Abstract
Enteroendocrine cells (EECs) play a key role in nutrient digestion and absorption, and are essential for normal life. Recently, EEC function has received considerable attention because several gastrointestinal hormones modulate insulin secretion and food intake; and, gut hormone‐based therapies have been developed to treat diabetes mellitus. Despite these advances, the regulation of EECs remains poorly understood. The development of transgenic mouse models that express green fluorescent proteins (GFP) under specific hormone promoters (e.g., peptide YY‐GFP) is shedding light onto previously overlooked features of EECs. These cells have prominent cytoplasmic processes that extend underneath enterocytes, and in some EECs, such as the L cell of the distal ileum, the basal process can be over 50 μm long. These basal cytoplasmic processes resemble axons and end in synaptic‐like bouton. The location and anatomy of these processes suggest two functions: (1) to monitor absorbed nutrients at the base of enterocytes; and (2) to convey electrochemical information through cell–cell connections with subepithelial myofibroblasts and/or nerves located directly beneath in the lamina propria. Understanding how EECs communicate with cells in the lamina propria may provide novel ways to treat metabolic disorders such as obesity and diabetes. Clin Trans Sci 2011; Volume 4: 387–391
Keywords: enteroendocrine cells, gut hormones, enteric neurons, obesity
Introduction
Enteroendocrine cells (EECs) form part of the neuroendocrine system of the gastrointestinal tract, which produces more than 100 hormonally active peptides. 1 These peptides are secreted by EECs in response to nutrients, flavors, and bacterial byproducts. 2 Recently, EECs have been shown to be essential for normal life. Blockade of EEC differentiation in mice by induced mutations in neurogenin‐3 results in impaired lipid absorption, reduced weight gain, and high mortality rates during the first week after birth. 3 In humans, mutations of neurogenin‐3 are associated with lack of EECs in the gut and pancre§§§as, and life‐threatening diarrhea due to impaired synthesis of gut hormones. 4 These hormones regulate critical physiological functions including: gastric emptying and nutrient absorption (e.g., cholecystokinin, CCK; peptide tyrosine tyrosine, PYY), 5 satiety and appetite (e.g., PYY; CCK; ghrelin; oxyntomodulin), 6 , 7 and insulin release (e.g., glucagon‐like peptide‐1, GLP‐1; glucose‐dependent insulinotropic polypeptide, GIP). 8 , 9 The physiological effects of individual gut hormones have made EECs attractive targets for the treatment of metabolic disorders, particularly obesity and diabetes. Yet, efforts to design effective treatments have been hindered in part by poor understanding of EEC biology.
Traditionally, EECs of the small and large intestines have been difficult to characterize because of their dispersed and scarce nature among other intestinal epithelial cells. Thus, EECs have been studied largely through indirect methods, such as: (1) measurements of plasma hormone levels in response to a meal, nutrients, or drugs applied to the whole intestinal mucosa; (2) the use of immortal cell lines (e.g., enteroendocrine cell line [STC‐1 cells]), which share common features with EECs but are not arranged in a continuous epithelial layer and have an altered phenotype; or (3) the identification of EECs by their immunoreactivity with antibodies against hormone peptides, which is largely limited by the number of primary antibodies that can be used at a time (at most three). Moreover, hormone vesicles are often located at the base of the cell and therefore immunolabeling does not delineate the full anatomy of the cell. As a consequence, EECs are generally thought of as spindle‐ or flask‐shaped epithelial cells capable of containing only one or two hormones. 10 , 11
The recent development of transgenic animal models that express fluorescent proteins (e.g., yellow fluorescent protein, YFP; or green fluorescent protein, GFP) driven by specific hormone promoters (e.g., CCK‐eGFP, GIP‐eYFP, GLP‐1‐eYFP, PYY‐eGFP) 12 , 13 , 14 , 15 is shedding light on some previously overlooked features of EECs ( Figure 1 ). Most of these findings relate to the EECs ability to “sense” nutrients and flavors, and have already been reviewed by several experts in the field. 2 , 11 , 16 , 17 Instead, this review will focus on the discovery and characterization of cytoplasmic processes that extend from the basal surface of EECs to contact neighboring cells in the lamina propria of the intestine. Although we use the CCK‐secreting I cells, and the GLP‐1‐ and PYY‐secreting L cells 13 , 18 as examples, the characteristics described are likely to apply to other EECs in the gut.
Figure 1.
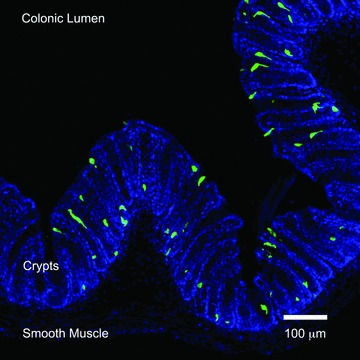
L cells in the distal colon. Immunofluorescence photomicrograph of colonic tissue from a transgenic PYY‐eGFP mouse. PYY‐eGFP cells are green and cell nuclei stained with DAPI appear blue.
Axon‐Like Basal Processes in EECs
The existence of cytoplasmic processes in EECs was first recognized in somatostatin cells. 19 Such processes are long, branched, and with bulbous ends, and serve to deliver somatostatin directly onto gastrin and parietal cells of the stomach through a paracrine action. 19 Several other EECs are known to have basal processes, including the serotonin‐secreting enterochromaffin cell, the GIP‐secreting K cell, the CCK‐secreting I cell, and the L cell. 15 , 18 , 20 , 21 , 22 L cells, in particular, are of great interest in diabetes and obesity research because they secrete the insulin‐stimulating hormone GLP‐1 and the satiety‐inducing hormone PYY. We recently developed a PYY‐eGFP transgenic mouse model 13 that allows us to isolate L cells from other epithelial cells by means of fluorescence‐activated cell sorting, and to identify them in intestinal tissue using fluorescence microscopy ( Figure 1 ). Under the fluorescence microscope, prominent basal processes were detected in all L cells. 13 We have characterized these processes in tissue sections (at least 15 μm thick) using high‐resolution laser scanning confocal microscopy and specialized software (e.g., Volocity® [PerkinElmer, Waltham, MA, USA] or Imaris [Bitplane Inc., Saint Paul, MN, USA]) that allows three‐dimensional reconstruction of image stacks. Using this method, we observed that the characteristics of L cell basal processes vary along the small intestine and colon ( Figure 2 ). For instance, the rare L cells that exist in the proximal small intestine (e.g., jejunum) have short (approximately 10 μm) and multiple (up to three) basal processes per cell. Each basal process is filled with hormone‐containing vesicles and typically enters the lamina propria immediately below the body of the cell (Bohórquez and Liddle, unpublished observations). These features are similar to those described in I cells of the jejunum. 18 L cells in the jejunum are different from those in the distal small intestine (e.g., ileum) and colon. Compared to the jejunum, cells in the ileum and colon have longer basal processes. Some basal processes in the ileum are over 50 μm long. 13 Unlike cells in the jejunum, processes in the ileal L cells typically run underneath several other epithelial cells. Interestingly, the anatomy of the basal process resembles an axon with synaptic‐like ends ( Figure 3 ).The location and anatomy of these processes suggest a dual function to monitor absorbed nutrients at the base of enterocytes; and to convey electrochemical information through cell–cell connections to cells located below the intestinal epithelium.
Figure 2.
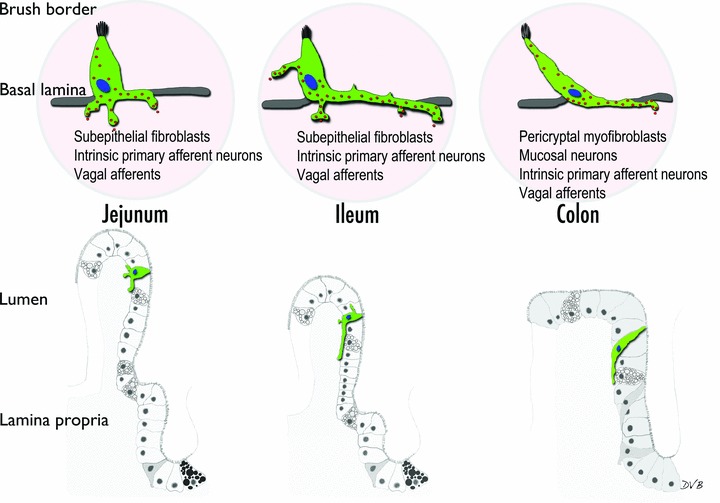
Representation of L cell basal processes in different parts of the digestive tract. In the jejunum, cells typically have more than one process, whereas in the ileum there is typically one main process that runs underneath several epithelial cells before entering the lamina propria. In the colon, cells are spindle‐shaped and have a long process at their base. The long processes in the ileum and colon often resemble axons with synaptic‐like ends. The potential targets of the processes are listed below the cells in the upper panel.
Figure 3.
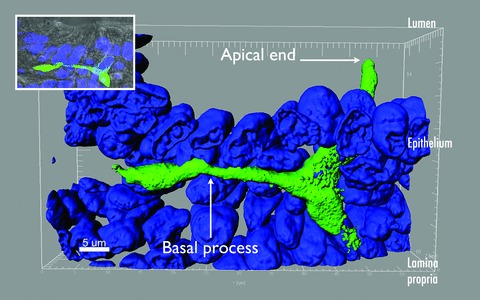
Axon‐like basal process in L cell of the ileum. Three‐dimensional reconstruction of an L cell in the ileum. The apical portion of the cell opens to the lumen, whereas the basal process runs underneath several epithelial cells. The ends of basal processes often have bulbous ends that resemble synaptic buttons. Nuclei were stained with DAPI and are colored blue. Inset shows the location of the same L cell with respect to the brush border of the gut epithelium.
Nutrient and Bacterial Sensing in EECs: Two Sides–Two Pathways?
The apical surface of most EECs is exposed to the lumen. Thus, it has long been assumed that nutrients present in the lumen come in direct contact with the apical surface of EECs to stimulate hormone secretion. 10 , 11 , 23 However, some reports suggest that absorbed nutrients may also stimulate gut hormone release. 24 Because basal processes are apposed just underneath enterocytes, they may serve to sense absorbed nutrients via specific receptors. 13 , 18 EECs are known to have receptors for nutrients, flavors, and gut bacteria. 15 , 21 , 25 , 26 , 27 Considering that the apical regions of L cells are approximately 3 μm in length compared to basal processes over 50 μm long, it is fair to hypothesize that the basal process may harbor at least some of the nutrient receptors that stimulate hormone secretion. 13 This hypothesis is supported by the fact that certain nutrients such as fats must be digested first before inducing hormone secretion in the distal gut. 24 Also, it has been shown that luminal perfusion of the rat’s small intestine with lipids stimulates CCK secretion and that this effect is blocked by inhibition of chylomicron formation. 28 Moreover, immunohistochemical experiments have shown that some of the receptors for fatty acids are actually inside enterocytes and mostly on the basal portion of the EECs. 29 , 30 This evidence suggests that some nutrients may need to access the basal portion of EECs in order to stimulate hormone secretion.
In addition to possibly sensing luminal nutrients, the apical portion of EECs open to the gut lumen may sense bacterial inputs. Some of the family members of bacterial toll‐like receptors (e.g., TLRs 4, 5, and 9) are expressed in the EEC line STC‐1, and stimulation with bacterial ligands (e.g., lipopolysaccharide or flagellin) results in secretion of CCK, keratinocyte‐derived chemokine, and β‐defensin‐2. 27 Remarkably, cytokines and defensins are secreted from STC‐1 cells only in response to bacterial ligands and not to fatty acids. Silencing of the TLR mediator MyD88 reduces CCK secretion stimulated by bacterial ligands but not by fatty acids. Interestingly, genetic deletion of TLR5 in mice results in a phenotype with hyperphagia and all of the characteristics of the metabolic syndrome. 31 This evidence suggests that there are two different sensing pathways in EECs, one for bacteria at the apical portion and one for nutrients perhaps at the base of EECs. Thus, identifying the sensing mechanisms and location of the receptors on EECs may facilitate the design of drugs that target specific receptors, and modulate the secretion of hormones involved in appetite regulation and insulin secretion.
Targets of Axon‐Like Basal Processes
Anatomically, the axon‐like processes in EECs may serve to exchange electrochemical information with neurons and other cells in the lamina propria. Although early attempts failed to find a synapse between EECs and neurons, those experiments relied solely on transmission electron microscopy (TEM). 32 TEM is limited by the fact that tissue sections are less than 100 nm in thickness. Thus, using TEM to find a structure that is sometimes 50 μm away from the body of the cell can be extremely challenging. As a consequence, EECs are thought to interact indirectly with nerves by secreting products that must navigate through the lamina propria to find their targets. 10 , 11 , 17 However, the anatomy of axon‐like basal processes in EECs strongly indicates a very efficient mechanism of communication through cell–cell connections. Two potential targets of such connections are enteric neurons and intestinal subepithelial myofibroblasts (ISEMFs).
Immediately beneath the intestinal epithelium exists a syncytium of myofibroblasts. Those surrounding the crypts are known as perycriptal myofibroblasts, whereas those beneath the villi epithelium are known as ISEMFs. 33 ISEMFs, in particular, are responsible for contractile activity of villi. 34 Recent data indicate that these cells are also important regulators of epithelial cell proliferation and restitution of the intestinal mucosa. 35 The gut hormone GLP‐2 is a major inducer of epithelial cell proliferation and its receptors (GLP‐2R) are located in ISEMFs. 36 , 37 , 38 GLP‐2 is thought to bind to GLP‐2R in ISEMFs and cause the release of insulin‐like growth factors, which stimulate epithelial cell proliferation and mucosal restitution. 39 One source of GLP‐2 is L cells, which are separated from ISEMFs only by the basal lamina. 37 Although a physical connection between EECs and ISEMFs has yet to be described, this relationship is very likely to exist considering that basal processes from EECs penetrate the basal lamina. Moreover, in villi but not crypts, the basal lamina has fenestrations that appear to provide access for epithelial processes to enter the lamina propria and interact with ISEMFs. 40 Uncovering the mechanisms of communication between EECs and cells in the lamina propria will help to elucidate how chemosensation in the gut lumen is coupled to gastrointestinal motility and epithelial cell proliferation.
Besides ISEMFs, the lamina propria of the intestinal mucosa is densely innervated by intrinsic and extrinsic neurons, some of which actually lie adjacent to epithelial cells ( Figure 4 ). 41 , 42 Neuronal afferents of the gut mucosa, in particular those of the vagus nerve, have been shown to express receptors for several gut hormones, including GLP‐1 and PYY. 43 , 44 , 45 In general, secretion of these two hormones is stimulated by nutrients reaching the distal gut. 15 However, plasma levels of GLP‐1 and PYY rise immediately within 15 minutes after a meal but before nutrients arrive in the distal small intestine and colon. 46 , 47 , 48 The mechanism that coordinates nutrient sensing in the foregut with hormone secretion in the distal gut is thought to be neuronal because neurotransmitters such as calcitonin‐gene related peptide can cause an immediate and dramatic release of GLP‐1 and PYY in the distal gut. 49 , 50 The existence of long basal processes in EECs of the distal gut may serve to coordinate the presence of nutrients in the proximal jejunum with hormone secretion in the distal ileum. Indeed, there is evidence that the expression of receptors for gut hormones in the distal gut adapts quickly to the nutritional status of the animal. For instance, expression of the PYY receptor Y2R decreases with fasting and increases after refeeding. This adaptation of Y2R appears to be induced by the foregut hormone CCK through the receptor CCK1R. 43 Because PYY release induces broad physiological changes including satiety, it will be of major interest to understand how an altered metabolic state such as obesity affects the relationship between L cells and nerves in the gut. Interestingly, inducing obesity in rats by a high‐fat diet causes the proopiomelanocortin (POMC) neurons that regulate appetite in the brain to lose synaptic connections. 51 What is more, the POMC neurons of rats that are resistant to diet‐induced obesity actually gain synaptic connections. 51 If, in a similar manner, obesity alters connections in the intestine, this would potentially alter the connection between nutrient chemosensation in the gut lumen and appetite regulation in the brain.
Figure 4.
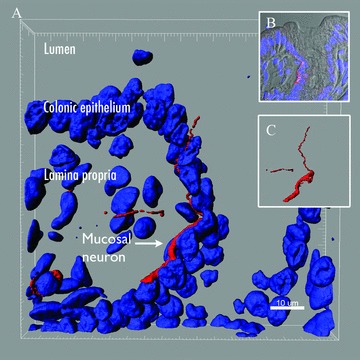
Mucosal neuron underneath the colon epithelium. (A) Colonic tissue from a mouse was stained with the neuronal marker medium size neurofilament (NFm) (red). Z‐stack images obtained with a laser confocal scanning microscope were three‐dimensionally reconstructed using Imaris. The body of the NFm positive neuron (red) is underneath the epithelial layer and one of the neurites actually pierces through the epithelium but does not reach the lumen. Cell nuclei are colored blue and were stained with DAPI. (B) Composite image showing the location of the mucosal neuron (red) with respect to epithelial cell nuclei (blue) and the lumen of the colon (gray). (C) Three‐dimensional reconstruction showing NFm positive neuron by itself.
Conclusions
EECs have prominent basal cytoplasmic processes that increase in length from the proximal to the distal intestine. These cytoplasmic processes run underneath the absorptive epithelium, are filled with hormone vesicles, and resemble an axon that ends in a synapse‐like bulb. The location and anatomy of these processes suggest two functions: (1) their location at the base of enterocytes may serve to monitor absorbed nutrients; and (2) their axon‐like anatomy may convey electrochemical information onto nerves and ISEMFs in the lamina propria through cell–cell connections. Because the gut is the first center to integrate inputs from nutrients and bacteria, future efforts to understand how EECs communicate with cells in the lamina propria may provide new insights for treating metabolic disorders.
Acknowledgment
Sources Of Financial Support
This work was supported by National Institutes of Health grant DK38626.
References
- 1. Rehfeld J. The new biology of gastrointestinal hormones. Physiol Rev. 1998; 78(4): 1087–1108. [DOI] [PubMed] [Google Scholar]
- 2. Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010; 153: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest. 2010; 120(5): 1708–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang J, Cortina G, Wu S, Tran R, Cho J, Tsai M, Bailey T, Jamrich M, Ament M, Treem W. Mutant neurogenin‐3 in congenital malabsorptive diarrhea. N Engl J Med. 2006; 355(3): 270–280. [DOI] [PubMed] [Google Scholar]
- 5. Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest. 1986; 77(3): 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batterham R, Cowley M, Small C, Herzog H, Cohen M, Dakin C, Wren A, Brynes A, Low M, Ghatei M. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002; 418(6898): 650–654. [DOI] [PubMed] [Google Scholar]
- 7. Stacher G, Bauer H, Steinringer H. Cholecystokinin decreases appetite and activation evoked by stimuli arising from the preparation of a meal in man. Physiol Behav. 1979; 23(2): 325–331. [DOI] [PubMed] [Google Scholar]
- 8. Gutniak M, Ørkov C, Holst J, Ahrén B, Efendi S. Antidiabetogenic effect of glucagon‐like peptide‐1 (7–36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992; 326(20): 1316–1322. [DOI] [PubMed] [Google Scholar]
- 9. Dupre J, Ross S, Watson D, Brown J. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metabol. 1973; 37(5): 826–828. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz T, Holst B. An enteroendocrine full package solution. Cell Metabol. 2010; 11(6): 445–447. [DOI] [PubMed] [Google Scholar]
- 11. Bertrand P. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009; 3(48): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samuel B, Shaito A, Motoike T, Rey F, Backhed F, Manchester J, Hammer R, Williams S, Crowley J, Yanagisawa M, Gordon J. Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Nat Acad Sci. 2008; 105(43): 16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohórquez D, Chandra R, Samsa L, Vigna S, Liddle R. Characterization of basal pseudopod‐like processes in ileal and colonic PYY cells. J Mol Histol. 2011; 1: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nivoit P, Morens C, Van Assche F, Jansen E, Poston L, Remacle C, Reusens B. Established diet‐induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009; 52(6): 1133–1142. [DOI] [PubMed] [Google Scholar]
- 15. Reimann F, Habib A, Tolhurst G, Parker H, Rogers G, Gribble F. Glucose sensing in L cells: a primary cell study. Cell Metabol. 2008; 8(6): 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karra E, Batterham R. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol cell endocrinol. 2010; 316(2): 120–128. [DOI] [PubMed] [Google Scholar]
- 17. Cummings D, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007; 117(1): 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandra R, Samsa L, Vigna S, Liddle R. Pseudopod‐like basal cell processes in intestinal cholecystokinin cells. Cell Tis Res. 2010; 341: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsson L, Goltermann N, de Magistris L, Rehfeld J, Schwartz T. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979; 205(4413): 1393–1395. [DOI] [PubMed] [Google Scholar]
- 20. Wade P, Westfall J. Ultrastructure of enterochromaffin cells and associated neural and vascular elements in the mouse duodenum. Cell and Tissue Research. 1985; 241(3): 557–563. [DOI] [PubMed] [Google Scholar]
- 21. Parker H, Habib A, Rogers G, Gribble F, Reimann F. Nutrient‐dependent secretion of glucose‐dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009; 52(2): 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lundberg J, Tatemoto K, Terenius L, Hellström, P , Mutt V Hökfelt, T , Hamberger B. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Nat Acad Sci. 1982; 79(14): 4471–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engelstoft M, Egerod K, Holst B, Schwartz T. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metabol. 2008; 8(6): 447–449. [DOI] [PubMed] [Google Scholar]
- 24. Feinle‐Bisset C, Patterson M, Ghatei M, Bloom S, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metabol. 2005; 289(6): E948–E953. [DOI] [PubMed] [Google Scholar]
- 25. Rozengurt N, Wu S, Chen M, Huang C, Sternini C, Rozengurt E. Colocalization of the α‐subunit of gustducin with PYY and GLP‐1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006; 291(5): G792–G802. [DOI] [PubMed] [Google Scholar]
- 26. Jang H, Kokrashvili Z, Theodorakis M, Carlson O, Kim B, Zhou J, Kim H, Xu X, Chan S, Juhaszova M. Gut‐expressed gustducin and taste receptors regulate secretion of glucagon‐like peptide‐1. Proc Nat Acad Sci. 2007; 104(38): 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, Zanobbio L, Arnaboldi F, Shirai YF, Serrao G. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. 2007; 178(7): 4296–4303. [DOI] [PubMed] [Google Scholar]
- 28. Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol-Reg Int Comp Physiol. 1998; 274(6): R1834–R1838. [DOI] [PubMed] [Google Scholar]
- 29. Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness J, Kuwahara A. Short‐chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell and Tissue Research. 2006; 324(3): 353–360. [DOI] [PubMed] [Google Scholar]
- 30. Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short‐chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008; 39(2): 135–142. [DOI] [PubMed] [Google Scholar]
- 31. Vijay‐Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE Gewirtz, AT . Metabolic syndrome and altered gut microbiota in mice lacking Toll‐like receptor 5. Science. 2010; 328(5975): 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundberg J, Dahlström A, Bylock A, Ahlman H, Pettersson G, Larsson I, Hansson H, Kewenter J. Ultrastructural evidence for an innervation of epithelial enterochromaffine cells in the guinea pig duodenum. Acta Physiol Scand. 1978; 104(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 33. Powell D, Pinchuk I, Saada J, Chen X, Mifflin R. Mesenchymal cells of the intestinal lamina propria. Ann Rev Physiol. 2011; 73(1): 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joyce N, Haire M, Palade G. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterol. 1987; 92(1): 68–81. [DOI] [PubMed] [Google Scholar]
- 35. Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011; 300(5): G684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon‐like peptide 2. Proc Nat Acad Sci. 1996; 93(15): 7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL Drucker, DJ . Enteroendocrine localization of GLP‐2 receptor expression in humans and rodents. Gastroenterol. 2000; 119(3): 744–755. [DOI] [PubMed] [Google Scholar]
- 38. Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP‐2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP‐2 receptors. Regul Pept. 2005; 124(1–3): 105–112. [DOI] [PubMed] [Google Scholar]
- 39. Leen JLS, Izzo A, Upadhyay C, Rowland KJ, Dube PE, Gu S, Heximer SP, Rhodes CJ, Storm DR Lund, PK . Mechanism of action of glucagon‐like peptide‐2 to increase IGF‐I mRNA in intestinal subepithelial fibroblasts. Endocrinol. 2011; 152(2): 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desaki J, Shimizu M. A re‐examination of the cellular reticulum of fibroblast‐like cells in the rat small intestine by scanning electron microscopy. J Electron Microsc. 2000; 49(1): 203–208. [DOI] [PubMed] [Google Scholar]
- 41. Berthoud H, Kressel M, Raybould H, Neuhuber W. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI‐tracing. Anat Embryol. 1995; 191(3): 203–212. [DOI] [PubMed] [Google Scholar]
- 42. Furness JB. The enteric nervous system. Malden , MA : Wiley‐Blackwell; 2006. [Google Scholar]
- 43. Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG Dockray, GJ . Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008; 28(45): 11583–11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K. The role of the vagal nerve in peripheral PYY3–36‐induced feeding reduction in rats. Endocrinol. 2005; 146(5): 2369–2375. [DOI] [PubMed] [Google Scholar]
- 45. Mannon P, Kanungo A, Mannon R, Ludwig K. Peptide YY/neuropeptide Y Y1 receptor expression in the epithelium and mucosal nerves of the human colon. Regul Pept. 1999; 83(1): 11–19. [DOI] [PubMed] [Google Scholar]
- 46. Adrian T, Ferri GL, Bacarese‐Hamilton A, Fuessl H, Polak J, Bloom S. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985; 89(5): 1070–1077. [DOI] [PubMed] [Google Scholar]
- 47. Batterham R, Heffron H, Kapoor S, Chivers J, Chandarana K, Herzog H Le Roux C, Thomas E, Bell J, Withers D. Critical role for peptide YY in protein‐mediated satiation and body‐weight regulation. Cell Metabolism. 2006; 4(3): 223–233. [DOI] [PubMed] [Google Scholar]
- 48. Taylor I. Distribution and release of peptide YY in dog measured by specific radioimmunoassay. Gastroenterol. 1985; 88(3): 731–737. [DOI] [PubMed] [Google Scholar]
- 49. Dumoulin V, Dakka T, Plaisancie P, Chayvialle J, Cuber J. Regulation of glucagon‐like peptide‐1‐(7–36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinol. 1995; 136(11): 5182–5188. [DOI] [PubMed] [Google Scholar]
- 50. Plaisancie P, Bernard C, Chayvialle JA, Cuber JC. Regulation of glucagon‐like peptide‐1‐(7–36) amide secretion by intestinal neurotransmitters and hormones in the isolated vascularly perfused rat colon. Endocrinol. 1994; 135(6): 2398–2403. [DOI] [PubMed] [Google Scholar]
- 51. Horvath T, Sarman B, GarcÌa‐C·ceres C, Enriori P, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen J Perez‐Tilve D. Synaptic input organization of the melanocortin system predicts diet‐induced hypothalamic reactive gliosis and obesity. Proc Nat Acad Sci. 2010; 107(33): 14875–14880. [DOI] [PMC free article] [PubMed] [Google Scholar]


