Abstract
Adenosquamous cell carcinoma (Ad-SCC) of the colon is rare. The pathogenesis of Ad-SCC is unclear, however, several hypotheses have been suggested. The clinical presentation and gross findings of Ad-SCC of the colon are similar to those of adenocarcinoma of the colon, but Ad-SCC has a more aggressive clinical course and a poorer prognosis. We report on two cases of Ad-SCC of the colon with obstruction; a collision-type Ad-SCC that has not only obstruction but also numerous hepatic metastases, and a composite-type Ad-SCC treated with left hemicolectomy followed by an adjuvant chemotherapy.
Keywords: Adenosquamous cell carcinoma, Collision, Composite, Colon cancer
INTRODUCTION
Adenosquamous carcinoma (Ad-SCC) of the colon, defined as a neoplasm comprising adenocarcinoma and squamous cell carcinoma, accounts for 0.025% and 0.1% of all colon cancers. Coexistence of squamous carcinomatous components in carcinoma of the colon usually indicates a poorer prognosis than adenocarcinoma alone [1-5].
Histologically, such mixed tumors are classified into two subgroups: composite type tumors, in which both components appear to be mixed haphazardly, and collision-type tumors, which represent the coexistence of two adjacent but histologically distinct tumors in an organ [6,7].
Generally, the epithelium near the dentate line is anatomically capable of differentiating into both glandular and squamous epithelium, but the pathogenesis of squamous cell carcinoma components of the colon remains unclear [2-4].
We report here on two cases of Ad-SCC of the colon which have obstruction. Each case had different histological aspects of Ad-SCC of the colon. Various suggestions of histogenesis are considered along with a review of the literature.
CASE REPORTS
Case 1
A 66-year-old man visited the emergency room complaining of diffuse abdominal pain and distension for 3 days. He had no significant medical history except hypertension. On physical examination, he had direct and rebound tenderness of the entire abdomen with abdominal distension.
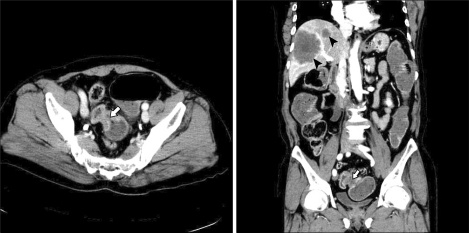
Laboratory test results showed a high white blood cell (WBC) count of 16,500/mm3. The carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 were 55.3 ng/mL (normal range, 0 to 5.0 ng/mL) and 367.5 ng/mL (normal range, 0 to 37 ng/mL). Plain radiography of the abdomen showed colonic dilatation. An abdominopelvic CT showed an obstructive sigmoid colon mass with numerous metastatic hepatic masses on both lobes (Fig. 1).
Fig. 1.
Abdominopelvic computed tomography shows obstructive enhancing mass on the sigmoid colon (arrow) with multiple hepatic metastatic masses on both lobes (arrowheads).
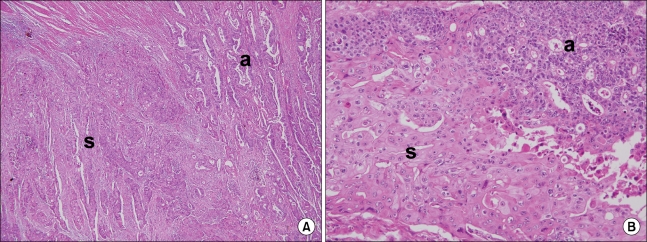
An emergency anterior resection and intraoperative colonic irrigation was performed without diverting ileostomy or colostomy. Histopathological examination revealed 4.0 × 5.5 × 1.0 cm sized collision-type Ad-SCC of the sigmoid colon invading the pericolic adipose tissue (pT3) (Figs. 2, 3). Two out of 12 lymph nodes contained metastatic tumor (pN1) and lymphatic, venous and perineural invasion were present. The postoperative course was uneventful. The patient was discharged after the 2 weeks of the operation and was lost to follow-up.
Fig. 2.
Macroscopic finding (left) shows the cut space of adenosquamous carcinoma of the sigmoid colon, and schematic diagram of the area (right) of the adenocarcinoma (gray area) and squamous cell carcinoma of sigmoid colon (black area).
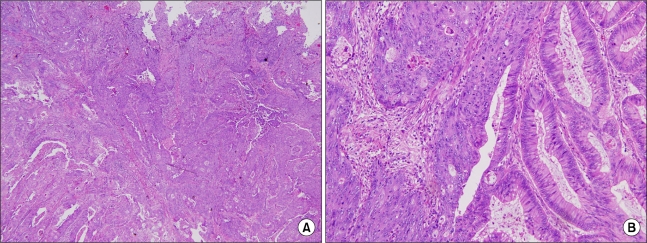
Fig. 3.
Histopathological views reveal adenosquamous carcinoma of the sigmoid colon, showing the feature of collision between adenocarcinoma (a) and squamous cell carcinoma (s). (A) (H&E, ×40), and (B) (H&E, ×200).
Case 2
A 69-year-old man visited the hospital complaining of constipation, hematochezia, and left lower quadrant (LLQ) abdominal pain. He had been treated for hypertension and chronic obstructive pulmonary disease for several years. On physical examination, he had mild direct tenderness of the LLQ.
Laboratory examinations revealed hemoglobin 9.7 g/dL, WBC 9.5 × 103/mm3, Platelet 230 × 103/µL (normal range, 150 to 450 × 103/µL), and chemistry findings were within normal limits.
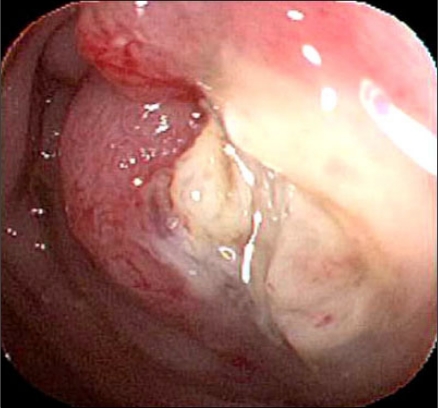
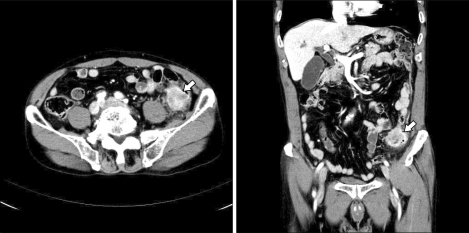
The serum CEA was 45.8 ng/mL (normal range, 0 to 5.0 ng/mL) and CA 19-9 was within normal limits. Sigmoidoscopic findings showed a circumferential obstructive mass at the sigmoid-descending (SD) junction which prohibited the passage of the colonoscopy (Fig. 4). An abdominopelvic CT showed an obstructive and circumferential enhancing wall mass at the SD junction without distant metastases (Fig. 5).
Fig. 4.
Sigmoidoscopic finding shows circumferential obstructive mass at the sigmoid-descending junction.
Fig. 5.
Abdominopelvic computed tomography shows obstructive and circumferential enhancing wall mass at the sigmoid-descending junction (arrow) without distant metastasis.
We performed a left hemicolectomy and intraoperative colonic irrigation without diverting ileostomy or colostomy. Histopathological examination revealed 4.0 × 7.0 × 0.9 cm sized composite-type Ad-SCC of the SD junction invading the pericolic adipose tissue (pT3) (Fig. 6). Two out of 26 lymph nodes contained metastatic tumor (pN1) and lymphatic, venous and perineural invasion were not identified. The patient was treated with adjuvant chemotherapy with a FOLFOX regimen, and is disease-free 1 year after the operation.
Fig. 6.
Histopathological views reveal adenosquamous carcinoma of the sigmoid-descending junction, showing the composite feature between adenocarcinoma and squamous cell carcinoma. (A) H&E, ×40, (B) H&E, ×200.
DISCUSSION
The first case of colorectal Ad-SCC was reported by Herxheimer [8] in 1907 and was described as a tumor consisting of both an adenocarcinoma and a squamous cell carcinoma component. In Korea, Kim et al. [5] first reported Ad-SCC of the colon located on the cecum and thought to be composite type Ad-SCC of the colon.
The histogenesis of colorectal Ad-SCC is not clearly understood, but four hypotheses have been suggested: 1) ectopic squamous cells in the colonic mucosa may be directly transformed into squamous malignant cells; 2) undifferentiated or reserve cells in the colonic epithelium may change directly into squamous cell carcinoma; 3) normal glandular cells may transform into a malignant squamous neoplasm; 4) adenocarcinomas in situ can directly transform into malignant squamous cells [2-4].
Histologically, mixed tumors are classified into two subgroups: composite type tumors, in which both components appear to be mixed haphazardly, and collision-type tumors, which represent the coexistence of two adjacent but histologically distinct tumors, in an organ without any histological admixture, considered as double tumors with a "side by side" or "one upon another" pattern [6,7].
The difference in histologic features between the composite and collision Ad-SCC may be explained by these various suggestions of Ad-SCC histogenesis.
The diagnosis of primary colorectal Ad-SCC requires the exclusion of other possible causes of epidermoid carcinoma that occur in the large intestine. A lack of continuity between the colorectal tumor and the normal squamous epithelium must first be demonstrated. Secondly, the patient must have no evidence of squamous cell carcinoma in other organs which could potentially involve the colon or rectum through either local extension or metastasis. Finally, the diagnosis requires the exclusion of a squamous cell lined fistulous tract, reported to initiate squamous cell carcinoma [9].
The clinical signs and symptoms of colonic Ad-SCC are the same as those for a typical colonic adenocarcinoma, but the squamous cell component has been reported to have greater metastatic potential than the glandular cell component. Colonic Ad-SCC patients may experience a more aggressive clinical course and worse prognosis than colonic adenocarcinoma patients [2-4].
Cagir et al. [1] described the clinicopathological features and prognosis of Ad-SCC of the colon, rectum, and anus. A high incidence of metastases was observed, and the overall rate of regional metastases and distant metastases were reported to be 46.0% and 42.4%, respectively. The overall 5-year survival rate was only 31%, whereas it is 66% in adenocarcinoma cases. The unfavorable outcome may be related to metastases to distant sites, such as the liver and lung, which occur in nearly half of the patients. Both of our patients not only had regional metastases and colonic obstruction, one patient had multiple hepatic metastases.
Surgical resection is considered to be the definitive treatment for colorectal Ad-SCC, and the exact role of adjuvant chemotherapy remains unclear because of its rarity [2-4,9]. Similarly, although postoperative radiation therapy has often been effective in the treatment of squamous cell carcinoma and has been used to treat Ad-SCC in several cases, its effectiveness has also not yet been adequately evaluated [9]. The most commonly used adjuvant chemotherapeutic agents are semustine, 5-fluorouracil, carmustine, and methotrexate. Adjuvant therapy for anorectal Ad-SCC has been used based on the Nigro protocol of 5-fluorouracil and mitomycin with radiation therapy (4,500 cGy). The efficacy of treating rectal Ad-SCC the same as epidermoid cancers of the anal canal is unknown [9,10]. We used the adjuvant chemotherapeutic agents FOLFOX (5-FU, leucovorin, and oxaliplatin) for the patient of composite-type Ad-SCC, because he had an advanced stage and the greater part of Ad-SCC is adenocarcinoma component and the patient is disease-free 1 year after surgery.
In conclusion, colonic Ad-SCC is rare and associated with a worse prognosis than adenocarcinoma alone. We report on two different histological features with a composite or collision colonic Ad-SCC with the hope of spurring further histogenesis investigation and improvements in adequate adjuvant treatment.
ACKNOWLEDGEMENTS
This paper was supported by Wonkwang University 2009.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Cagir B, Nagy MW, Topham A, Rakinic J, Fry RD. Adenosquamous carcinoma of the colon, rectum, and anus: epidemiology, distribution, and survival characteristics. Dis Colon Rectum. 1999;42:258–263. doi: 10.1007/BF02237138. [DOI] [PubMed] [Google Scholar]
- 2.Frizelle FA, Hobday KS, Batts KP, Nelson H. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum. 2001;44:341–346. doi: 10.1007/BF02234730. [DOI] [PubMed] [Google Scholar]
- 3.Nozoe T, Anai H. Adenosquamous carcinoma of the sigmoid colon: report of a case. Surg Today. 2001;31:830–832. doi: 10.1007/s005950170059. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Wang J, Ma H, Zhou H, Lu G, Zhou X. Primary adenosquamous carcinoma of the colon: report of five cases. Surg Today. 2009;39:619–623. doi: 10.1007/s00595-008-3884-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Han SM, An CH, Kim JS, Yoo SJ, Chae HS, et al. A case of adenosquamous carcinoma of the cecum. J Korean Soc Coloproctol. 2000;16:204–208. [Google Scholar]
- 6.Levendoglu H, Cox CA, Nadimpalli V. Composite (adenocarcinoid) tumors of the gastrointestinal tract. Dig Dis Sci. 1990;35:519–525. doi: 10.1007/BF01536929. [DOI] [PubMed] [Google Scholar]
- 7.Fukui H, Takada M, Chiba T, Kashiwagi R, Sakane M, Tabata F, et al. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours? Gut. 2001;48:853–856. doi: 10.1136/gut.48.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herxheimer G. Ober heterologue cancroide. Beitr Pathol Anat. 1907;41:348–412. [Google Scholar]
- 9.Schneider TA, 2nd, Birkett DH, Vernava AM., 3rd Primary adenosquamous and squamous cell carcinoma of the colon and rectum. Int J Colorectal Dis. 1992;7:144–147. doi: 10.1007/BF00360355. [DOI] [PubMed] [Google Scholar]
- 10.Petrelli NJ, Valle AA, Weber TK, Rodriguez-Bigas M. Adenosquamous carcinoma of the colon and rectum. Dis Colon Rectum. 1996;39:1265–1268. doi: 10.1007/BF02055120. [DOI] [PubMed] [Google Scholar]