Summary
— A synthesis of 2,4-bis-(4-amidinophenyl)pyrimidine 6, 2,4-bis-[(4-imidazolin-2-yl)phenyl)]pyrimidine 7, 2,4-bis[(4-tetrahydropyrimidinyl-2-yl)phenyl]pyrimidine 8, 2,4-bis[(4-N-n-propylamidino)phenyl]pyrimidine 9, 2,4-bis[(4-N-isopropylamidino)-phenyl]pyrimidine 10 and 2,4-bis[(4-N-isobutylamidino)phenyl]pyrimidine 11 starting from 4-bromobenzamidine and 4-bromoaceto-phenone is reported. A synthesis of 2-(4-amidinophenyl)-4-(2-methoxy-4-amidinophenyl)pyrimidine 20, 2-[4-(imidazolin2-yl)-phenyl]-4-[2-methoxy-4-(imidazolin-2-yl)phenyl]pyrimidine 21, and 2-[4-(N-iso-propylamidino)phenyl]-4-[2-methoxy-4-(N-isopropylamidino)phenyl]pyrimidine 22 beginning with 4-bromobenzamidine and 2-methoxy-4-bromoacetophenone is described. Compounds 6–11 and 20–22 all bind strongly to DNA. Compounds 6, 9–11, and 20 given at 5 mg/kg are more active and less toxic than pentamidine at its effective dose when evaluated against Pneumocystis carinii pneumonia (PCP) in the immunosuppressed rat model. Several compounds in this series are being evaluated further as potential new anti-PCP agents.
Keywords: Pneumocystis carinii pneumonia, diaryldiamidine, DNA
Introduction
Investigations from our laboratories have focused upon diaryldiamidines as dications which exhibit both strong binding to DNA [1–4] and useful biological properties [5, 6]. The precise mode of binding of these types of dicationic molcules with DNA has been found to be extremely sensitive to structure and dramatic differences in binding mode and specificity have resulted from relatively minor changes in structure [1–4]. The aromatic diamidines berenil and stilbamidine exhibit strong minor-groove binding at sites which have three or more consecutive AT base pairs [7, 9]. However, the well-known DNA stain DAPI, 2-[4′-amidinophenyl]-6-amidinoindole, long known to bind in the minor groove of DNA at AT sites, has also been found to intercalate at GC sites [2]. In a related study, the nucleic acid binding properties of a series of 2,5-diphenylfuran dications were found to vary in interaction with DNA from AT selective minor-groove binding, to classical intercalation at GC sites, to threading intercalation at GC sites, depending upon the intrinsic nature of the cationic groups [3]. Recent reports [10, 11] have suggested that 2,5-bis[(4-amidino)phenyl]furan also binds in the major groove of DNA in addition to binding in the minor groove, however this view has been questioned [12]. The crystal structure of a complex between 2,5-bis[(4-amidino)phenyl]furan and d(CGCGAATTCGCG)2 was recently solved and clearly illustrates the minor-groove-binding mode [13]. A method of calculating the radius of curvature of groove-binding molecules has been developed by Cory et al [14], which expands upon the isohelicity concept [16]. Furthermore, the study suggested that the curvature of the small molcule is an important factor in determining the effectiveness of the interaction of groove-binding dications with DNA [14]. Cory et al suggested that dicationic molcules with calculated radius of curvature values between 10 and 100 Å should effectively bind to the minor groove of DNA. We recently evaluated the effect of structural modification on the nucleic acid binding of dicationic 2,4-diarylpyrimidines which have calculated radius of curvature values of approximately 85 Å [17]. The binding affinities of these dicationic 2,4-diarylpyrimidines were sensitive to steric requirements and torsion angles of the cationic centers [17].
The binding of such dicationic molecules to the DNA minor groove has been associated with the ability of these molecules to inhibit DNA-related enzymes such as topoisomerase II [18]. Pentamidine and its analogs [19], dicationic bis-benzimidazoles [20, 21], and more recently dicationic 2,5-diarylfurans [13] (the latter give calculated radius of curvature values of approximately 15 Å) have been shown to be effective in treating Pneumocystis carinii pneumonia (PCP) and topoisomerase II inhibition may be involved in their mode of action. Collectively, these observations led us to evaluate dicationic 2,4-diarylpyrimidines, which give radius of curvature values in the range predicted to be effective DNA binding agents [17] and which have been demonstrated to strongly bind to AT-rich DNA [14], for effectiveness against PCP. In this paper we describe the synthesis and biological activity of several dicationic 2,4-diarylpyrimidines.
Chemistry
The synthetic route employed to make the dicationic pyrimidines is outlined in scheme 1. The key intermediate, 2,4-bis(4-bromophenyl)pyrimidine 4, was obtained in good yield by base-promoted condensation of 4-bromobenzamidine and 1-dimethylamino-3-dimethylimmonio-1-(4-bromophenyl)-1-propene 3 using the approach described by Wagner and Jutz [22]. The bisnitrile 5 was readily obtained by the action of copper(I) cyanide on 4 in refluxing DMF in a standard manner [23]. The bis-nitrile 5 was converted into the corresponding imidate ester using applied Pinner methodology [24]. The dicationic molcules 6–11 were obtained by reacting the imidate ester with ammonia or the appropiate amine using conventional approaches.
Scheme 1.
a) DMF, POCl3; b) (CH3)2NH; c) p-bromobenzamidine, NaOEt, EtOH; d) CuCN, DMF; e) HCl, EtOH; f) NH3, NH2CH2CH2NH2, NH2CH2CH2CH2NH2, NH2-n-Pr, NH2-iPr or NH2-iBu.
The monocationic 2-phenyl-4-[4-(imidazolin-2-yl)-phenyl]pyrimidine 15 was prepared to serve as a control compound for the dicationic pyrimidines for studies of both their interaction with DNA and their properties as anti-PCP agents. The method employed for synthesis of 15 parallels that used for the preparation of the dicationic molcules and is outlined in scheme 2.
Scheme 2.
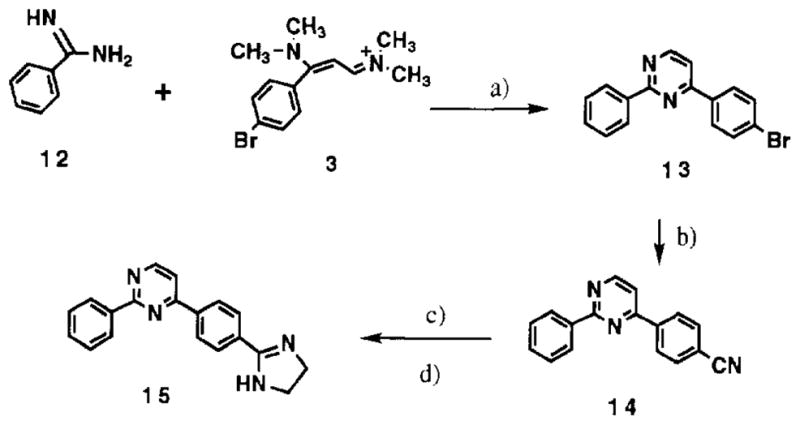
a) NaOEt; b) CuCN, DMF; c) HCl, EtOH; d) NH2CH2CH2NH2.
The synthesis of the methoxy-substituted pyrimidines 20–22 is outlined in scheme 3. The required 2-methoxy-4-bromoacetophenone was obtained by methylation of 4-bromo-2-hydroxyacetophenone which was the product of the Fries rearrangement of 3-bromo-phenyl acetate [25]. Pyrimidine ring formation was achieved by the base-promoted condensation between 4-bromobenzamidine and 3-dimethylamino-1-(4-bromo-2-methoxyphenyl)prop-2-ene-1-one 17. Conversion of 2-(4-bromophenyl)-4-(2-methoxy-4-bromophenyl)-pyrimidine 18 into the corresponding bisnitrile 19 and thence on to the dicationic target compounds 20–22 was achieved using the approach described above for the preparation of the parent 2,4-diarylpyrimidine series.
Scheme 3.

a) (CH3O)2CHN(CH3)2; b) p-bromobenzamidine, NaOEt, EtOH; c) CuCN, DMF; d) HCl, EtOH; e) NH3, NH2CH2CH2NH2, or NH2-iPr.
Biological results and discussion
The results from the evaluation of the DNA binding characteristics and the in vivo anti-PCP activity of the pyrimidines are recorded in table I. All of the dicationic pyrimidines effectively bind to DNA as indicated by the ΔTm values recorded in table I. The ΔTm differences between the parent series (6, 7) and the methoxy-substituted series (20, 21) have been previously attributed to the effect of structural changes on the pyrimidine systems on binding in the minor groove [17]. Variation of the alkyl group on an amidino nitrogen causes some reduction in ΔTm values; compare values for 6 with those for 9–11. Interestingly, the changes are smaller than noted for substitution effects on the aryl rings [17]; compare values for 6, 10 and 20. As expected, the monocation 15 does not effectively interact with DNA and it shows no activity against PCP in the immunosuppressed rat model. Compound 8 exhibits the strongest interaction with DNA in this series; analogous results have been noted for the tetrahydropyrimidine cationic center in other similar series of dicationic molcules [20, 21]. There is no apparent direct relationship between topoisomerase II inhibition, modeled using the enzyme from Gardia lamblia, and DNA binding or in vivo anti-PCP activity. The dicationic pyrimidines 7 and 8, which have the amidine unit incorporated in a five- and six-membered ring system, exhibit strong binding to DNA but show only moderate activity in vivo against PCP at dosages of 5.0 and 2.5 mg/kg respectively. Compound 8 was toxic at the 5 mg/kg dosage. Substitution on the amidine group with a single alkyl group yields improved anti-PCP activity in the parent pyrimidine system; compare the results for 9 and 10 to that of 6. Several of the dications including 6, 9–11, and 20 screened at 5 mg/kg have comparable or improved anti-PCP activities compared to pentamidine, tested at 10 mg/kg, with apparently reduced overt toxicity. Further studies are underway to examine dose–response effects for these compounds on activity and toxicity.
Table I.
Biological evaluation of dicationic diaryl pyrimidines.
| Compound | ΔTm(DNA)a | Topo II G lamblia IC50 (mM)b | In vivo activity against P carinii
|
||
|---|---|---|---|---|---|
| Dosagec (mg/kg) | Toxicityc | Cystsc (%) | |||
| Pentamidine | 12.8 | NDd | 10 | 2+ | 3.3 |
| Saline | 100e | ||||
| 6 | 21.5 | 6–12 | 10.0 | 1+ | 0.9 |
| 5.0 | 0 | 4.3 | |||
| 7 | 22.7 | 3–6 | 5.0 | 1+ | 86.4 |
| 8 | >25 | 3–12 | 5.0 | 3+ | f |
| 2.5 | 0 | 31.0 | |||
| 9 | 19.1 | 6–12 | 5.0 | 1+ | 0.05 |
| 10 | 17.8 | 3–6 | 5.0 | 0 | 1.3 |
| 11 | 16.4 | 6 | 5.0 | 1+ | 4.3 |
| 15 | 0.6 | 100 | 5.0 | 1+ | 131 |
| 20 | 14.7 | 15 | 5.0 | 2+ | 0.5 |
| 21 | 18.9 | 200 | NDd | NDd | NDd |
| 22 | 13.9 | 3–6 | 5.0 | 3+ | f |
| 2.5 | 0 | 1.1 | |||
Thermal melting increase of poly dA.dT; see ref [26].
Inhibition of topoisomerase II isolated from G lamblia, see ref [27].
Evaluation of iv dosage of the pyrimidines against P carinii in rats as described in ref [21].
Not determined.
Mean cyst count for pooled saline controls = 3.8 × 107 cysts/g lung tissue.
After toxicity was noted, dosage was lowered to 2.5 mg/kg and the evaluation was continued, results for the lower dosage shown on the next line.
Experimental protocols
General
Melting points were recorded using a Thomas Hoover (Uni-Melt) capillary melting point apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded employing a Varian VXR400 spectrometer and chemical shifts (δ) are in ppm relative to TMS; coupling constants are in hertz. High resolution mass spectra were recorded with a VG Instruments 70-SE spectrometer (Georgia Institute of Technology, Atlanta, GA); others were recorded by a Shimadzu GC-MS 5000 instrument at 70 eV chamber voltage on a direct inlet system. IR spectra were recorded using a Michelson 100 (Bomem, Inc) instrument. Elemental analysis were obtained from Atlantic Microlab Inc (Norcross, GA) and are within ±0.4% of the theoretical values. All chemicals and solvents were purchased from Aldrich Chemical Co or Fisher Scientific.
Chemistry
1-Dimethylamino-3-dimethylimmonio-1-(4-bromophenyl)-1-propene perchlorate 3
To a cooled and stirred solution of dry DMF (73 g, 1.0 mol) in 150 mL dry chloroform was added, drop wise, freshly distilled phosphorus oxychloride (122.8 g, 0.8 mol). The solution turned reddish pink during addition and was stirred for 1 h. 4-Bromo-acetophenone (39.8 g, 0.2 mol) in 100 mL dry chloroform was added to the previously prepared solution and the mixture was heated to 50–60 °C for 3 h. After the mixture was allowed to cool it was poured into stirred ice-cold water (external cooling required). The aqueous solution was extracted with 100 mL ether, and aqueous dimethylamine (40% soln) was added slowly with cooling to the aqueous layer. A thick yellow precipitate formed was filtered and was resuspended in 200 mL water. A solution of sodium perchlorate (34 g, 0.28 mol) in 50 mL water was added to the suspension, and a thick yellow precipitate was obtained. The precipitate was filtered and dried in vacuo at 50 °C for 12 h. The yellow solid was dissolved in 400 mL boiling absolute ethanol. After cooling the off-white crystalline precipitate was filtered, washed with dry ether and dried in vacuo at 50 °C for 24 h, to yield 50.5 g (66%) mp 160–162 °C. IR (KBr) 2931, 1630, 1561, 1394, 836 cm−1. 1H-NMR (DMSO-d6) 7.76 (d. 2H. J = 8), 7.27 (d, 2H, J = 8), 6.91 (d, 1H, J = 12), 5.56 (d, 1H, J = 12), 3.31 (s, 3H), 3.13 (s, 3H), 3.11 (s, 3H), 2.87 (s, 3H). 13C-NMR (DMSO-d6) 169.4, 160.9, 131.9, 130.8, 130.5, 123.9, 91.8, 45.8, 42.8. 40.8, 38.1. MS m/e 281 (M+ – HClO4).
4-Bromobenzamidine
A stirred mixture of 4-bromobenzonitrile (36.4 g, 0.2 mol) and ammonium benzenesulfonate (45 g, 0.26 mol) was gradually heated to 260–270 °C (bath temperature) for 2.5 h; at this temperature the melt becomes a clear liquid (both boiling and sublimation of the mixture is observed). The bath was removed and the melt was slowly and carefully added to 600 mL acetone to dissolve unreacted nitrile and also to prevent the melt from becoming a hard solid mass. Any lumps were broken and the solid was filtered. The solid was thoroughly ground and then slurried with water to remove any excess ammonium benzene sulfonate. The solid was slurried with acetone and filtered, washed with dry ether and the gray-brown solid dried in vacuo at 100 °C for 12 h. The yield of 4-bromobenzamidine benzenesulfonate was 33 g (51% based on recovered nitrile), mp 253 °C (lit [28] 250–260 °C). The acetone extract yielded unreacted p-bromobenzonitrile (12.8 g). IR (KBr) 3325, 3126, 1681, 1597 cm−1. 1H-NMR (DMSO-d6) 9.15 (s, 4H), 7.8 (q, 4H), 7.32 (m, 2H), 7.23 (m, 3H). 13C-NMR (DMSO-d6) 164.8, 147.8, 131.9, 130.0, 128.4, 127.5, 127.2, 125.3.
To a stirred mixture of 4-bromobenzamidine benzenesulfonate (3.57 g, 0.01 mol) in 10 mL water was added 6–7 mL 5 M NaOH and stirring was maintained for 10 min; the solid was filtered, washed with water, dissolved in acetone (50 mL), treated with charcoal, filtered and the acetone removed under reduced pressure. The white crystalline solid obtained was washed with dry ether and dried in vacuo at 40 °C (12 h). Yield: 1.5 g (75%), mp 168–169 °C (lit [28] 159 °C). IR (KBr) 3420, 3326, 3240, 3050, 1649 cm−1. 1H-NMR (DMSO-d6, 45 °C) 7.74 (d, 2H, J = 8.2), 7.62 (d, 2H, J = 8.2), 6.43 (br, 3H). 13C-NMR (DMSO-d6, 45 °C) 161.3, 135.4. 130.7, 128.5, 123.0. MS m/e 198 (M+).
2,4-Bis(4-bromophenyl)pyrimidine 4
To a stirred mixture of p-bromobenzamidine benzenesulfonate (10.7 g, 0.03 mol) and 1-dimethylamino-3-dimethylimmonio-1-(4-bromophenyl)-1-propene perchlorate (8.4 g, 0.022 mol) in 100 mL absolute ethanol was added 0.055 mol sodium ethoxide (prepared from 1.26 g Na and 70 mL ethanol). After stirring for 30 min at room temperature a further equivalent of sodium ethoxide was added and the mixture was heated under reflux with efficient stirring for 3 h. The solvent was removed under reduced pressure, and the yellow residue was triturated with 100 mL water. The resulting solid was filtered and washed with water. The yellow cake, after drying, was recrystallized from ether/hexane (2:3) to give colorless needles, 7.37 g (86% yield), mp 165–166 °C. IR (KBr) 1585, 1578, 1555, 816, 765 cm−1. 1H-NMR (CDC13, 35 °C) 8.71 (d, 1H, J = 5.5), 8.39 (d, 2H, J = 8.5), 8.25 (d, 2H, J = 8.5), 7.63 (d, 2H, J = 6.7), 7.6 (d, 2H, J = 6.7), 7.5 (d, 1H, J = 5.5). 13C-NMR (CDCl3, 35 °C) 163.9, 162.8, 158.0, 136.7, 135.7, 132.2, 131.7, 129.9, 128.6, 125.8, 125.6, 114.3. MS m/e 390 (M+).
2,4-Bis(4-cyanophenyl)pyrimidine 5
A mixture of 2,4-bis(4-bromophenyl)pyrimidine (7.8 g, 0.02 mol) and copper(I) cyanide (4.45 g, 0.05 mol) in 50 mL dry DMF was heated under reflux for 30–35 h, during which time the color changed to dark brown. When the mixture was poured into 300 mL ice-cold water, a brown solid precipitated. The mixture was stirred for 3 h with 300 mL 10% NaCN solution; the solid was filtered, washed with water (1.5 L) and dried. The brown solid cake was placed in a soxhlet device and treated with acetone for 36 h. The solvent was removed and the light brown solid was chromatographed over neutral Al2O3. Elution with acetone/hexane (7:3) (20 × 50 mL fractions) and evaporation of the solvent yielded pale yellow fluffy needles which were dried in vacuo at 100 °C (6–7 h). Yield: 2.7 g (48%), mp 239–241 °C. IR (KBr) 2223, 1580, 1550, 831, 798 cm−1. 1H-NMR (DMSO-d6, 35°C) 9.08 (d, 1H, J = 5.4), 8.65 (d, 2H, J= 8.8), 8.5 (d, 2H, J = 8.3), 8.15 (d, 2H, J = 5.4), 8.03 (d, 2H, J = 8.8), 8.01 (d, 2H, J = 8.3). 13C-NMR (DMSO-d6, 35 °C) 161.7, 161.2, 159.0, 140.8, 139.8, 139.6, 132.6, 132.3, 128.2, 127.7, 118.2, 118.0, 116.3, 113.4, 113.1. MS m/e 282 (M+). Anal C18H10N4 (C, H, N).
2,4-Bis-(4-amidinophenyl)pyrimidine trihydrochloride 6
The bisnitrile 5 (2.8 g, 0.011 mol) was suspended in 250 mL absolute ethanol, cooled in an ice–salt bath and dry HCl gas was passed through it; the compound dissolved. The clear yellow solution was placed in a pressure bottle and left for 12 h with occasional shaking. The imidate ester hydrochloride which formed was filtered, washed with dry ether and dried in vacuo to yield 4.2 g. The dried imidate ester was shown to be free of contamination by the bisnitrile starting material by its IR spectrum.
Dry ammonia was passed through a cold suspension of 2.1 g (0.0043 mol) of imidate ester in 50 mL absolute ethanol until saturated. The mixture was shaken for 2 days; the solvent was removed, and the resulting solid was suspended in water and basified to pH > 9 with 2 M NaOH. The free base of 6 precipitated, was filtered and dried in vacuo at 70 °C for 12 h. The off-white solid was recrystallized from boiling ethanol. Yield: 1.1 g (69%), mp 237–239 °C (dec). IR (KBr) 3459, 3349, 3251, 1649, 1583 cm−1. 1H-NMR (CD3CO2D, 70 °C) 9.05 (d, 1H, J = 5.4), 8.71 (d, 2H, J = 8.8), 8.49 (d, 2H, J = 8.8), 8.08 (d, 2H, J = 8.8). 13C-NMR (CD3CO2D, 70 °C) 167.5, 167.3, 164.2, 163.8, 160.0, 143.7, 142.8, 131.4, 131.0, 130.1, 129.7, 129.3, 129.2, 117.5. MS m/e 316 (M+). FAB m/e 317 (M+ + H). Anal C18H16N6·0.5H2O (C, H, N).
The free base of 6 (0.8 g, 0.0025 mol) was suspended in 25 mL absolute ethanol, 25 mL saturated ethanolic HCl was added and the mixture was allowed to reflux for 1 h. After cooling, the volume was reduced to 15 mL. The off-white solid that formed was filtered and washed with dry ether. The salt was dried in vacuo at 80 °C for 24 h. Yield: 1.0 g (94%), mp >360 °C (sublimes). IR (KBr) 3356, 3249, 3007, 1684, 1609 cm−1. 1H-NMR (D2O/TSP/50 °C) 8.43 (d, 1H, J = 5.4), 7.87 (d, 2H, J = 7.8), 7.81 (d, 2H, J = 7.8), 7.57 (d, 4H, J = 7.8), 7.44 (d, 1H, J = 5). 13C-NMR (D2O/TSP/50 °C) 162.3, 162.2, 158.9, 158.3, 155.0. 137.8, 137.0, 126.5, 126.1, 125.7, 125.3, 125.1, 125.0, 113.4. MS FAB m/e 317 (M+ + H). Anal C18H16N6·3HCl (C, H, N).
2,4-Bis[(4-imidazolin-2-yl)phenyl]pyrimidine hydrochloride 7
The bis-imidate ester hydrochloride from 5 (2.1 g, 0.0043 mol) was suspended in 50 mL absolute ethanol, ethylene diamine (0.6 g, 0.01 mol) was added and the mixture was refluxed for 12 h. The solvent was evaporated and the resulting solid triturated with water. The off-white solid thus obtained was filtered, washed with water and dried. After treating with NaOH the resultant free base of 7 was boiled with 800 mL absolute ethanol and filtered to yield a beige crystalline solid. Yield: 1.3 g (82%), mp 337–339 °C (dec) (turns brown at 320 °C and black at mp). IR (KBr) 3190.2, 1615.4, 1602.6, 1575.8 cm−1. 1H-NMR (CD3CO2D) 65 °C 9.04 (d, 1H, J = 5.4), 8.72 (d, 2H, J = 8.3), 8.49 (d, 2H, J = 8.8), 8.16 (d, 2H, J = 8.3), 8.1 (d, 2H, J = 8.8), 7.98 (d, 1H, J = 5.4), 4.19 (s, 8H). 13C-NMR (CD3CO2D) 65 °C 167.02, 166.8, 163.9, 163.6, 144.1, 143.0, 139.4, 130.4, 130.1, 130.0, 129.2, 125.5, 125.1, 117.6, 46.1. MS EI (electron capture) 368.2 (M+). Anal C22H20N6 (C, H, N).
The free base of 7 (1.0 g, 0.0027 mol) was suspended in 35 mL saturated ethanolic HCl and allowed to reflux for 1 h (not all the solid dissolved). The solvent was reduced, in vacuo, to a volume of to 5–6 mL, diluted with dry ether and filtered, washed with dry ether and dried in vacuo at 100 °C 24 h. Yield: 1.2 g, mp > 360 °C. IR (KBr) 3405, 3060, 2945, 1618, 1607, 1572 cm−1. 1H-NMR (D2O/dioxane 45 °C) 8.73 (d, 1H, J = 5.5), 8.13 (d, 2H, J = 7.9), 8.03 (d, 2H, J = 7.9), 7.71 (m, 5H), 4.02 (s, 4H), 4.01 (s, 4H). 13C-NMR (D2O/dioxane/45 °C) 164.7, 164.5, 161.2, 158.3, 141.2, 140.2, 128.4, 128.2, 127.7, 123.6, 123.3, 116.2, 44.5. MS EI m/e 368.2 (M+). Anal C22H20N6·3HCl·1.5H2O (C, H, N).
2,4-Bis[(4-tetrahydropyrimidinyl-2-yl)phenyl]pyrimidine hydrochloride 8
Reaction of the bisimidate ester hydrochloride from 5 with 1,3-propanediamine as described above for 7 gave a 53% yield of the free base of 8, mp 165–166 °C. IR (KBr) 3350, 3179, 3035, 2945, 1633, 1575, 1438, 1367, 1197, 832, 695 cm−1. 1H-NMR (DMSO-d6, 35 °C) 8.96 (d, 1H, J = 5.4), 8.52 (d, 2H, J = 8.3), 8.35 (d, 2H, J = 8.3), 8.04 (d, 2H, J = 5.4), 7.95 (d, 2H, J = 8.3), 3.41–3.37 (m, 8H), 1.73–1.72 (m, 4H). 13C-NMR (DMSO-d6, 35 °C) 162.9, 162.4, 158.6, 153.1, 152.7, 138.8, 138.2, 138.1, 136.8, 127.4, 126.7, 126.4, 115.3, 41.4, 41.3, 20.4, 20.3. MS m/e 396 (M+).
The free base of 8 was converted into 8 (mp 267–268 °C) as described above for 7 in an 82% yield. IR (KBr) 3425, 3179, 3025, 1640, 1620, 1574, 1400, 1013, 832, 693 cm−1. 1H-NMR (DMSO-d6/45 °C) 10.5 (s, 2H), 10.43 (s, 2H), 9.08 (d, 1H, J = 5.4), 8.68 (d, 3H, J = 8.3), 8.55 (d, 2H, J = 8.3), 8.21 (d, 1H, J = 5.4), 8.08 (d, 2H, J = 8.4), 8.03 (d, 2H, J = 8.8), 3.52 (m, 6H), 2.0 (m, 4H). 13C-NMR (DMSO-d6/40 °C) 162.2, 161.7, 159.2, 158.5, 158.3, 141.0, 139.9, 130.5, 130.3, 128.5, 128.2, 128.0, 127.4, 116.44, 38.9, 17.6. Anal C24H24N6·3HCl·0.5H2O (C, H, N).
2,4-Bis[(4-N-n-propylamidino)phenyl]pyrimidine dihydrochloride 9
Freshly distilled n-propylamine (0.265 g, 0.0045 mol) was added to a suspension of the imidate ester from 5 (0.73 g, 0.0015 mol) in 15 mL ethanol and stirred under nitrogen at room temperature for 12 h. The solvent was removed under vacuum, and the residue was triturated with dry ether and decanted. The residue was dissolved in cold water (20 mL) and basified with 2 M NaOH which led to the precipitation of the free base of 9. The solid was filtered, washed with water, dried, and dissolved in CHC13. The solution was dried over Na2SO4, the volume was reduced under vacuum and the solid was collected by filtration. Recrystallization from CHCl3/ether gave an off-white solid (0.5 g, 83%), mp 182–183 °C. 1H-NMR (DMSO-d6) 8.97 (d, 1H, J = 5.4), 8.51 (d, 2H, J = 8.4), 8.34 (d, 2H, J = 5.4), 8.03 (d, 1H, J = 5.4), 7.93 (t, 4H), 6.38 (br, 4h), 3.12 (t, 4H, J = 7.2), 0.97 (t, 6H, J = 7.2). MS m/e 380 (M+).
Ethanol saturated with hydrogen chloride (5 mL) was added to a solution of 0.4 g (0.001 mol) of the free base of 9 in 10 mL ethanol, stirred at 60 °C for 16 h and the solvent removed under vacuum to yield a solid. The solid was triturated with dry ether, filtered, dried under vacuum at 80 °C for 24 h to yield 0.43 g (89 %), mp 228–231 °C. IR (KBr) 3410, 3330, 3190, 2950, 1663, 1600, 1570, 1380, 1115 cm−1. 1H-NMR (DMSO-d6) 10.33 (s, 1H), 10.27 (s, 1H), 9.81 (s, 1H), 9.8 (s, 1H), 9.47 (s, 1H), 9.07 (d, 1H, J = 5.4), 8.63 (d, 2H, J = 8.7), 8.51 (d, 2H, J = 8.2), 8.2 (d, 1H, J = 5.4), 8.06 (t, 4H, J = 8.7), 3.51 (brq, 4H. J = 6.3), 1.72 (sext, 4H, J = 7.2). 1.0 (t, 6H, J = 7.2). 13C-NMR (DMSO-d6) 162.1, 161.9, 159.0, 141.0, 139.9, 130.9, 130.7, 128.8, 128.6, 127.7, 127.1, 116.3, 44.1, 20.6, 10.8. Anal C24H28N6·2HCl·0.5H2O (C, H, N).
2,4-Bis[(4-N-isopropylamidino)phenyl]pyrimidine hydrochloride 10
Reaction of isopropylamine with the bisimidate ester hydrochloride from 5 as described above for 9 gave a 71% yield of the free base of 10, mp 198–199 °C. IR (KBr) 3352, 3020, 2930, 1620, 1571, 1380 cm−1. 1H-NMR (DMSO-d6/D2O) 9.01 (d. 1H, J = 5.37), 8.59 (d. 2H, J = 8.3), 8.40 (d, 2H, J = 8.3), 8.06 (d, 1H, J = 5.37), 7.89 (d, 2H, J = 7.3), 7.86 (d, 2H, J = 7.81), 3.92 (sept, 2H, J = 6.35), 1.25 (d, 6H, J = 6.35), 1.24 (d, 6H, J = 6.35). MS m/e 400 (M+).
The free base of 10 was converted into 10 (mp 258–260 °C) as described above for 9 in an 83% yield. IR (KBr) 3420, 3348, 3221, 2988, 1670, 1618, 1573, 1437,1392, 1128 cm−1. 1H-NMR (D2O/DMSO-d6) 9.86 (brt, 2H, J = 10.2, J = 8.3), 9.68 (brd, 2H, J = 8.77), 9.41 (brd, 2H, J = 8.3), 9.1 (d, 1H, J = 5.37), 8.67 (d, 2H, J = 8.3), 8.53 (d, 2H, J = 8.3), 8.22 (d, 1H, J = 5.37), 7.98 (t, 4H, J = 7.8), 4.2 (sept. 2H, J = 8.35), 1.32 (d, 12H, J = 6.35). 13C-NMR (D2O/DMSO-d6) 162.3, 161.8, 161.3, 161.1. 159.2, 141.0, 139.9, 131.4, 131.1,129.1, 128.8, 127.2, 116.4, 45.2, 21.1. Anal C24H28N6·2HCl·0.5H2O (C, H, N).
2,4-Bis[(4-N-isobutylamidino)phenyl]pyrimidine dihydrochloride 11
Reaction of isobutylamine with the bisimidate ester hydrochloride from 5 as described above for 9 gave a 86% yield of the free base of 11, mp 190–192 °C. IR (KBr) 3384, 3234, 3032, 2952, 1672, 1627, 1564, 1437, 1387, 1180 cm−1. 1H-NMR (DMSO-d6/D2O) 9.05 (d, 1H, J = 5.37), 8.61 (d, 2H, J = 8.3), 8.45 (d, 2H, J = 8.3), 8.13 (d, 1H, J = 5.37), 7.97 (t, 4H, J = 8.3). MS m/e 428 (M+).
The free base of 11 was converted into 11 (mp 240–242 °C dec) as described above for 9 in an 90% yield. IR (KBr) 3420, 3219, 3056, 2955, 2864, 1672, 1624, 1573. 1438, 1016 cm−1. 1H-NMR (DMSO-d6) 10.16–10.07 (brm, 2H), 9.73(s, 1H), 9.70 (s, 1H), 9.39 (s, 1H), 9.36 (s, 1H). 9.12 (d, 1H. J = 5.37), 8.69 (d, 2H, J = 8.79), 8.56 (d, 2H. J = 8.79), 8.23 (D, 1H, J = 8.79), 8.02 (t, 4H, J = 8.3), 3.34 (d, 4H), 3.08 (sept, 2H, J = 6.35), 1.0 (d, 12H, J = 6.35). 13C-NMR (DMSO-d6) 162.7, 162.4, 162.3, 161.8, 159.2, 141.1, 140.0, 131.2, 130.9, 128.9, 128.7, 127.9, 127.3, 116.5, 49.6, 26.9, 19.7. Anal C26H33N6·2HCl·H2O (C, H, N).
2-Phenyl-4-(4-bromophenyl)pyrimidine 13
Benzamidine hydrochloride and 1-dimethylamino-3-dimethyl-immino-1-(4-bromophenyl)-1-propene perchlorate 3 were allowed to react as described above for the preparation of 4. After work-up and recrystallization from CHCl3 ethyl ether (1:3), 72% yield of a white crystalline solid was obtained, mp 107–108 °C. IR (KBr) 2925, 1597, 1562, 1540, 1427 cm−1. 1H-NMR (CDCl3) 8.65 (d, 1H, J = 5.3). 8.55–8.52 (m, 2H), 8.02 (d, 2H, J = 8.3), 7.6 (d, 2H, J = 8.8), 7.5–7.46 (m, 3H), 7.44 (d, 1H, J = 5.3). 13C-NMR (CDCl3) 164.6, 162.5, 157.9, 137.6, 135.7, 132.1, 130.8, 128.6, 128.5, 128.2, 125.6, 114.1. MS m/e 311 (M+).
2-Phenyl-4-(4-cyanophenyl)pyrimidine 14
2-Phenyl-4-(4-bromophenyl)pyrimidine 13 was converted into 14 as described above for 5 to give, after recrystallization from CHCl3/ethyl ether (1:3), a white crystalline solid in a 55% yield, mp 125–126 °C. IR (KBr) 2235, 2277, 1592, 1562, 1425 cm−1. 1H-NMR (DMSO-d6/90 °C) 9.0 (d, 1H, J = 5.3), 8.5–8.45 (m, 2H), 8.4 (d, 2H. J = 8.3), 8.04 (d, 1H, J = 5.3), 8.01 (d, 2H, J = 8.8), 7.55–7.53 (m, 3H). 13C-NMR (DMSO-d6/90°C) 163.4, 161.0, 159.0, 140.0, 136.9, 132.8, 130.9, 128.6, 127.8, 118.3, 115.7, 113.3. MS m/e 257 (M+).
2-Phenyl-4-(4-imidazolin-2-yl)phenylpyrimidine hydrochloride 15
The imidate ester from 14 was allowed to react with ethylene diamine as outlined above for 7 and a 70% yield of the free base of 15 (white solid, recrystallization from ethanol, mp 200–202 °C) was obtained. IR (KBr) 3176, 2925, 2865, 1606, 1562, 1422 cm−1. 1H-NMR (CDCl3/40 °C) 8.84 (d, 1H. J = 5.5), 8.8–8.55 (m, 2H), 8.25 (d, 2H, J = 8.6), 7.95 (d, 2H, J = 8.6), 7.6 (d, 1H, J = 5.5), 3.83 (s, 4H). 13C-NMR (DMSO-d6/50 °C) 163.3, 162.9, 162.1, 158.5, 139.7, 137.5, 137.1, 132.9, 130.6, 128.4, 127.7, 127.5, 126.7, 115.0, 49.5. MS m/e 299 (M+). The free base of 15 was converted into the hydrochloride salt by the standard procedure to yield a white solid (85%, mp > 300 °C). IR (KBr) 3033, 2876, 2710, 1607, 1587 cm−1. 1H-NMR (DMSO-d6/50 °C) 11.25 (s, 2H), 8.95 (d, 1H, J = 5.5), 8.54–8.44 (m, 2H), 8.52 (d, 2H, J = 8.6), 8.37 (d, 2H, J = 8.6), 8.15 (d, 1H, J = 5.5), 7.75 (s, 1H. NH), 7.55–7.53 (m, 3H), 4.02 (s, 4H). 13C-NMR (DMSO-d6/50 °C) 163.9, 163.3, 161.1, 158.7, 141.2, 136.8, 130.7, 129.4, 128.4, 127.7, 127.3, 124.0, 115.6, 44.2. Anal C19H15N4·2HCl·0.5H2O (C, H, N).
2(4-Bromophenyl)-4-(2-methoxy-4-bromophenyi)pyrimidine 18
Reaction of 3-dimethylamino-1-(4-bromo-2-methoxyphenyl) propenone and 4-bromobenzamidine as described above for 4 yielded an off-white crystalline solid (62%), mp 153–155 °C. IR (KBr) 3040, 2920, 1592, 1560, 1433, 1387, 1235, 1039, 859, 807 cm−1. 1H-NMR (CDC13) 8.74 (d, 1H, J = 5.4), 8.39 (d, 2H, J = 8.8), 8.10 (d, 1H, J = 8.3), 7.83 (d, 1H, J = 5.4), 7.61 (d, 2H, J = 8.3), 7.28 (dd, 1H, J = 1.95), 7.17 (s, 1H), 3.91 (s, 3H). 13C-NMR (CDCl3) 163.4, 161.6, 158.6, 157.1, 136.9, 132.3, 131.7, 129.7, 125.7, 125.3, 125.1, 124.4, 119.6, 115.1, 55.9. MS m/e 420 (M+).
2-(4-Cyanophenyl)-4-(2-methoxy-4-cyanophenyl)pyrimidine 19
Reaction of the dibromo compound 18 as described above for 5 yielded a white solid (40%), mp 239–240 °C. IR (KBr) 3085, 2229, 1579, 1556, 1406, 1274, 1027, 819 cm−1. 1H-NMR (CDCl3) 8.88 (d, 1H, J = 4.4), 8.65 (d, 2H, J = 8.3), 8.3 (d, 2H, J = 8.3), 7.94 (d, 1H, J = 4.4), 7.79 (d, 2H, J = 8.3), 7.46 (dd, 1H, J = 8.3, J = 1.4), 7.3 (s, 1H), 3.99 (s, 3H). 13C-NMR (CDCl3) 162.9, 161.0, 158.1, 157.6, 141.7, 132.3, 132.0, 130.5, 124.9, 120.6, 118.7, 118.2, 115.0 114.2, 56.2. MS m/e 312 (M+). Anal C19H12N4O (C, H, N).
2-(4-Amidinophenyl)-4-(2-methoxy-4-amidinophenyl)pyrimidine trihydrochloride 20
The dinitrile 19 was converted into the free base of 20 as described above for 6 to yield a white crystalline solid (73%), mp 148–149 °C dec. IR (KBr) 3380, 3290, 3212, 1645, 1570, 1420, 1256, 1185, 1026, 829 cm−1. 1H-NMR (DMSO-d6/D2O) 8.84 (d, 1H, J = 4.4), 8.42 (d, 2H, J = 8.4), 8.09 (d, 1H, J = 4.4), 7.9 (d, 1H, J = 5.2), 7.66 (d, 2H, J = 8.4), 7.53 (brs, 1H), 7.47 (d, 1H, J = 8.8). 13C-NMR (DMSO-d6/D2O) 163.8, 163.5, 163.3, 162.2, 158.3, 158.2, 139.8, 139.4, 138.2, 131.1, 128.2, 127.6, 127.3, 120.5, 119.7, 111.1, 56.5. MS m/e 346 (M+).
The free base of 20 was converted into the trihydrochloride salt of 20 (mp 255–257 °C dec) as described above for 6 in a 75% yield. IR (KBr) 3380, 3270, 3137, 1677, 1597, 1257, 1019, 735 cm−1. 1H-NMR (DMSO-d6) 9.79 (s, 2H), 9.65 (s, 2H), 9.49 (s, 2H), 9.44 (s, 2H), 9.0 (d, 1H, J = 6.8), 8.6 (d, 2H, J = 8.4), 8.25 (d, 1H, J = 8.0), 8.04 (m, 3H), 7.77 (s, 1H), 7.67 (d, 1H, J = 8.0). 13C-NMR (DMSO-d6) 165.4, 164.9, 162.1, 161.0, 158.2 157.6, 141.8, 131.1, 130.9, 129.8 129.7, 128.7, 128.0, 120.7, 120.5, 112.4, 56.6. Anal C19H18N6O·3HCl·1.5H2O (C, H, N).
2-(4-[Imidazolin-2-yl]phenyl)-4-(2-methoxy-4-[imidazolin-2-yl]-phenyl)pyrimidine 21
Reaction of the bisimidate ester hydrochloride from 19 with 1,2-ethanediamine as described above for 7 gave a 74% yield of the free base of 21, mp 150–151 °C. IR (KBr) 3375, 3214, 2938, 2866, 1606, 1567, 1429, 1275, 1233, 1027. 986 cm−1. 1H-NMR (DMSO-d6/45 °C) 8.93 (d, 1H, J = 5.4), 8.51 (d, 2H, J = 8.3), 8.21 (d, 1H, J = 7.8), 8.0 (d, 2H, J = 7.8), 7.98 (d, 1H, J = 2.4), 7.67 (s, 1H), 7.63 (d, 2H, J = 7.8), 3.97 (s, 3H), 3.67 (s, 4H), 3.65 (s, 4H). 13C-NMR (DMSO-d6/45 °C) 163.2, 163.0, 162.7, 161.4, 157.6, 157.5, 138.8, 134.0, 132.50, 130.3, 127.4, 127.3, 126.7, 119.7, 119.5 110.8, 55.8, 49.5. MS m/e 398 (M+).
The free base of 21 was converted into the salt of 21 (mp 237–240 °C dec) by the standard method in an 84% yield. IR (KBr) 3361 (br), 3113, 1617, 1590, 1258, 1024, 791 cm−1. 1H-NMR (D2O/DMSO-d6/45 °C) 8.78 (d, 1H, J = 4.9), 8.32 (d, 2H, J = 6.9), 8.08 (d, 1H, J = 5.8), 7.98 (d, 1H, J = 5.3), 7.88 (d, 2H, J = 6.8), 7.44 (d, 1H, J = 8.3), 7.42 (s, 1H), 4.1 (s, 4H), 4.08 (s, 4H). 13C-NMR (D2O/DMSO-d6) 166.3, 166.0, 162.7, 162.0, 159.6, 159.0, 143.2, 132.8, 130.8, 129.8, 126.1, 124.8, 122.1, 121.7, 112.8, 57.5, 45.9. Anal C23H22N6·3HCl· 1.5H2O (C, H, N).
2-(4-[N-Isopropylamidino]phenyl)-4-(2-methoxy-4-[N-isopropylamidino]phenyl)pyrimidine 22
Reaction of isopropylamine with the bisimidate ester hydrochloride from 19 as described above for 10 gave a 75% yield of the free base of 22, mp 179–180 °C. IR (KBr) 3257, 3058, 2966, 1597, 1566, 1429, 1386, 1251, 1183, 801 cm−1. 1H-NMR (DMSO-d6/D2O) 8.9 (d, 1H, J = 4.9), 8.46 (d, 2H, J = 8.5), 8.12 (d, 1H, J = 8.5), 7.94 (d, 1H, J = 5.5), 7.85 (d, 2H, J = 8.5), 7.48 (s, 1H), 7.46 (d, 1H, J = 4.9), 3.96 (s, 3H) 3.82 (brm, 2H), 1.15 (d, 6H, J = 6.7), 1.13 (d, 6H, J = 6.1). 13C-NMR (DMSO-d6/D2O) 162.8, 161.6, 157.5, 157.2, 157.1, 141.0, 139.3, 138.2, 130.0, 127.2, 126.8, 126.0, 119.5, 119.1, 110.4, 55.8, 43.7, 22.8. MS m/e 430 (M+).
The free base of 22 was converted into 22 (mp 224–226 °C) by the standard approach to give a 79% yield. IR (KBr) 3422, 3218, 3068, 1667, 1619, 1571, 1394, 1264, 1129, 1003, 738 cm−1. 1H-NMR (D2O/DMSO-d6/45 °C) 8.95 (d, 1H, J = 5.4), 8.55 (d, 2H, J = 8.3), 8.18 (d, 1H, J = 8.79), 8.0 (d, 1H, J = 5.4), 7.83 (d, 2H, J = 8.3), 7.45 (s, 1H), 7.44 (d, 1H, J = 5.4), 3.96 (br, 2H), 3.89 (s, 3H), 1.29 (d, 6H), 1.27 (d, 6H). 13C-NMR (D2O/DMSO-d6/45 °C) 163.0, 162.4, 162.1, 158.9, 158.3, 142.0, 132.74, 131.9, 131.5, 130.1, 129.3, 128.8, 121.4, 121.2, 112.6, 57.0, 46.0, 46.0, 21.6. Anal C25H30N6O·3HCl·H2O (C, H, N).
Acknowledgments
This work was supported by NIH Grant NIAID AI-33363 and the Georgia Research Alliance. An award by the Chemical Instrumental Program of NSF (CHE 8409599) provided partial support for acquisition of the Varian VXR400 spectrometer. We appreciate the technical assistance of W Brake with the animal model for Pneumocystis carinii.
References
- 1.Wilson WD, Tanious FA, Buczak H, et al. Structure and Function. In: Sarma RH, Sarma MH, editors. Nucleic Acids. Vol. 1. Adenine; New York: 1992. pp. 83–105. [Google Scholar]
- 2.Wilson WD, Tanious FA, Barton HJ, Strekowski L, Boykin DW, Jones RL. J Am Chem Soc. 1989;111:5008–5010. [Google Scholar]
- 3.Wilson WD, Tanious FA, Buczak H, Venkatramanan MK, Das BP, Boykin DW. In: Jerusalem Symposia on Quantum Chemistry and Biochemistry. Pullman B, Jortner J, editors. Vol. 23. Kluwer Academic; The Netherlands: 1990. pp. 331–353. [Google Scholar]
- 4.Kumar A, Rhodes AR, Spychala J, et al. Eur J Med Chem. 1995;30:99–106. doi: 10.1016/0223-5234(96)88214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das BP, Boykin DW. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 6.Tidwell RR, Jones SK, Geratz JD, Ohemeng KA, Cory M, Hall JE. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer C, Wahnert U. Prog Biophys Mol Biol. 1986;47:31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]
- 8.Neidle S, Pearl LH, Shelly JV. Biochem J. 1987;243:1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunn MN, Jenkins TC, Neidle S. Biochemistry. 1993;32:13838–13843. doi: 10.1021/bi00213a012. [DOI] [PubMed] [Google Scholar]
- 10.Jansen K, Lincoln P, Nordeen B. Biochemistry. 1993;32:6605–6612. doi: 10.1021/bi00077a013. [DOI] [PubMed] [Google Scholar]
- 11.Nordeen B, Kurucsev T. J Mol Recogn. 1994;7:141–156. doi: 10.1002/jmr.300070211. [DOI] [PubMed] [Google Scholar]
- 12.Tanious FA, Spychala J, Kumar A, Greene K, Boykin DW, Wilson WD. J Biomol Struct Dyn. 1994;11:1063–1083. doi: 10.1080/07391102.1994.10508053. [DOI] [PubMed] [Google Scholar]
- 13.Boykin DW, Kumar A, Spychala J, et al. J Med Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 14.Cory M, Tidwell RR, Fairley T. J Med Chem. 1992;35:431–438. doi: 10.1021/jm00081a003. [DOI] [PubMed] [Google Scholar]
- 15.Cain BF, Atwell GJ, Seelye RN. J Med Chem. 1969;12:199–206. doi: 10.1021/jm00302a001. [DOI] [PubMed] [Google Scholar]
- 16.Goodsell D, Dickerson RE. J Med Chem. 1986;29:727–733. doi: 10.1021/jm00155a023. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Zhao M, Wilson WD, Boykin DW. Bio Med Chem Lett. 1994;4:2913–2918. [Google Scholar]
- 18.Douc-Rasy S, Kayser A, Riou JF, Riou G. Proc Natl Acad Sci USA. 1986;83:7152–7156. doi: 10.1073/pnas.83.19.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SK, Hall JE, Allen MA, et al. Antimicrob Agents Chemother. 1990;34:1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairley TA, Tidwell RR, Donkor I, et al. J Med Chem. 1993;36:1746–1753. doi: 10.1021/jm00064a008. [DOI] [PubMed] [Google Scholar]
- 21.Tidwell RR, Jones SK, Naiman NA, et al. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner RM, Jutz C. Chem Ber. 1971;104:2975–2983. doi: 10.1002/cber.19711041002. [DOI] [PubMed] [Google Scholar]
- 23.Spychala J, Wilson WD, Boykin DW, et al. Eur J Med Chem. 1994;29:363–367. doi: 10.1016/0223-5234(94)90061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das BP, Boykin DW. J Med Chem. 1977;20:1219–1221. doi: 10.1021/jm00219a023. [DOI] [PubMed] [Google Scholar]
- 25.Chen FC, Chang CT. J Chem Soc. 1958:146–150. [Google Scholar]
- 26.Wilson WD, Ratmeyer L, Zhao M, Strekowski L, Boykin DW. Biochemistry. 1993;32:4098–4104. doi: 10.1021/bi00066a035. [DOI] [PubMed] [Google Scholar]
- 27.Bell CA, Dykstra CC, Naiman NA, Cory M, Fairley TA, Tidwell RR. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oxley P, Short WF. J Chem Soc. 1947:497–505. [PubMed] [Google Scholar]



