihydrosphingomyelin (DHSM) and the most abundant GPs are unusual 1-O-alkyl-ether linked PE and PS [58]. In the lower mass range, m/z 350–650 cholesterol, LPE, Cer and Cer1P species were observed, within the mass range from m/z 675 to m/z 975 SM, PE, PS, PC and LacCer species were identified in the positive ion mode. Analysis by DESI-MS provides a comprehensive overview of the lipid composition of the human lens in a single measurement. DESI-MS was also employed to created 2D images of human lens tissue in order to show the abundance distributions of the broad range of lipids detected [58]. SM(d18:0/16:0) and SM(d18:24:1) were found to be more abundant in the outer and barrier regions of the lens. The most abundant 1-O-alkyl GP, PE(18:1e/18:1) was shown to be predominantly distributed in a thin ring in the outer, metabolically active region of the lens, while a related lipid, PS(18:1e/18:1) along with all other low abundance 1-O-alkyl PE and PS lipids were shown to have an identical distribution. Cholesterol is distributed almost homogenously throughout the lens with the lowest signal intensity seen in the innermost region. Particular lipids, such as Cer(d18:0/16:0), Cer1P(d18:0/16:0), Cer(d18:0/24:1), Cer(d18:1/16:0) and Cer(d18:1/24:1) were found to be concentrated in the nucleus of the lens [58]. An interesting distribution was observed for LPE(18:1e) in that it had an annular distribution that is concentrated inside that of PE(18:1e/18:1), the conversion occurring in vivo either by enzymatic or chemical pathways, with one possibility being that LPE(18:1e) could be produced via hydrolysis of the acyl 18:1 FA in PE(18:1e/18:1). The composition and distribution of human lens lipids are altered with age, and the DESI-MS lens images from 28 and 50 and 58 year-old individuals suggest increasing concentrations of Cer(d18:0/16:0) in the inner region of the lens with age. In order to make a comparison between the generated DESI-MS ion image and the absolute amount of particular lipids, the lipids were extracted from each region, outer, barrier, inner and core, and quantified by ESI-MS. For PE(18:1e/18:1), the regional concentrations determined by ESI-MS closely matched the relative abundances determined by DESI-MS. The spatial and intensity distributions determined by DESI-MS imaging appear to be representative of the actual lipid distribution within the eye lens [58].
Disease State Evaluation
DESI-MS imaging of biological tissue samples was initially applied to animal tissues, but the first experiments aimed at diagnosis of disease were made using human liver [51]. The lipid profiles acquired in the positive ion mode allowed the discrimination between healthy and disease tissues [51]. In this as in subsequent work, the reference method was standard histopathological examination, usually using hematoxylin and eosin (H&E) stained tissue. While the potential for DESI-MS imaging to distinguish cancer from normal tissues was indicated, much work with a larger and more diverse cohort was needed (Fig. 9).
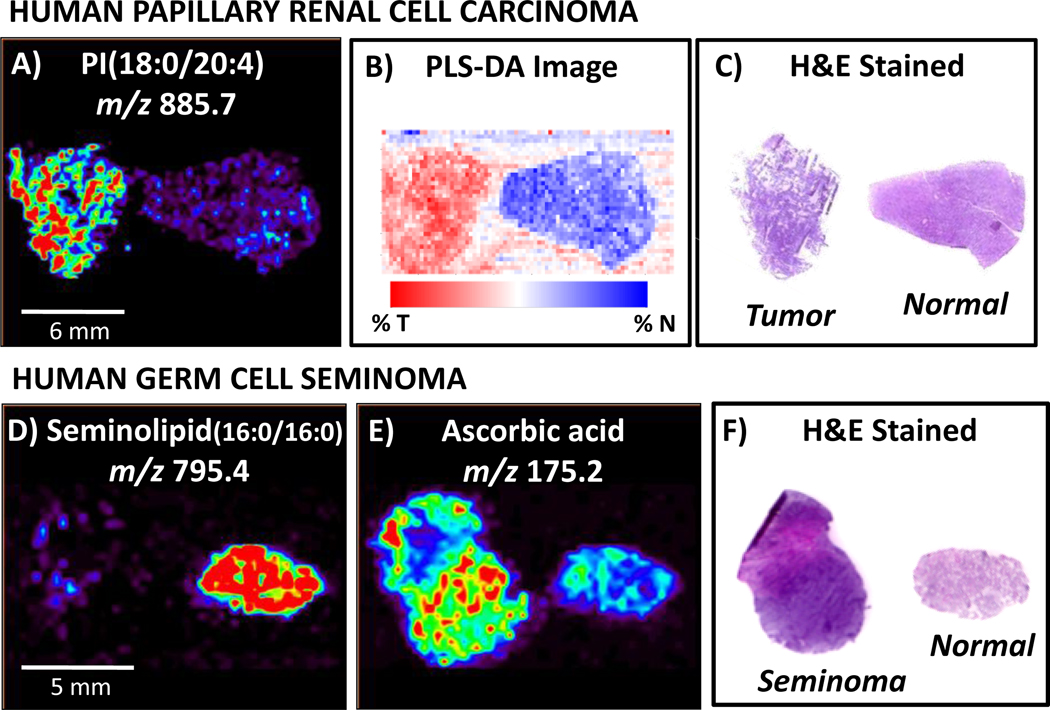
Fig. 9.
DESI-MS imaging has been used to analyze human cancerous tissue, including human papillary renal cell carcinoma and human germ cell seminoma. Agreement is seen between A) DESI-MS ion image of m/z 885.7, identified as PI(18:0/20:4), B) PLS-DA synthetic image using all ions and C) H&E stained tissue sections, enabling cancer diagnosis. Human germ cell tissue analysis by DESI imaging revealed hat D), while seminolipid (16:0/16:0), m/z 795.4, occurs in abundance in normal tubules, E)ascorbic acid, m/z 175.2, was present at higher intensities in cancerous tissue. This distinction between cancerous and normal germ tissue is consistent with F) diagnosis obtained from pathological analysis of H&E stained tissue sections. Adapted from references 4 and 55
DESI-MS imaging has been applied to the analysis of canine bladder cancer and adjacent normal tissues where it was used to successfully distinguish tumor from normal tissue on the basis of multiple marker lipids [53]. In the negative ion mode PS(36:1), PG(36:2), PI(36:1), PI(34:1), PS(36:2), FA(18:1), FA(18:2) and FA(20:4) were all found to give signals of increased intensity within the cancerous regions of the tissue as compared to the adjacent normal tissues [53]. Sodium and potassium adducts of PC(34:1) and PC(36:2) were detected at increased intensities within the diseased tissue sections when imaged in the positive ion mode, while higher intensities of SM(34:1) was seen within the normal tissue sections. In addition to the conventional DESI-MS experiments, the spray solvent was doped with silver ions in order to enhance the detection of olefins through formation of cationic adducts [60]. In the positive ion mode the primary compounds detected, silver adducts of free FAs, oleic acid and arachidonic acid, showed increased abundances in cancerous tissue. In addition TAGs were detected in the mass range greater than m/z 900, one particular TAG, TG(52:3) was detected within the canine bladder tissue, but was distributed throughout the tissues and not useful in cancer diagnosis [60]. All of the tissue sections analyzed and diagnosed by DESI-MS were compared to serial sections stained with H&E for pathological diagnosis.
DESI-MS imaging experiments have been conducted in many types of human cancerous tissues, providing successful diagnosis of disease. It is striking that while many of the same lipid species are present in multiple types of normal tissues, the aberrant metabolism that occurs during malignancy is unique to each type of cancer, resulting in distinct profiles of lipid species that can be easily recognized by DESI-MS and definitively associated with a specific disease. For human bladder cancer, tissue samples from 20 different patients including tumor and adjacent normal tissue from each were imaged using DESI-MS [10, 81]. Particular species, such as PS(36:1), PI(38:4), FA(18:1) and FA (as the monomers and dimers), were observed at increased intensities in cancerous tissue in the negative ion mode, and function as putative molecular markers of disease. The increased intensities of the FAs and especially their dimers could be indicative of the presence of lipid droplets, the co-localization of increased amounts of FAs resulting in increased dimer formation. Lipid droplets are thought to be key regulators of inflammatory disease and of many types of cancer, with increased lipid droplet accumulation found in cancerous cells [82]. These lipid droplets have complex functions within the cell, controlling cellular processes important to malignancy such as, cell activation, migration, proliferation and apoptosis [82]. The DESI-MS lipid profile of human bladder tissue allowed a clear visual distinction between cancerous and normal tissues. Multivariate statistical analysis was used to visualize and create a diagnostic rule for characterizing the tissue sections, using the methods of principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA). While interpretation of ion images alone is often valuable, statistical evaluation of the data is much more advantageous for diagnostics applications. The statistical methods rely on the entire set of mass spectral data acquired, not on selected ion images. The DESI-MS ion images correlate well with the statistically generated images, which correlate with the pathological diagnosis in 88% of the examined cases of bladder cancer [10].
In subsequent DESI-MS studies, cancerous and adjacent normal tissue from two types of human kidney cancer were analyzed [4]. Imaging DESI was used to interrogate the lipid profiles of thin tissue sections of 11 sample pairs of human papillary renal cell carcinoma (RCC) and adjacent normal tissue and 9 sample pairs of clear cell RCC and adjacent normal tissue. The DESI-MS images showing the varying spatial distributions of particular GPs and free FAs between cancerous and normal tissue in the negative ion mode were compared to serial tissue sections stained with H&E. For papillary RCC, the cancerous tissue exhibited increased absolute intensities for the lipid species at m/z 885.7 (PI(18:0/20:4)), m/z 788.5 (PS(18:0/18:1)), m/z 773.5 (PG(18:1/18:1)) and m/z 913.5 (PI(22:4/18:0)) and the normal tissue exhibited increased absolute intensity of FA species at m/z 215.3 (FA(12:0)). Unlike the papillary RCC samples, for the clear cell RCC tissue samples there were no noticeable visual trends in the absolute or relative intensities of the lipid species for either the cancerous or normal tissue, highlighting the need for a multivariate statistical analysis using the entirety of the mass spectral data. Multivariate statistical analysis using orthogonal projection to latent structures treated PLS-DA was used for visualization and classification of the tissue pairs using the full mass spectra as predictors. PLS-DA successfully distinguished tumor from normal tissue for both papillary and clear cell RCC with misclassification rates obtained from the validation set of 14.3% and 7.8%, respectively. It was also used to distinguish between papillary and clear cell RCC from each other and from the combined normal tissues with a reasonable misclassification rate of 23%, as determined from the validation set. Overall DESI-MS imaging combined with multivariate statistical analysis was able to distinguish both types of kidney cancer from paired normal tissue as well as distinguish between each type of cancer and the pooled normal samples on the basis of GP profiles.
Human prostate cancer tissue specimens from 34 different patients were also examined by DESI-MS imaging in the negative ion mode [54]. There were differences in the distributions of lipids and a single molecule, cholesterol sulfate (CS), was found to be over-expressed in precancerous lesions or cancer while giving little or no signal in the adjacent normal tissue. Many biological roles for CS in prostate cancer progression have been hypothesized, including its ability to influence cell signaling, cellular differentiation and apoptosis [83–85]. Similarly to prostate cancer, human seminoma tissue and adjacent normal tissues were analyzed using DESI-MS imaging in the negative ion mode [55]. A single class of compounds, the seminolipids (seminolipid(32:0) and seminolipid(30:0)), were found exclusively in normal tubule tissue, allowing for the identification of this tissue type. In addition, within the majority of cancerous tissue, ascorbic acid gave signals of significantly increased intensities, providing a single compound by which to identify cancerous tissue [55].
Glioma specimens from seven human subjects encompassing WHO grade II, grade III and grade IV (glioblastoma) were examined using DESI-MS in the negative and positive ion modes [5]. The major ions observed in the negative ion mode mass spectrum from a grade II astrocytoma correspond to lipids of classes ST, PS and PI. By contrast, the spectrum from a grade IV glioblastoma (GBM) showed the highest negative ion abundances for lipids of the classes PS, PI and plasmenyl-PE. The ST ions observed for the lower grade astrocytomas are completely absent in the small number of GBM tissues analyzed. While the lipid profiles observed for grade II and grade IV astrocytomas are distinctive, the negative ion mode mass spectrum obtained from a grade III astrocytoma, shows a transitional lipid composition [5]. Similar trends in tissue lipid profiles with increasing malignancy were observed in the positive ion mode spectra. A series of ions identified as galactoceramides (GalCer) was observed most abundantly in the positive mode spectrum for grade II astrocytomas, in lower abundance in the spectrum obtained for grade III astrocytomas and they are absent in the spectra obtained from the GBM samples. Standard histological H&E staining was performed on serial sections in order to confirm the diagnosis obtained through DESI-MS analysis. These preliminary findings indicate that it is possible to establish three different lipid profiles corresponding to each degree of malignancy II, III, and IV in astrocytic tumors by negative and positive ion DESI-MS analysis. Since these distinctive lipids profiles are easily assessed using DESI-MS imaging, the approach might possibly be translatable to in situ analysis of gliomas to assist in intraoperative surgical decision making [5].
In addition to attempts to establish a base of data needed to diagnose cancer, DESI-MS has been used to characterize tissues of other disease states. DESI-MS was used for the chemical profiling and imaging of human arterial plaques [52]. Since atherosclerosis is associated with the accumulation of lipoproteins within the wall of blood vessels, there is a desire to identify the lipid composition as well as their spatial distributions within the plaques. PCs, SMs and lysoglycerophosphocholines (LPC) were detected in both positive and negative ion modes, as sodium and chloride adducts, respectively. Addition of ammonium chloride to the DESI spray allowed the detection and imaging of CE in the positive ion mode. The ability to detect these CEs is important since they comprise a major fraction of the lipid-rich plaque and their abundance has been associated with plaque rupture. Cholesterol itself was also imaged in plaque tissue using the BA reactive DESI experiment. The lipid composition of plaques varies and can change over time, so it is valuable to be able to characterize and image these lipids to gain a better understanding of atherosclerosis.
4. Common complications in DESI-MS analysis
One general limitation of imaging mass spectrometry is the occurrence of matrix effects. Biological tissues contain a wide range of lipids belonging to different chemical classes as well as many other compound types. The presence of salts or even different classes of lipids in the same region of the tissue being investigated can change the efficiency of ionization of particular lipids [86]. Therefore, as is the case in other imaging techniques, it is not possible to be sure that the observed lipids accurately and completely represent the tissue constituents without performing extraction and quantitation in parallel by LC-MS. This is because ion suppression effects can be minimized by pre-separation steps which come at the cost of loss of information on the spatial distribution. This was demonstrated by the analysis of lipids from porcine brain extract by DESI-MS after 2D–TLC separation, which allowed the identification of a much larger number of lipids than did direct analysis of the extract without any prior separation [12]. Ion suppression makes the analysis of lipids not fully quantitative. Whilst DESI-MS imaging is not yet a quantitative technique, the signal intensities observed for particular species have been shown to correlate with the absolute amounts present in the tissues; both for endogenous lipids in human lens tissue and for exogenous drug metabolites in animal tissues [58, 76]. The qualitative results obtained in the investigation of lipid compounds in human diseased and normal tissue by DESI-MS imaging have not yet been followed-up by parallel LC-MS experiments. Nonetheless, if the imaging conditions allow the detection of biomarker molecules of interest, the degree to which the data is representative of the all the species in the sample is an interesting but secondary issue. The main requirement is that one can obtain comparative data from different tissue seconds. Also relevant to lipid analysis by MS is the precise attribution of structures to the lipid species. As with other ionization techniques performed without prior separation, DESI spectra include contributions from isobaric (same nominal mass) and isomeric compounds. The bewildering array of different lipid molecules includes numerous structural and stereoisomers. As an example, the GPs, which are highly relevant complex lipids, have as building blocks FA that most commonly contain between twelve and twenty-two carbon atoms, which can be in the sn-1 or sn-2 position in the glycerol backbone, and may be unsaturated in one or more carbon-carbon bonds at different carbons on the FA chain. Furthermore, isomerism of the double bonds in the free FA complicates precise structural assignment; this is the main reason why FA are normally tentatively assigned in DESI experiments based on the mass of the molecular ion in the negative ion mode [50]. The tentative assignment is based on prior knowledge of isomers occurring in related samples. There is a large likelihood that more than one lipid species may be detected at a given m/z value in DESI-MS spectra, especially when using the unit mass resolution provided by ion trap analyzers. The difficulties of structural attribution in lipid analysis have however not prevented lipid mapping by DESI-MS. For most MS-imaging applications, the precise attribution of the isomer species is not crucial as it would be for protein secondary structure determination. This is due to the fact that in most of the diagnostic studies performed by DESI imaging, identification of the lipids present in the mass spectra is not necessary for an empirical correlation of the histopathology and MS data [10]. Moreover, when the goal is to use multivariate statistical analysis for visualization and classification of MS data, the need for precise lipid identification is even further decreased [4]. Nonetheless, identification and structural characterization of the lipid species is important since understanding the role that specific lipids play in normal cells can lead to an understanding of how lipids function in disease state.
5. Perspectives and Conclusions
Lipids are readily detected by DESI imaging experiments and are of unequivocal diagnostic value due to their diverse physiological and pathological roles, their molecular diversity, and their structural variety. Fundamental studies with potential to advance DESI imaging technology are being pursued. Since solvent composition, doping and polarity influence lipid ionization by DESI-MS, expanding the lipidomic and metabolomic information from biological tissue may result from optimizing solvent combinations for analysis and evaluating solvent-dependent DESI morphological effects on tissues, as exemplified in Fig. 3. This combination is indispensable for microscopic analysis of sections previously submitted to DESI analysis and for intraoperative DESI applications. Another fundamental technical issue relates to expanding reactive DESI applications to specifically target lipid classes which not readily ionized in conventional DESI or to suppress ionization of particular classes so as to increase analytical specificity. There may also be value in implementing the recent observation of acceleration of reaction rates by several orders of magnitude in the microdroplets splashed from the surface during reactive DESI experiments to reaction systems not yet explored [61].
Fast screening of normal or pathological tissue samples is a major goal in DESI imaging studies, and this approach is exemplified in this manuscript in the DESI imaging of tissue microarrays (Fig. 2). High-throughput chemical analysis of tissue microarrays could be performed by the addition of internal standards to allow lipid quantification and this work could be done in parallel with DESI imaging. While the standard spatial resolution of ~ 200 um obtained by DESI imaging is sufficient for disease diagnosis and information on tumor margins, there are increasing indications that for many other applications where DESI imaging could be useful this spatial resolution will be a significant limitation. For this reason, we are currently working on significantly increasing the spatial resolution of DESI and will report on this soon. Another area of investigation is the evaluation of the effects of drugs on animal models or for lipid mapping among multiple animal species. In addition, direct tissue analysis of metabolites and their alterations in different pathologies has not been extensively addressed by DESI-imaging.
The review has concerned only the single ambient ionization imaging method of DESI. The relationship of DESI imaging to other MS imaging methods was noted in the Introduction. There are many parallels between the systems studied and the data obtained by DESI and by MALDI imaging in particular. For example, gangliosides have been detected by both MALDI [87] and DESI [56] in the negative ion mode after planar chromatographic separation of brain lipid extracts. Cholesterol is detected at m/z 369.3 by MALDI as a product of its dehydration [M+H–H2O]+ in biological fluids [88] while dehydrated cholesterol has also been observed in human lens DESI-imaging [58]. Detection of protonated and sodiated forms of PCs and SMs from human liver tissue has also been reported by both DESI[51] and MALDI [89]. Imaging of rat brain sections in the negative ion mode by both MALDI [90] and DESI [7] have shown preferential distribution of ST (24:1) in the white matter region. In addition to DESI, other ambient ionization methods are likely to be increasingly used for imaging. Already there is a substantial literature on the use of the laser-based methods such as laser ablation electrospray ionization (LAESI) [91], laser ablation coupled to flowing atmospheric-pressure afterglow (LA-FAPA) [92] and atmospheric pressure femtosecond laser imaging mass spectrometry (AP fs-LDI IMS) [93], for ambient imaging, amongst others. One must also note that DESI and other ambient ionization methods need not be used in an imaging mode. Experiments on biological tissue in which one examines the lipid constituents directly from the tissue sample or from a TLC extract have been reported [56]. Intrinsically non-imaging experiments, like paper spray ionization, also have a role in biological tissue analysis [94]. For example, tissue sections can be examined for their lipid composition by placing the section on a paper triangle and applying a high voltage [95]. Another recently developed non-imaging technique, rapid evaporative ionization mass spectrometry (REIMS), allows in vivo and in situ analysis of tissue by MS during surgical interventions [96]. This technique interrogates the lipid composition of surgical smoke produced by direct electrothermal desorption of tissue. A tissue identification system was developed using spectral library matching and PCA, allowing real-time diagnosis and identification of tissue features.
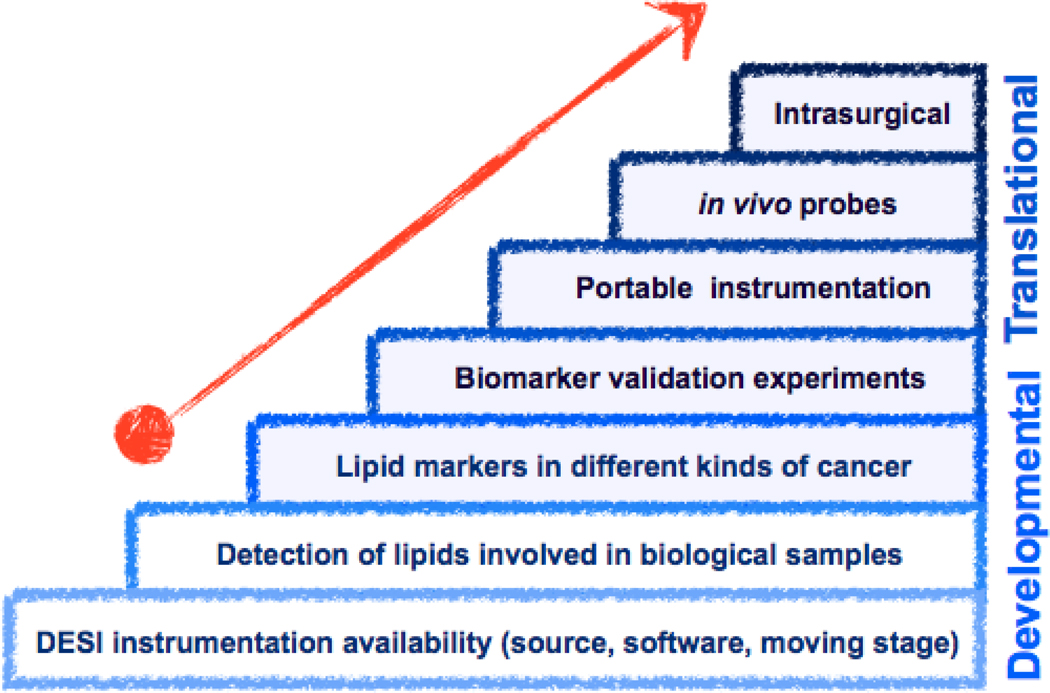
Fig. 10 summarizes DESI lipid imaging benchmarks in technological development and the beginning of its “translational steps” into routine medical applications by screening other cancerous tissues and lipid-related diseases and validating biomarkers in large sets of samples. DESI ionization sources are commercially available for many types of mass spectrometers. Portable instrumentation that includes capabilities for DESI imaging is likely to become available. As high resolving power is not fundamental for lipid analysis by MS, miniaturized instrumentation presents many advantages [97–99].
Fig. 10.
Visual representation of milestones related to development and translation of DESI imaging technology. Developmental "steps" are detailed in this review and should be followed by a translational research stage aimed at routine medical diagnostic use for tumor classification or progression, and also for in vivo DESI imaging applications
Developments in the analysis of fresh tissue should pave to way to the most challenging DESI application, its intraoperative use in surgery. Surgery remains the most important and usually first treatment modality for the majority of human solid tumors. While maximal surgical excision of gross total tumor resection is desirable, in practice, delineation of margins is very difficult because tumors can closely resemble normal tissue [5]. Therefore, information on boundaries between normal and cancerous tissue is very important during surgery for most types of tumors, especially brain gliomas. The development of a DESI based probe for in vivo tissue analysis during tumor resection or minimally invasive surgery could contribute to the unmet need of localizing tumor boundaries and then resecting tumors based on chemical tissue composition on the mm scale.
In conclusion, DESI lipid imaging applications in biomedical and life sciences should include the diagnosis of human diseases associated with aberrant lipid metabolism, biomarkers for cancerous tissue localization, grading and intraoperative surgical margin evaluation. The specific distribution of particular lipid classes within tissues is not well understood [100], so that basic science and biotechnologies related to embryology and tissue engineering should also benefit from DESI lipid imaging.
Table 1.
Biological applications of DESI-MS for lipid analysis
| Biological Sample |
DESI solvent system | Lipid classes detected | Imaging | Ref | |
|---|---|---|---|---|---|
| Negative ion mode | Positive ion mode | ||||
| Rat brain | MeOH:H2O (1:1) + 1% acetic acid | --- | PC, SM | No | [51] |
| MeOH:H2O (1:1) | FA, plasm-PE, PE, PS, ST, LPA, PI | --- | Yes | [7] | |
| ACN:H2O:DMF (8:3:1) + 65 ppm BA (reactive) | --- | Cholesterol, PC | Yes | [63] | |
| MeOH:H2O (1:1) | FA, PS, PI, ST | PC, PE, SM, Cer | Yes | [79] | |
| 1. MeOH:H2O (1:1) 2. MeOH:H2O (1:1) + 0.1% NH4HCO2 |
1. PS, PI, ST 2. PS, PI, ST, PC |
--- | No | [38] | |
| MeOH:CHCl3 (3:1) | PE, plasm-PE, PI, PS, hST, ST, GM1, GD1, GT1, GQ1 |
--- | No | [56] | |
|
Porcine brain extract |
1. MeOH:H2O (1:1) 2. MeOH:H2O (1:1) + 0.1% NH4HCO2 |
1. PS, PI, ST 2. PS, PI, ST, PC |
1. PC | No | [38] |
| 1. MeOH 2. ACN + 20 ppm BA (reactive) |
1. PC, PS, SM, PI, PE, plasm-PE | 1. PC, SM 2. Cholesterol |
Yes | [12] | |
|
Porcine & rabbit adrenal gland |
1. MeOH:H2O (1:1) 2. ACN:CHCl3 (1:2) + 50 ppm BA (reactive) |
1. FA, DHCer, plasm-PE, PE, PS, PI |
1. PC 2. Cholesterol |
Yes | [69] |
| Mouse brain | MeOH:H2O (1:1) | FA, PE, PG, PS, plasm-PE, ST, PI | --- | Yes | [45] |
| Mouse pancreas | MeOH:H2O (1:1) + 1% acetic acid | --- | PC, SM | No | [51] |
|
Mouse lung extract |
MeOH:H2O (1:1) + 0.1% acetic acid | PG, PI | PC | No | [42] |
| Rat spinal cord | MeOH:H2O (7:3) | FA, plasm-PE, SM, PC, PE, ST, PS, PG, PI |
PC, SM, PE, plasm-PE | Yes | [50] |
|
Injured Rat spinal cord |
MeOH:H2O (1:1) | FA, plasm-PE, SM, PC, PE, ST, PS, PG, PI, PGF2α, LPG, LST, DAG |
--- | Yes | [66] |
| Chicken Heart | MeOH:H2O (1:1) + 1% acetic acid | FA | --- | No | [51] |
| Bacteria | MeOH:H2O (1:1) | FA, LPE, LPG, PG | LPE, PE | No | [39] |
| MeOH:H2O (1:1) | --- | PE, acylium ions of FA | No | [80] | |
| Canine bladder | ACN:H2O (1:1) | FA, PS, PG, PI | PC, SM | Yes | [53] |
| ACN + 5.5 µg/mL AgNO3 | --- | FA, TAG | Yes | [60] | |
| Human liver | MeOH:H2O (1:1) + 0.1% NH4OH | --- | PC, SM | No | [51] |
| Human brain | MeOH:H2O (1:1) | Plasm-PE, PE, PS, ST, PI | PC, SM, GalCer | Yes | [5] |
|
Human atherosclerotic plaque |
1. MeOH:H2O + 40 ppm formic acid 2. MeOH:H2O (3:1) 3. MeOH:H2O (3:1) + 0.01% NH4Cl 4. ACN:H2O:DMF (8:3:1) + 65 ppm BA (reactive) |
2. LPC, SM, PC | 1. LPC, SM, PC 2. LPC, SM, PC 3. CE 4. Cholesterol |
Yes for solvents 1 & 4 |
[52] |
| Human bladder | ACN:H2O (1:1) | PS, PI, FA | PC | Yes | [10, 81] |
| Human kidney | ACN:H2O (1:1) | FA, PI, PS, PG | --- | Yes | [4] |
| Human testis | ACN:H2O (1:1) | FA, PI, PS, seminolipid | --- | Yes | [55] |
| Human prostate | ACN:H2O (1:1) | FA, PI, PS, CS | --- | Yes | [54] |
| Human lens | MeOH:H2O (4:1) + 0.05% HCl | --- | Cholesterol, LPE, Cer, SM, PE, PS, PC, LacCer, Cer1P |
Yes | [58] |
Acknowledgement
Support of the work on which this review is based by the U.S. National Institutes of Health (Grant 1R21 EB00 9459-01), National Science Foundation (CHE 08–48650) and the Alfred Mann Institute for Biomedical Development at Purdue University (AMI) is acknowledged.
Abbreviations
- DESI-MS
desorption electrospray ionization mass spectrometry
- HPLC
high performance liquid chromatography
- GC
gas chromatography
- TLC
thin-layer chromatography
- SIMS
secondary ion mass spectrometry
- MALDI
matrix assisted laser desorption ionization
- FA
fatty acids
- GP
glycerophospholipids
- GL
glycerolipids
- SP
sphingolipids
- ST
sterol lipids
- SM
sphingomyelin
- PS
glycerophosphoserine
- GalCer
galactoceramide
- H&E
hematoxylin and eosin
- PE
glycerophosphoethanolamines
- plasm-PE
plasmenyl glycerophosphoetanolamines
- PI
glycerophosphoinositols
- PG
glycerophosphoglycerols
- ST
sulfatides
- PC
glycerophosphocholine
- CE
cholesteryl esters
- LIT
Linear ion traps
- hST
hydroxylated sulfatide
- DHSM
dihydrosphingomyelin
- TAGs
triacylglycerides
- DAG
diacylglycerols
- PCA
principal component analysis
- PLS-DA
partial least squares discriminant analysis
- RCC
renal cell carcinoma
- GBM
glioblastoma
- LPC
lysoglycerophosphocholines
- LAESI
laser ablation electrospray ionization
- LA-FAPA
laser ablation coupled to flowing atmospheric-pressure afterglow
- AP fs-LDI
atmospheric pressure fentosecond laser desorption ionization
- REIMS
rapid evaporative ionization mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ifa DR, Wu C, Ouyang Z, Cooks RG. Desorption electrospray ionization and other ambient ionization methods: current progress and preview. Analyst. 2010;135:669–681. doi: 10.1039/b925257f. [DOI] [PubMed] [Google Scholar]
- 2.Venter A, Nefliu M, Cooks RG. Ambient desorption ionization mass spectrometry, TrAC. Trends Anal. Chem. 2008;27:284–290. [Google Scholar]
- 3.Alberici RM, Simas RC, Sanvido GB, Romao W, Lalli PM, Benassi M, Cunha IBS, Eberlin MN. Ambient mass spectrometry: bringing MS into the "real world". Anal. Bioanal. Chem. 2010;398:265–294. doi: 10.1007/s00216-010-3808-3. [DOI] [PubMed] [Google Scholar]
- 4.Dill AL, Eberlin LS, Zheng C, Costa AB, Ifa DR, Cheng LA, Masterson TA, Koch MO, Vitek O, Cooks RG. Multivariate statistical differentiation of renal cell carcinomas based on lipidomic analysis by ambient ionization imaging mass spectrometry. Anal. Bioanal. Chem. 2010;398:2969–2978. doi: 10.1007/s00216-010-4259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberlin LS, Dill AL, Golby AJ, Ligon KL, Wiseman JM, Cooks RG, Agar NYR. Discrimination of Human Astrocytoma Subtypes by Lipid Analysis Using Desorption Electrospray Ionization Imaging Mass Spectrometry. Angew. Chem. Int. Ed. 2010;49:5953–5956. doi: 10.1002/anie.201001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agar NYR, Golby AJ, Ligon KL, Norton I, Mohan V, Wiseman JM, Tannenbaum A, Jolesz FA. Development of Stereotactic Mass Spectrometry for Brain Tumor Surgery. Neurosurgery. 2011;68:280–290. doi: 10.1227/NEU.0b013e3181ff9cbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiseman JM, Ifa DR, Song QY, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew. Chem. Int. Ed. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman JM, Ifa DR, Venter A, Cooks RG. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat. Protoc. 2008;3:517–524. doi: 10.1038/nprot.2008.11. [DOI] [PubMed] [Google Scholar]
- 9.Dill AL, Ifa DR, Manicke NE, Zheng OY, Cooks RG. Mass spectrometric imaging of lipids using desorption electrospray ionization. J. Chromatogr. B. 2009;877:2883–2889. doi: 10.1016/j.jchromb.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dill A, Eberlin LS, Costa AB, Zheng C, Ifa DR, Cheng L, Masterson TA, Koch MO, Vitek O, Cooks RG. Multivariate Statistical Identification of Human Bladder Carcinomas using Ambient Ionization Imaging Mass Spectrometry. Chem. -Eur. J. 2011 doi: 10.1002/chem.201001692. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto-Inoue N, Hayasaka T, Taki T, Gonzalez TV, Setou M. A new lipidomics approach by thin-layer chromatography-blot-matrix-assisted laser desorption/ionization imaging mass spectrometry for analyzing detailed patterns of phospholipid molecular species. J. Chromatogr. A. 2009;1216:7096–7101. doi: 10.1016/j.chroma.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 12.Paglia G, Ifa DR, Wu CP, Corso G, Cooks RG. Desorption Electrospray Ionization Mass Spectrometry Analysis of Lipids after Two-Dimensional High-Performance Thin-Layer Chromatography Partial Separation. Anal. Chem. 2010;82:1744–1750. doi: 10.1021/ac902325j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hove ERA, Smith DF, Heeren RMA. A concise review of mass spectrometry imaging. J. Chromatogr. A. 2010;1217:3946–3954. doi: 10.1016/j.chroma.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Pol J, Strohalm M, Havlicek V, Volny M. Molecular mass spectrometry imaging in biomedical and life science research. Histochem. Cell Biol. 2010;134:423–443. doi: 10.1007/s00418-010-0753-3. [DOI] [PubMed] [Google Scholar]
- 15.Pacholski ML, Winograd N. Imaging with mass spectrometry. Chem. Rev. 1999;99 doi: 10.1021/cr980137w. 2977-+. [DOI] [PubMed] [Google Scholar]
- 16.Boxer SG, Kraft ML, Weber PK. Advances in Imaging Secondary Ion Mass Spectrometry for Biological Samples. Annu. Rev. Biophys. 2009;38:53–74. doi: 10.1146/annurev.biophys.050708.133634. [DOI] [PubMed] [Google Scholar]
- 17.Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 18.Li LJ, Sweedler JV. Peptides in the Brain: Mass Spectrometry-Based Measurement Approaches and Challenges. Annu. Rev. Anal. Chem. 2008;1:451–483. doi: 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- 19.Reyzer ML, Caprioli RM. MALDI-MS-based imaging of small molecules and proteins in tissues. Curr. Opin. Chem. Biol. 2007;11:29–35. doi: 10.1016/j.cbpa.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Zhang QC, Zou HF, Guo BC, Ni JY. A method for the analysis of low-mass molecules by MALDI-TOF mass spectrometry. Anal. Chem. 2003;75 doi: 10.1021/ac010979m. (vol 74, pg 1637, 2002) 707-707. [DOI] [PubMed] [Google Scholar]
- 21.Blanksby SJ, Mitchell TW. Advances in Mass Spectrometry for Lipidomics. Annu. Rev. Anal. Chem. 2010;vol. 3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 22.Kates M. Techniques in Lipology: Isolation, analysis and identification of Lipids. New York: Elsevier; 1986. [Google Scholar]
- 23.Cowart LA. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009;20:34–42. doi: 10.1016/j.tem.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Podo F, Sardanelli F, Iorio E, Canese R, Carpinelli G, Fausto A, Canevari S. Abnormal choline phospholipid metabolism in breast and ovary cancer: Molecular bases for noninvasive imaging approaches. Curr. Med. Imaging Rev. 2007;3:123–137. [Google Scholar]
- 25.Sakai K, Okuyama H, Yura J, Takeyama H, Shinagawa N, Tsuruga N, Kato K, Miura K, Kawase K, Tsujimura T, Naruse T, Koike A. Composition and turnover of phospholipids and neutral lipids in human breast cancer and reference tissues. Carcinogenesis. 1992;13:579–584. doi: 10.1093/carcin/13.4.579. [DOI] [PubMed] [Google Scholar]
- 26.Yamaji-Hasegawa A, Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol. Pharm. Bull. 2006;29:1547–1553. doi: 10.1248/bpb.29.1547. [DOI] [PubMed] [Google Scholar]
- 27.Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- 28.Dobrzynska I, Szachowicz-Petelska B, Sulkowski S, Figaszewski Z. Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol. Cell. Biochem. 2005;276:113–119. doi: 10.1007/s11010-005-3557-3. [DOI] [PubMed] [Google Scholar]
- 29.Shimma S, Sugiura Y, Hayasaka T, Hoshikawa Y, Noda T, Setou M. MALDI-based imaging mass spectrometry revealed abnormal distribution of phospholipids in colon cancer liver metastasis. J. Chromatogr. B. 2007;855:98–103. doi: 10.1016/j.jchromb.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Beljebbar A, Dukic S, Amharref N, Bellefqih S, Manfait M. Monitoring of Biochemical Changes through the C6 Gliomas Progression and Invasion by Fourier Transform Infrared (FTIR) Imaging. Anal. Chem. 2009;81:9247–9256. doi: 10.1021/ac901464v. [DOI] [PubMed] [Google Scholar]
- 31.Engelbrecht AM, Louw L, Cloete F. Comparison of the fatty acid compositions in intraepithelial and infiltrating lesions of the cervix: part II - free fatty acid profiles. Prostag. Leukotr. Ess. 1998;59:253–257. doi: 10.1016/s0952-3278(98)90138-7. [DOI] [PubMed] [Google Scholar]
- 32.Louw L, Engelbrecht AM, Cloete F. Comparison of the fatty acid compositions in intraepithelial and infiltrating lesions of the cervix: part I - total fatty acid profiles. Prostag. Leukotr. Ess. 1998;59:247–251. doi: 10.1016/s0952-3278(98)90137-5. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J, Park IS, Lee SK, Kim SY, Chung EJ, Kim J, Kim DJ, Yoon S, Lee-Kim YC. Fatty acid patterns in gastric mucosa of stomach cancer patients. Yonsei Med. J. 2001;42:220–226. doi: 10.3349/ymj.2001.42.2.220. [DOI] [PubMed] [Google Scholar]
- 34.Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Rad. Bio. Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 35.Pratico D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Rokach J, Maclouf J, Violi F, Fitzgerald GA. Localization of distinct F-2-isoprostanes in human atherosclerotic lesions. J. Clin. Invest. 1997;100 doi: 10.1172/JCI119735. (vol 100, pg 2028, 1997) 2637-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa AB, Cooks RG. Simulated splashes: Elucidating the mechanism of desorption electrospray ionization mass spectrometry. Chem. Phys. Lett. 2008;464:1–8. [Google Scholar]
- 37.Venter A, Sojka PE, Cooks RG. Droplet dynamics and ionization mechanisms in desorption electrospray ionization mass spectrometry. Analytical Chemistry. 2006;78:8549–8555. doi: 10.1021/ac0615807. [DOI] [PubMed] [Google Scholar]
- 38.Manicke NE, Wiseman JM, Ifa DR, Cooks RG. Desorption electrospray ionization (DESI) mass Spectrometry and tandem mass spectrometry (MS/MS) of phospholipids and sphingolipids: Ionization, adduct formation, and fragmentation. J. Am. Soc. Mass Spectrom. 2008;19:531–543. doi: 10.1016/j.jasms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JI, Talaty N, Costa AB, Xia Y, Tao WA, Bell R, Callahan JH, Cooks RG. Rapid direct lipid profiling of bacteria using desorption electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2010 In Press, Corrected Proof. [Google Scholar]
- 40.Jackson AU, Tata A, Wu CP, Perry RH, Haas G, West L, Cooks RG. Direct analysis of Stevia leaves for diterpene glycosides by desorption electrospray ionization mass spectrometry. Analyst. 2009;134:867–874. doi: 10.1039/b823511b. [DOI] [PubMed] [Google Scholar]
- 41.Lane AL, Nyadong L, Galhena AS, Shearer TL, Stout EP, Parry RM, Kwasnik M, Wang MD, Hay ME, Fernandez FM, Kubanek J. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA. 2009;106:7314–7319. doi: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basile F, Sibray T, Belisle JT, Bowen RA. Analysis of lipids from crude lung tissue extracts by desorption electrospray ionization mass spectrometry and pattern recognition. Anal. Biochem. 2011;408:289–296. doi: 10.1016/j.ab.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ifa DR, Wiseman JM, Song QY, Cooks RG. Development of capabilities for imaging mass spectrometry under ambient conditions with desorption electrospray ionization (DESI) Int. J. Mass Spectrom. 2007;259:8–15. [Google Scholar]
- 44.Takats Z, Wiseman JM, Cooks RG. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms applications in forensics, chemistry, and biology. J. Mass Spectrom. 2005;40:1261–1275. doi: 10.1002/jms.922. [DOI] [PubMed] [Google Scholar]
- 45.Eberlin LS, Ifa DR, Wu C, Cooks RG. Three-Dimensional Vizualization of Mouse Brain by Lipid Analysis Using Ambient Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2010;49:873–876. doi: 10.1002/anie.200906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ifa DR, Wu CP, Ouyang Z, Cooks RG. Desorption electrospray ionization and other ambient ionization methods: current progress and preview. Analyst. 2010;135:669–681. doi: 10.1039/b925257f. [DOI] [PubMed] [Google Scholar]
- 47.Kertesz V, Van Berkel GJ. Improved imaging resolution in desorption electrospray ionization mass spectrometry. Rapid Comm Mass Spectrom. 2008;22:2639–2644. doi: 10.1002/rcm.3662. [DOI] [PubMed] [Google Scholar]
- 48.Badu-Tawiah A, Bland C, Campbell DI, Cooks RG. Non-Aqueous Spray Solvents and Solubility Effects in Desorption Electrospray Ionization. J. Am. Soc. Mass Spectrom. 2010;21:572–579. doi: 10.1016/j.jasms.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Green FM, Salter TL, Gilmore IS, Stokes P, O'Connor G. The effect of electrospray solvent composition on desorption electrospray ionisation (DESI) efficiency and spatial resolution. Analyst. 2010;135:731–737. doi: 10.1039/b924208b. [DOI] [PubMed] [Google Scholar]
- 50.Girod M, Shi YZ, Cheng JX, Cooks RG. Desorption Electrospray Ionization Imaging Mass Spectrometry of Lipids in Rat Spinal Cord. J. Am. Soc. Mass Spectrom. 2010;21:1177–1189. doi: 10.1016/j.jasms.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 51.Wiseman JM, Puolitaival SM, Takats Z, Cooks RG, Caprioli RM. Mass spectrometric profiling of intact biological tissue by using desorption electrospray ionization. Angew. Chem. Int. Ed. 2005;44:7094–7097. doi: 10.1002/anie.200502362. [DOI] [PubMed] [Google Scholar]
- 52.Manicke NE, Nefliu M, Wu C, Woods JW, Reiser V, Hendrickson RC, Cooks RG. Imaging of Lipids in Atheroma by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2009;81:8702–8707. doi: 10.1021/ac901739s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dill AL, Ifa DR, Manicke NE, Costa AB, Ramos-Vara JA, Knapp DW, Cooks RG. Lipid Profiles of Canine Invasive Transitional Cell Carcinoma of the Urinary Bladder and Adjacent Normal Tissue by Desorption Electrospray Ionization Imaging Mass Spectrometry. Anal. Chem. 2009;81:8758–8764. doi: 10.1021/ac901028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eberlin LS, Dill AL, Costa AB, Ifa DR, Cheng L, Masterson T, Koch M, Ratliff TL, Cooks RG. Cholesterol Sulfate Imaging in Human Prostate Cancer Tissue by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010;82:3430–3434. doi: 10.1021/ac9029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masterson TA, Dill AL, Eberlin LS, Mattarozzi M, Cheng L, Koch MO, Bianchi F, Cooks RG. Distinctive Glycerophospholipid Profiles of Human Seminoma and Adjacent Normal Tissues by Desorption Electrospray Ionization Imaging Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2010 doi: 10.1007/s13361-011-0134-8. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiseman JM, Li JB. Elution, Partial Separation, and Identification of Lipids Directly from Tissue Slices on Planar Chromatography Media by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010;82:8866–8874. doi: 10.1021/ac1016453. [DOI] [PubMed] [Google Scholar]
- 57.Badu-Tawiah A, Cooks RG. Enhanced Ion Signals in Desorption Electrospray Ionization Using Surfactant Spray Solutions. J. Am. Soc. Mass Spectrom. 2010;21:1423–1431. doi: 10.1016/j.jasms.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Ellis SR, Wu CP, Deeley JM, Zhu XJ, Truscott RJW, Panhuis MIH, Cooks RG, Mitchell TW, Blanksby SJ. Imaging of Human Lens Lipids by Desorption Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2010;21:2095–2104. doi: 10.1016/j.jasms.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Deeley JM, Hankin JA, Friedrich MG, Murphy RC, Truscott RJW, Mitchell TW, Blanksby SJ. Sphingolipid distribution changes with age in the human lens. J. Lipid Res. 2010;51:2753–2760. doi: 10.1194/jlr.M007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson AU, Shum T, Sokol E, Dill AL, Cooks RG. Enhanced detection of olefins using ambient ionization mass spectrometry: Ag(+) adducts of biological relevant alkenes. Anal. Bioanal. Chem. 2011;399:367–376. doi: 10.1007/s00216-010-4349-5. [DOI] [PubMed] [Google Scholar]
- 61.Girod M, Moyano E, Campbell DI, Cooks RG. Accelerated bimolecular reactions in microdroplets studied by desorption electrospray ionization mass spectrometry. Chem. Sci. 2011 Advance Article. [Google Scholar]
- 62.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Rapid screening of anabolic steroids in urine by reactive Desorption Electrospray ionization. Anal. Chem. 2007;79:8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 63.Wu CP, Ifa DR, Manicke NE, Cooks RG. Rapid, Direct Analysis of Cholesterol by Charge Labeling in Reactive Desorption Electrospray Ionization. Anal. Chem. 2009;81:7618–7624. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, Cotte-Rodriguez I, Cooks RG. cis-diol functional group recognition by reactive desorption electrospray ionization (DESI) Chem. Commun. 2006:597–599. doi: 10.1039/b516448f. [DOI] [PubMed] [Google Scholar]
- 65.Sparrapan R, Eberlin LS, Haddad R, Cooks RG, Eberlin MN, Augusti R. Ambient Eberlin reactions via desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 2006;41:1242–1246. doi: 10.1002/jms.1088. [DOI] [PubMed] [Google Scholar]
- 66.Girod M, Shi YZ, Cheng J, Cooks RG. Mapping Lipid Alterations in Traumatically Injured Rat Spinal Cord by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2011;83:207–215. doi: 10.1021/ac102264z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian Q, Failla ML, Bohn T, Schwartz SJ. High-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry determination of cholesterol uptake by Caco-2 cells. Rapid Commun. Mass Spectrom. 2006;20:3056–3060. doi: 10.1002/rcm.2700. [DOI] [PubMed] [Google Scholar]
- 68.Johnson DW, ten Brink HJ, Jakobs C. A rapid screening procedure for cholesterol and dehydrocholesterol by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2001;42:1699–1705. [PubMed] [Google Scholar]
- 69.Wu CP, Ifa DR, Manicke NE, Cooks RG. Molecular imaging of adrenal gland by desorption electrospray ionization mass spectrometry. Analyst. 2010;135:28–32. doi: 10.1039/b919816d. [DOI] [PubMed] [Google Scholar]
- 70.Han XL, Cheng H. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J. Lipid Res. 2005;46:163–175. doi: 10.1194/jlr.D400022-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Hsu FF, Turk J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: A mechanistic study. J. Am. Soc. Mass Spectrom. 2000;11:986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 72.Hsu FF, Turk J. Studies on phosphatidylglycerol with triple quadrupole tandem mass spectrometry with electrospray ionization: Fragmentation processes and structural characterization. J. Am. Soc. Mass Spectrom. 2001;12:1036–1043. [Google Scholar]
- 73.Hsu FF, Turk J. Studies on sulfatides by quadrupole ion-trap mass spectrometry with electrospray ionization: Structural characterization and the fragmentation processes that include an unusual internal galactose residue loss and the classical charge-remote fragmentation. J. Am. Soc. Mass Spectrom. 2004;15:536–546. doi: 10.1016/j.jasms.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Berry KAZ, Murphy RC. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass Spectrom. 2004;15:1499–1508. doi: 10.1016/j.jasms.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Ifa DR, Jackson AU, Paglia G, Cooks RG. Forensic applications of ambient ionization mass spectrometry. Anal. Bioanal. Chem. 2009;394:1995–2008. doi: 10.1007/s00216-009-2659-2. [DOI] [PubMed] [Google Scholar]
- 76.Wiseman JM, Ifa DR, Zhu YX, Kissinger CB, Manicke NE, Kissinger PT, Cooks RG. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc. Natl. Acad. Sci. USA. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manicke NE, Dill AL, Ifa DR, Cooks RG. High-resolution tissue imaging on an orbitrap mass spectrometer by desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 2010;45:223–226. doi: 10.1002/jms.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eberlin LS, Abdelnur PV, Passero A, de Sa GF, Daroda RJ, de Souza V, Eberlin MN. Analysis of biodiesel and biodiesel-petrodiesel blends by high performance thin layer chromatography combined with easy ambient sonic-spray ionization mass spectrometry. Analyst. 2009;134:1652–1657. doi: 10.1039/b817847j. [DOI] [PubMed] [Google Scholar]
- 79.Manicke NE, Dill AL, Ifa DR, Cooks RG. High-resolution tissue imaging on an orbitrap mass spectrometer by desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 2010;45:223–226. doi: 10.1002/jms.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song YS, Talaty N, Tao WA, Pan ZZ, Cooks RG. Rapid ambient mass spectrometric profiling of intact, untreated bacteria using desorption electrospray ionization. Chem. Commun. 2007:61–63. doi: 10.1039/b615724f. [DOI] [PubMed] [Google Scholar]
- 81.Cooks RG, Manicke NE, Dill AL, Ifa DR, Eberlin LS, Costa AB, Wang H, Huang G, Ouyang Z. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring Faraday Discuss. 2011;149:247–267. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bozza PT, Viola JPB. Lipid droplets in inflammation and cancer. Prostag. Leukotr. Ess. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Kuroki T, Ikuta T, Kashiwagi M, Kawabe S, Ohba M, Huh N, Mizuno K, Ohno S, Yamada E, Chida K. Cholesterol sulfate, an activator of protein kinase C mediating squamous cell differentiation: a review. Mutat. Res., Rev. Mutat. Res. 2000;462:189–195. doi: 10.1016/s1383-5742(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 84.Rearick JI, Stoner GD, George MA, Jetten AM. Cholesterol Sulfate Accumulation in Tumorigenic and Nontumorigenic Rat Esophageal Epithelial-Cells - Evidence for Defective Differentiation Control in Tumorigenic Cells. Cancer Res. 1988;48:5289–5295. [PubMed] [Google Scholar]
- 85.Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what's it all about? J. Lipid Res. 2003;44:1268–1278. doi: 10.1194/jlr.R300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Murphy RC, Hankin JA, Barkley RM. Imaging of lipid species by MALDI mass spectrometry. J Lipid Res. 2009;50 Suppl:S317–S322. doi: 10.1194/jlr.R800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivleva VB, Sapp LM, O'Connor PB, Costello CE. Ganglioside analysis by thin-layer chromatography matrix-assisted laser desorption/ionization orthogonal time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2005;16:1552–1560. doi: 10.1016/j.jasms.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Schiller J, Arnhold J, Glander HJ, Arnold K. Lipid analysis of human spermatozoa and seminal plasma by MALDI-TOF mass spectrometry and NMR spectroscopy - effects of freezing and thawing. Chem. Phys. Lipids. 2000;106:145–156. doi: 10.1016/s0009-3084(00)00148-1. [DOI] [PubMed] [Google Scholar]
- 89.Fujiwaki T, Tasaka M, Yamaguchi S. Quantitative evaluation of sphingomyelin and glucosylceramide using matrix-assisted laser desorption ionization time-of-flight mass spectrometry with sphingosylphosphorylcholine as an internal standard. Practical application to tissues from patients with Niemann-Pick disease types A and C, and Gaucher disease. J. Chromatogr. B. 2008;870:170–176. doi: 10.1016/j.jchromb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Wang HYJ, Post S, Woods AS. A minimalist approach to MALDI imaging of glycerophospholipids and sphingolipids in rat brain sections. Int. J. Mass Spectrom. 2008;278:143–149. doi: 10.1016/j.ijms.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nemes P, Barton AA, Li Y, Vertes A. Ambient molecular imaging and depth profiling of live tissue by infrared laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2008;80:4575–4582. doi: 10.1021/ac8004082. [DOI] [PubMed] [Google Scholar]
- 92.Shelley JT, Ray SJ, Hieftje GM. Laser Ablation Coupled to a Flowing Atmospheric Pressure Afterglow for Ambient Mass Spectral Imaging. Anal. Chem. 2008;80:8308–8313. doi: 10.1021/ac801594u. [DOI] [PubMed] [Google Scholar]
- 93.Coello Y, Jones AD, Gunaratne TC, Dantus M. Atmospheric Pressure Femtosecond Laser Imaging Mass Spectrometry. Anal. Chem. 2010;82:2753–2758. doi: 10.1021/ac9026466. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Liu JJ, Cooks RG, Ouyang Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew. Chem. Int. Ed. 2010;49:877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, Ouyang Z. Direct Analysis of Biological Tissue by Paper Spray Mass Spectrometry. Anal. Chem. 2011 doi: 10.1021/ac103150a. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schafer KC, Denes J, Albrecht K, Szaniszlo T, Balog J, Skoumal R, Katona M, Toth M, Balogh L, Takats Z. In Vivo, In Situ Tissue Analysis Using Rapid Evaporative Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2009;48:8240–8242. doi: 10.1002/anie.200902546. [DOI] [PubMed] [Google Scholar]
- 97.Ouyang Z, Cooks RG. Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 98.Sanders NL, Kothari S, Huang GM, Salazar G, Cooks RG. Detection of Explosives as Negative Ions Directly from Surfaces Using a Miniature Mass Spectrometer. Anal. Chem. 2010;82:5313–5316. doi: 10.1021/ac1008157. [DOI] [PubMed] [Google Scholar]
- 99.Sanders NL, Sokol E, Perry RH, Huang GM, Noll RJ, Duncan JS, Cooks RG. Hand-held mass spectrometer for environmentally relevant analytes using a variety of sampling and ionization methods. Eur. J. Mass Spectrom. 2010;16:11–20. doi: 10.1255/ejms.1036. [DOI] [PubMed] [Google Scholar]
- 100.Murphy RC, Hankin JA, Barkley RM. Imaging of lipid species by MALDI mass spectrometry. Journal of Lipid Research. 2009;50:S317–S322. doi: 10.1194/jlr.R800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]