Abstract
Objective
Reperfusion after stroke leads to infiltration of inflammatory cells into the ischemic brain. NADPH oxidase (NOX2) is a major enzyme system which generates superoxide in immune cells. We studied the effect of NOX2 derived from the immune cells in the brain and in blood cells in experimental stroke.
Methods
To establish whether NOX2 plays a role in brain ischemia, strokes were created in mice, then mice were treated with the NOX2 inhibitor, apocynin or vehicle and compared to mice deficient in NOX2's gp91 subunit and their wildtype littermates. To determine whether NOX2 in circulating cells versus brain resident cells contribute to ischemic injury, bone marrow chimeras were generated by transplanting bone marrow from wildtype or NOX2 deficient mice into NOX2 or wildtype hosts, respectively.
Results
Apocynin and NOX2 deletion both significantly reduced infarct size, blood-brain barrier disruption and hemorrhagic transformation of the infarcts, compared to untreated wildtype controls. This was associated with decreased MMP-9 expression and reduced loss of tight junction proteins. NOX2 deficient mice receiving wildtype marrow had better outcomes compared to the wildtype mice receiving wildtype marrow. Interestingly, wildtype mice receiving NOX2 deficient marrow had even smaller infarct sizes and less hemorrhage than NOX2 deficient mice receiving wildtype marrow.
Interpretation
This indicates that NOX2, whether present in circulating cells or brain resident cells, contributes to ischemic brain injury and hemorrhage. However, NOX2 from the circulating cells contributed more to the exacerbation of stroke than that from brain resident cells. These data suggest the importance of targeting the peripheral immune system for treatment of stroke.
Introduction
The inflammatory response accompanying stroke is recognized to contribute to secondary ischemic injury 1. Blood-brain barrier (BBB) disruption plays a crucial role in complicating ischemic brain damage, because it can lead to brain edema and cerebral hemorrhage. Prior reports have linked inflammation to BBB disruption because immune mediators open this barrier and worsen ischemic injury 2–5. Thus, immune responses are likely to contribute significantly to BBB disruption during stroke. Prior studies have implicated cytokines 4, matrix metalloproteinases (MMPs) 5 and endogenous tissue plasminogen activator (tPA) 6. Consistent with this, our lab previously showed that the addition of microglia to BBB constituents potentiated injury due to oxygen-glucose deprivation (OGD), and that this could be reversed by inhibiting phagocytic NADPH oxidase (NOX2), a major enzyme system in immune cells that generates superoxide 7.
Inflammation following focal cerebral ischemia consists of peripheral leukocyte influx into the brain and activation of endogenous microglia, leading to the generation of immune substances that may induce more ischemic damage including disruption of the BBB and extracellular matrix 1, 8–10. BBB disruption can further potentiate brain injury and contribute to secondary ischemic damage by permitting serum elements and blood cells to enter the brain 11, 12. An extreme consequence of BBB disruption can lead to the entry of blood into the ischemic brain, or hemorrhagic transformation (HT), and can be especially catastrophic in the setting of thrombolytic use 13.
The NADPH oxidases (NOX) are a group of plasma membrane-associated enzymes found in primarily in neutrophils and microglia 14, 15. Through electron transfer with NADPH as the donor, NOX generates superoxide. We and others found that pharmacologically inhibiting NOX2 or studying mice deficient in the same enzyme was protective against experimental stroke 16–22. We now show that NOX2 in the circulating immune cells contributes more to ischemic brain injury compared to NOX2 in the brain.
Methods
All studies involving laboratory animals received prior institutional approval, according to NIH guidelines.
Mouse stroke model
C57BL/6 male mice (Jackson Lab) or transgenic mice from the same genetic background (25–30 gm) were anesthetized with isoflurane by face mask and maintained at surgical planes of anesthesia. Throughout the procedure, body temperature, heart rate, and blood pressure were monitored. Mice were subjected to transient middle cerebral artery occlusion model (tMCAO) using an intraluminal filament for 2h followed by reperfusion 7, 16. Mice were euthanized at the end of the observation period by an overdose of isoflurane followed by decapitation.
Neurological deficit scores
Prior to euthanasia, mice were assessed for neurological deficits using a modified Bederson scale previously published by our group 16. A lower score indicated a less severe deficit than a higher score.
Infarct Volume and Gross Hemorrhage Assessment
After sacrifice, brains were removed, cut into 2mm coronal slices, sections were inspected for gross hemorrhage according to previously published methods 16. Only cerebral hemorrhages visible to the naked eye were scored on a scale of 0= no gross hemorrhage, to 4=the most severe hemorrhage. Brain sections were then fixed and prepared for histochemistry 23. Infarct volume was determined from hematoxylin and eosin (H&E) stained sections using previously published methods 24.
Detection of BBB disruption
BBB permeability was evaluated by detection of extravasated Evans blue dye (EBD) as previously described 7. The volume of dye extravasation was determined by tracing the region of blue-stained tissue from coronal sections. In order to establish that this method compares to a widely used method to assess BBB disruption that relies on a spectrophotometric readout 25, mice were subjected to 0 (n=1), 60 (n=2), 90 (n=1) and 120 (n=1) minutes of MCAO. EBD was injected, and brains were sectioned and scanned, then the ipsilateral brain sections were homogenized for spectrophotometric measurements. The amount of EBD contained in the brain sample was measured on a spectrophotometer (SpectraMax 340PC, Molecular Devices) at 610 nm. The tissue content of EBD was quantified from a linear standard curve derived from known amounts of the dye placed in adjacent wells. Areas of EBD extravasation were then measured from the scanned brain sections as described previously 7. Both methods were found to highly correlate (R=0.99); thus, subsequent BBB disruption was estimated from intact brain sections.
Genetic mouse models
Transgenic mice expressing green fluorescent protein (GFP) transgenic mice (C57BL/6-Tg(ACTB-EGFP)1Osb/J, Jackson Labs, Bar Harbor, ME) or LacZ (B6;129S-Gt(ROSA)26Sor/J, Jackson Labs) were also used as bone marrow donors.
Homozygote female and hemizygote male breeding pairs deficient in NOX2 were purchased from Jackson Lab (B6.129S6-Cybbtm1Din/J). These mice contain a null allele of the gp91 subunit of NOX2 involved in X-linked chronic granulomatous disease (X-CGD) 26. Mice were backcrossed for five generations and the hemizygous males were genotyped. Cerebral vasculature was delineated by injection of carbon black, and plasticity of the posterior communicating arteries was compared between knockout and wildtype littermates 27.
Bone marrow chimeras
4–5 week-old male mice were anesthetized with a cocktail of xylazine and ketamine (50 mg/kg ketamine & 10 mg/kg xylazine). All recipient mice received a 1.1Gy irradiation as described before 28. Briefly, irradiation was generated by a Philips RT250 X-ray machine (Philips Medical Systems, Hamburg, Germany). During irradiation, the heads of the mice were shielded with a lead bar which reduced 99.99% of irradiation. Irradiated mice were rescued with bone marrow from donor mice within 3h after irradiation by injecting 5 × 106 bone marrow cells through the tail vein.
To validate this model, chimeras generated from marrow of the GFP or lacZ transgenic mice were prepared. Blood samples were collected and subjected to flow cytometry as at 1, 2 and 3 months post bone marrow transplantation, and brains of the mice were harvested and stained for β-galactosidase (gene product of lacZ) or GFP. Chimeric animals were subjected to 2h MCAO, followed by 22h reperfusion, then sacrificed; brains were collected and sectioned as described above. LacZ or GFP positive cells in the brain were detected by immunohistochemistry 29 and viewed under a microscope.
Myeloperoxidase (MPO) staining and counting
Brain sections were processed and immunostained as described above using a primary antibody against MPO (Abcam, Cambridge, MA, USA. cat #: ab15484) followed by a biotin-conjugated polyclonal goat anti rabbit IgG (Abcam, Cambridge, MA, USA. cat #: ab6720). MPO positive cells were counted from within the infarct border as described elsewhere 23. Only MPO positive cells with characteristic neutrophil morphology were counted from six adjacent nonoverlapping 400X high-power and the cell densities were expressed as mean number of cells per field.
Western blots
22h after reperfusion, brains were harvested and the ipsi and contralateral hemispheres were separated and brain tissue was homogenized in 7 volumes of ice cold suspension buffer containing, protease (Sigma, Cat# P8340) and phosphatase inhibitors (Sigma, Cat #: 2850). Tissue lysates were then centrifuged at 14,000G for 10 minutes at 4°C. Supernatants were collected and protein concentrations were measured. 15 µg of protein was loaded into a 10–20 % Ready Gel Tris-HCl Gel (Bio-Rad, Hercules, CA) and subjected to SDS polyacrylamide gel electrophoresis, then transferred onto PVDF membranes, blocked and probed with primary antibodies against: MMP-9 (1:5000, Chemicon, AB19047), Occludin (1:100, Zymed, South San Francisco, CA, 71–1500), or ZO-1 (1:100, Zymed, South San Francisco, CA, 61–7300) followed by peroxidase-conjugated secondary antibody. Signals were visualized with enzyme chemiluminescence (ECL, GE Healthcare, Piscataway, NJ, RPN2132). Films were scanned with an HP scanner and the signals were quantified with NIH Image J software. In order to confirm equal protein loading, the membranes were stripped and stained with monoclonal anti-β-actin antibody produced in mouse (Sigma, A2228).
Superoxide assay
In order to estimate the capacity of circulating cells to generate reactive oxygen species (ROS), a superoxide assay was performed using the LumiMax Superoxide Anion Detection Kit (Agilent Technologies, Stratagene, La Jolla, CA, #204525) according to the manufacturer’s directions. This assay is based on the oxidation of luminal by superoxide anion. Whole blood was collected in anesthetized mice through an intracardiac approach. Leukocytes were then isolated using a method of centrifugation and chilling (to remove red blood cells) described elsewhere 30. The isolated leukocytes were resuspended and diluted cells in ROS assay solution (Agilent Technologies, Stratagene, La Jolla, CA Catalog #204525-25) to a concentration of 1.25×104/µl. Assay reagents were then added to the cell suspension according to manufacturer’s instructions, followed by stimulation with 100ng/ml PMA. After 80min, sample luminescence was read on a luminometer (Turner BioSystems, Inc. Cat#9300-002. Sunnyvale, CA). Relative luminescence was then normalized the number of leukocytes in each sample assayed.
Statistical Analysis
All statistical analyses were performed using Sigma Stat 3.1 (Systat Software, Inc., CA). Quantitative data were presented as mean ± S.E. Standard parametric tests were applied where appropriate, such as ANOVA with post hoc comparisons. Chi square or nonparametric tests were used for noncontinuous data. P<0.05 was considered statistically significant.
Results
NADPH oxidase inhibition or deficiency led to smaller infarcts, better neurological function, less BBB disruption and hemorrhage following ischemic brain injury
To establish the comparative effects of NOX2 inhibition or deficiency in our model, mice were exposed to middle cerebral artery occlusion (MCAO), then treated with apocynin 2.5mg/kg IV (apo) or vehicle (veh, an equivalent amount of DMSO) at the onset of reperfusion. Groups studied include 1) wild type (WT)+veh, 2) X-CGD+veh, 3) WT+ apo, and 4) X-CGD+apo. This dose of apocynin was based on our prior study where we had observed optimal neuroprotection and that apocynin itself does penetrate into the brain after system administration 16. Wildtype littermates of the X-CGD mice were also studied and were found to have similar infarct sizes as the non wildtype littermates; thus, data from these mice were pooled. By 24h post MCAO, mice treated with apocynin or mice lacking gp91 had smaller infarcts than vehicle treated, wildtype controls (Fig 1A). X-CGD mice given apocynin failed to have any further reduction in infarct size compared to untreated X-CGD mice, although they had somewhat smaller infarcts than wildtype mice given apocynin. To exclude the possibility that the smaller infarcts in the X-CGD mice were not due to differences in cerebrovascular anatomy, mice were perfused with carbon black, and posterior communicating artery plasticity was examined as described previously 27. No differences in cerebrovascular anatomy were observed (Suppl Fig. 1). There were also no differences between experimental groups in any physiological parameters monitored. Assessment of neurological deficits using a modified Bederson score also showed that mice with NOX2 deficiency/inhibition had better outcome (Fig. 1B), and that X-CGD mice treated with apocynin fared no better than X-CGD mice or wildtype mice given apocynin. Mortality was no different between groups, and ranged from 20–25%.
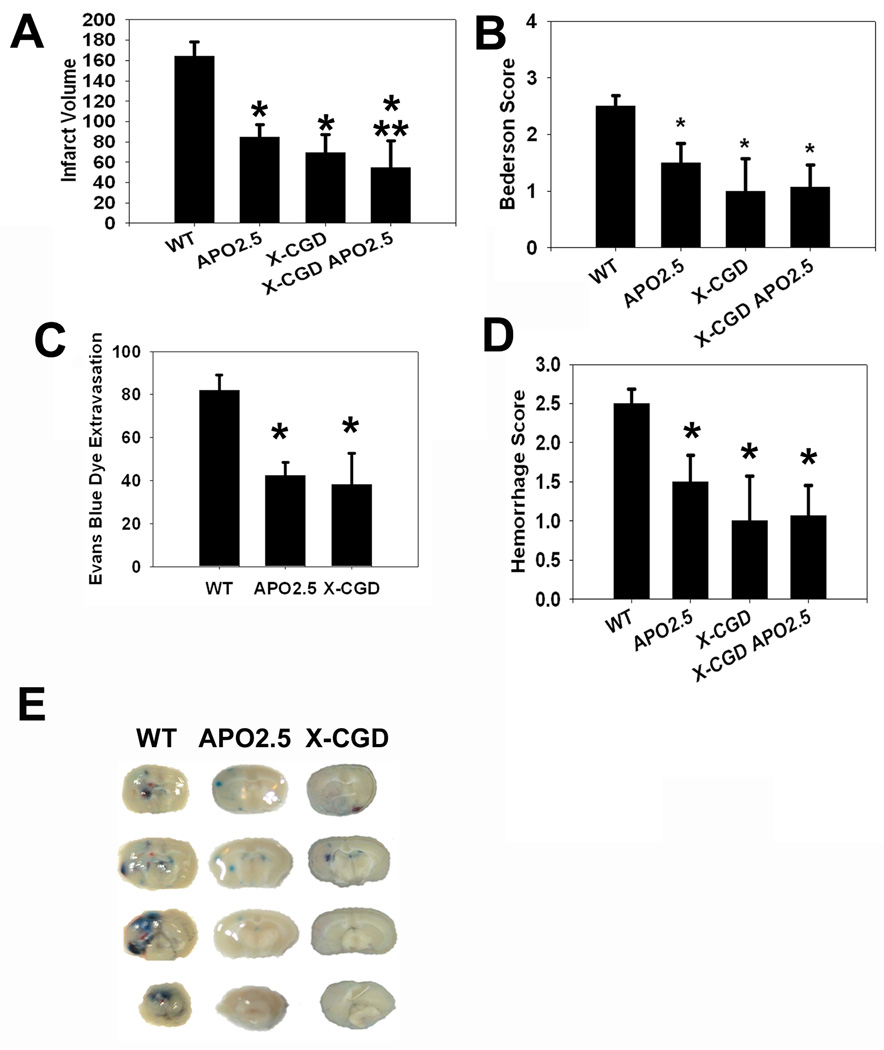
Fig 1. Mice lacking the NADPH oxidase subunit gp91 or treated with apocynin were protected from ischemic injury.
(A) Mice treated with apocynin (APO2.5) or those deficient in NOX2’s gp91 subunit (X-CGD) had smaller infarcts after MCAO compared to vehicle treated wildtype mice (WT). Apocynin treatment of X-CGD mice (X-CGD APO2.5) failed to further decrease infarct size compared to untreated X-CGD mice, but had somewhat smaller infarcts than APO2.5. (B) Bederson scores among APO2.5 and X-CGD mice were more favorable than WT, and X-CGD mice treated with apocynin (X-CGD APO2.5) fared no better than deficient mice or wildtype mice treated with apocynin. Evans blue dye extravasation (EBD) (C) and hemorrhage scores (D) were similarly reduced compared to untreated WT. (E) Representative images of EBD extravasation and hemorrhagic transformation from the different experimental groups. (n=6/group, *P<0.05 vs. WT, **P<0.05 vs. APO2.5)
NOX2 deficiency/inhibition reduces BBB disruption and hemorrhage
BBB disruption, as measured by assessing the amount of Evan’s blue dye (EBD) extravasation within the brain parenchyma, was decreased among X-CGD and apocynin treated mice compared to controls (Fig. 1C and E). NOX2 inhibition and deficiency also reduced the severity of hemorrhagic transformation compared to controls, and X-CGD mice treated with apocynin failed to have further reduction in brain hemorrhage severity (Fig. 1D–E).
NOX2 inhibition or deficiency decreased MMP-9 expression, and prevented tight junction protein loss
Consistent with our prior observations that NOX2 is a major factor in BBB disruption 7, we assessed the effect of NOX2 deficiency/inhibition on MMP-9 expression (a major MMP implicated in disruption of the extracellular matrix) and representative tight junction proteins. Compared with controls, apocynin treated and X-CGD mice demonstrated less MMP-9 expression after experimental stroke (Fig. 2A). Similarly, NOX2 inhibition/ deficiency led to preservation of occludin and ZO-1 (Fig. 2B–C).
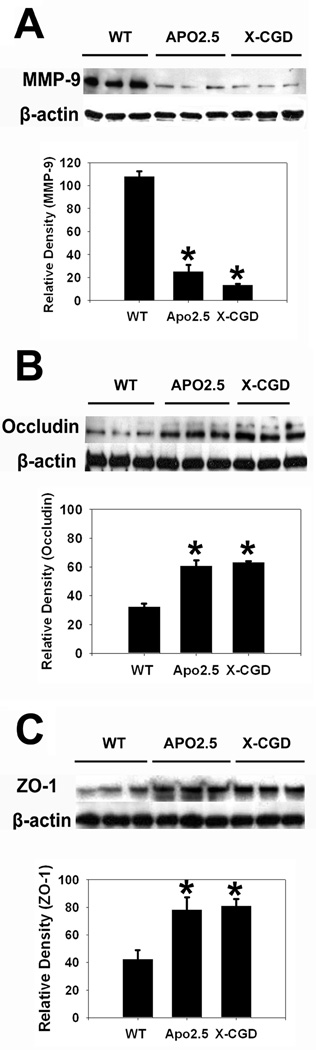
Fig 2. NOX2 inhibition/deletion reduces matrix metalloproteinase-9 (MMP-9) expression and prevents loss of tight junction components.
Compared to wild type mice (WT), mice that received apocynin 2.5 mg/kg (APO2.5) or NOX2 deficient mice (X-CGD) had decreased MMP-9 expression following experimental stroke (A). NOX2 inhibition/ deficiency led to reduced loss of tight junction proteins occludin (B) and zona occludens-1 (ZO-1) (C). (n=6/group). Data are expressed as the mean optical density normalized to that of the corresponding β-actin band. *P<0.05 vs. WT.
Validation of a modified bone marrow chimera model
In order to distinguish the effects of NOX2 derived from circulating immune cells and that from NOX2 expressing cells in the brain, we turned to a bone marrow chimera model where marrow from donor mice replaces that of the host mouse. In order to generate such chimeras, it is necessary to ablate the host’s marrow, often by lethal irradiation. Because whole body irradiation itself can cause brain cell death 31, microglial activation 32 and even BBB disruption 33, we shielded the heads of these mice during marrow ablation to minimize such complications. Marrow from GFP or LacZ transgenic mice was transplanted into wildtype hosts. Two months later, 70% or more of the donor marrow cells could be detected in the circulating cells of the recipient mice, but there was no turnover of any resident brain cells by the transplanted marrow (Fig. 3A). However, by 3 months, marrow derived cells began to incorporate into vascular structures of the brain. Thus, we chose to study chimeric mice 2 months post transplant when no marrow derived cells had yet entered the brain. These mice were subjected to 2 h MCAO and brains were harvested 24 h later. Immunostaining for donor marrow revealed numerous positive cells within the ischemic cortex (Fig. 3B, C) and surrounding vascular structures (Fig. 3D). In a control, non-transplanted animal subjected to MCAO, no donor derived cells were observed (Fig. 3E).
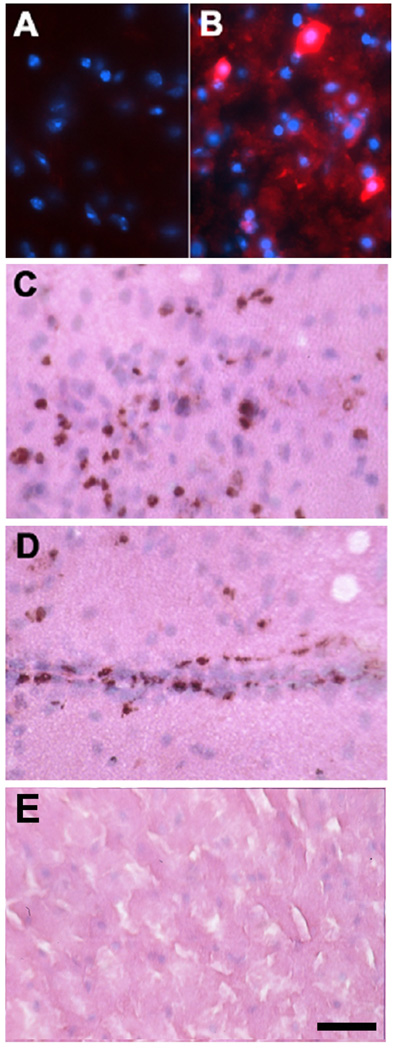
Fig 3. Bone marrow chimeras do not show turnover of resident brain cells 2 months post transplant by marrow derived cells, but leukocyte entry does occur after ischemic stroke.
C57BL/6 mice were transplanted with marrow from donor lacZ transgenic mice which expresses β-galactosidase (β-gal). Two months post transplantation, no marrow-derived cells were observed in nonischemic brain (A). In a mouse subjected to 2 h MCAO, immunostaining for β-gal shows numerous positive cells within the ischemic cortex 24 h post insult (B, C) and surrounding vascular structures (D). In a control, non-transplanted animal subjected to MCAO, no β-gal positive cells were observed in the brain (E). (A, B: β-gal (red) and DAPI (blue), C–E: β -gal stain with H & E counterstain, scale = 50 µm).
NOX2 deficiency in circulating immune cells protected from experimental stroke more than NOX2 deficiency in the brain
Three variations of bone marrow chimeric mice were generated: (1) Wildtype mice that received NOX2 deficient marrow from the X-CGD mice, (2) X-CGD mice that received wildtype marrow, and (3) wildtype mice that received wildtype marrow. These mice were subjected to experimental stroke as described above. Chimeric mice from groups (1) and (2) had smaller infarcts than (3), but mice from group (1) had even smaller infarcts than mice from group (2) (Fig. 4A–B). Similarly, neurological deficits were improved among group (1) mice compared to the other 2 groups (Fig. 4C). Mortality was no different between groups and ranged from 15–25%.
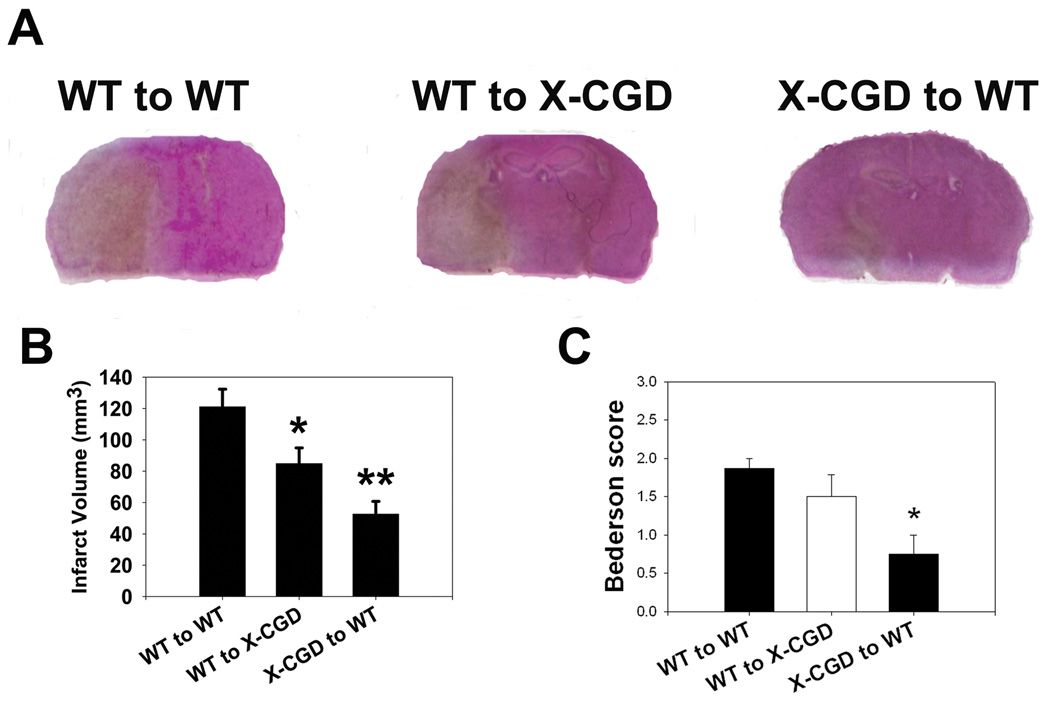
Fig. 4. Marrow derived NOX2 contributes more to brain ischemia than brain derived NOX2.
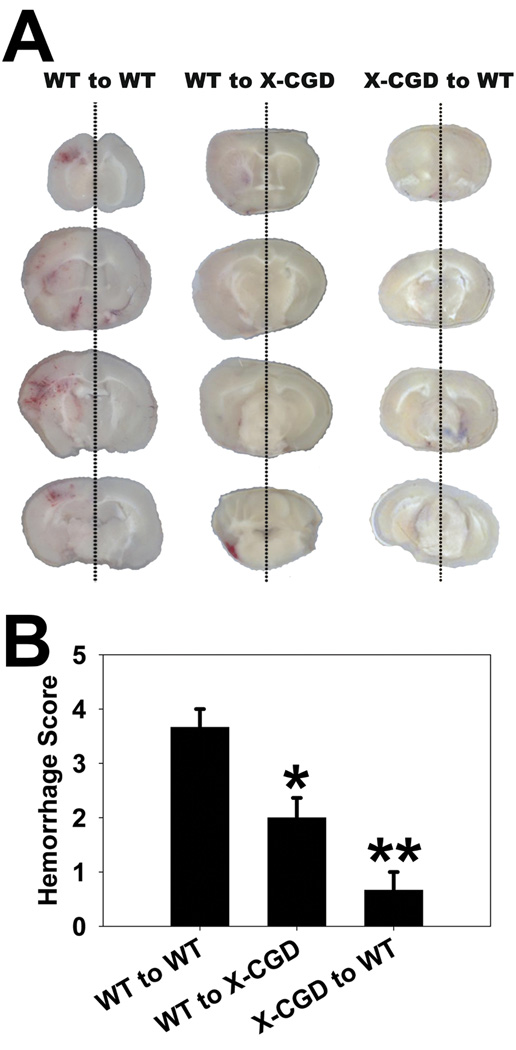
Wildtype (WT) mice receiving NOX2 deficient marrow (X-CGD to WT) and NOX2 deficient mice receiving WT marrow (WT to X-CGD) both had smaller infarcts than WT mice receiving WT marrow (WT to WT) (A, B). However, X-CGD to WT chimeras have even smaller infarcts than WT to X-CGD chimeras indicating that NOX2 generated in the circulating marrow derived cells contributes to ischemic damage more than NOX2 in the brain. Bederson scores indicate that X-CGD to WT chimeras had better neurological function compared to both WT to WT and WT to X-CGD chimeras (C). (n=6/group, *P<0.05 vs. WT to WT, **P<0.05 vs. WT to WT and WT to X-CGD)
Mice from groups (1) and (2) had less severe brain hemorrhage compared to group (3), but mice from group (1) had even less severe hemorrhage than mice from group (2) (Fig. 5). These observations indicate that NOX2 in circulating cells contributes more to ischemic injury than NOX2 in the brain.
Fig 5. NOX2 deficiency in marrow derived cells reduces hemorrhagic transformation more than NOX2 deficiency in the brain.
A: Representative images show the severity of hemorrhagic transformation (HT) after experimental stroke. Mice lacking NOX2 in marrow derived cells (X-CGD to WT) had the least severe HT compared to mice with intact NOX2 in marrow derived cells (WT to X-CGD and WT to WT). B: Mice lacking NOX2 in either the brain (WT to X-CGD) or marrow (X-CGD to WT) experienced less severe HT compared to wildtype mice receiving wildtype marrow (WT to WT), but X-CGD to WT mice had the least severe HT. (n=6/group, *P<0.05 vs. WT to WT, **P<0.05 vs. WT to WT and WT to X-CGD)
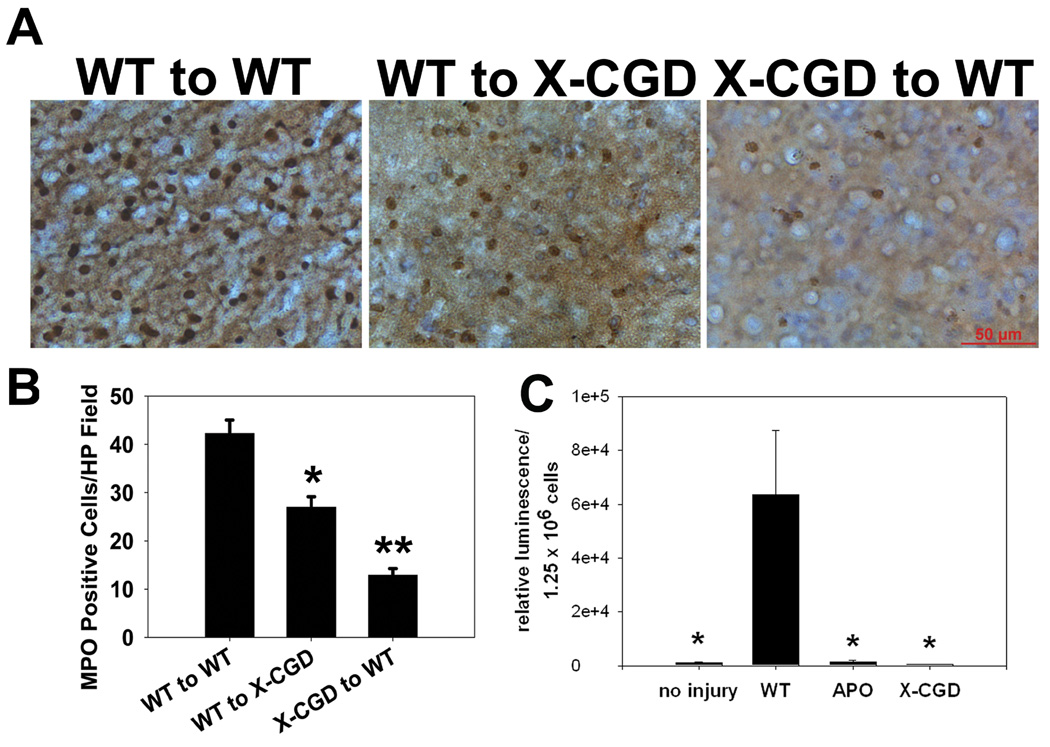
NOX2 deficiency in circulating cells led to fewer infiltrating neutrophils than NOX2 deficiency in the brain
From the above chimeric mice, brain sections were stained for myeloperoxidase to identify neutrophils. Compared to wild type mice that received wild type marrow (3), NOX2 deficient mice that received wild type marrow (2) had fewer numbers of invading neutrophils within the borderline of the infarct core. However, wild type mice that received NOX2 deficient marrow had smallest number of myeloperoxidase positive cells in the ischemic brain (Fig. 6A–B).
Fig 6. NOX2 deficiency in marrow derived cells reduces neutrophil infiltration into ischemic brain, and are less capable of generating reactive oxygen species (ROS) following experimental stroke.
A: Representative myeloperoxidase stains to identify neutrophils show the density of neutrophils that infiltrated into the brain parenchyma 24 hours after experimental stroke. Mice lacking NOX2 in marrow derived cells (X-CGD to WT) had fewer neutrophils present in ischemic brain compared to mice with intact NOX2 in marrow derived cells (WT to X-CGD, WT to WT). B: Mice lacking NOX2 in either the brain (WT to X-CGD) or marrow (X-CGD to WT) both had lower neutrophil densities compared to wildtype mice receiving wildtype marrow (WT to WT), but X-CGD to WT mice had the lowest neutrophil density of all (*P<0.05 vs. WT to WT, **P<0.05 vs. WT to WT and WT to X-CGD). C: Peak superoxide generation as detected by luminescence generated by isolated leukocytes following experimental stroke (WT) was markedly increased, but this was suppressed in leukocytes of animals deficient in NOX2 (X-CGD) or treated with apocynin (APO). An uninjured group (no injury) is shown for comparison. (n=6/group, *P<0.01 vs. WT)
NOX2 deficient leukocytes have decreased capacity to generate ROS compared to wildtype leukocytes
To determine whether NOX2 inhibition or deficiency led to reduced ROS generation, leukocytes were harvested 24 h post experimental stroke then stimulated with PMA to determine their capacity to produce superoxide. Experimental stroke markedly increased the ability of circulating leukocytes to generate superoxide, which was almost completely suppressed to baseline levels with apocynin treatment and in the X-CGD mice (Fig. 6C).
Discussion
We show that NOX2 inhibition or deficiency led to improved neurological outcome as well as decreased BBB disruption and brain hemorrhage. We also found that NOX2 inhibition or deficiency prevented the loss of several tight junction components, and is consistent with the notion that NOX2 contributes to ischemia-induced BBB disruption. To understand the relative contributions of NOX2 in circulating versus brain resident cells, we generated bone marrow chimeras using NOX2 deficient mice. While infarct size and BBB disruption was reduced whether NOX2 was absent in the brain or marrow-derived cells, the beneficial effect was more pronounced when NOX2 was absent in the circulating cells. This observation underscores the importance of the peripheral circulation in potentiating injury due to cerebral ischemia. Further, we found that ischemia itself increases the superoxide generating capacity of circulating leukocytes, and this is suppressed with NOX2 inhibition/deficiency.
A few other studies have also focused on the importance of the peripheral circulation and stroke 34–36. Interestingly, inhibition of this peripheral immune response through splenectomy seems to be protective. MMP-9 generated by circulating immune cells exacerbates brain injury more than that generated in the brain 5, and deletion of delta PKC in circulating leukocytes is similarly protective 37. These latter studies are in line with ours, not only because they focus on the circulating immune cells, but also because delta PKC is one of several kinases that phosphorylate and activate NOX2, and we show that NOX2 deficiency decreases MMP-9.
Translating this to the clinical level is relevant to the extent that many drugs do not penetrate the BBB, yet we demonstrate the importance of targeting the circulating immune cells. Thus, drugs may not need to have adequate CNS penetration to have a neuroprotective effect. While apocynin is an inhibitor of NOX2, it is not specific38. Some prior reports have suggested that it also acts as a direct anti-oxidant 39. Yet, any further beneficial effects in X-CGD mice treated with this compound were relatively small (~15% reduction in infarct size, but no significant differences with our other outcome measures).
In one prior study, bone marrow chimeras using this same X-CGD model were studied. The authors found that non-chimeric NOX2 deficient mice were protected from experimental stroke, but they failed to see any differences among any of the chimera combinations generated 22. The authors concluded that brain and circulating NOX2 must both be suppressed in order to achieve protection. However, these investigators did not shield the heads of the mice during irradiation, which could have added a significant confound to their observations, as irradiation can disrupt the BBB and injure or activate resident brain cells. They also studied mice 4–5 weeks post transplant, whereas we waited until 8 weeks before subjecting the chimeras to experimental stroke. These differences may have increased the overall vulnerability of their chimeras, and it should be noted that their study also had a somewhat higher mortality rate (~38%). We also generated chimeras where NOX2 deficient marrow was transplanted into NOX2 deficient mice. However, these chimeras did not survive more than 2 weeks after marrow transplantation, and may suggest that extreme immunodeficiency (NOX2 deficiency plus marrow irradiation) can be lethal rather than beneficial.
MMP-9 has been the focus of abundant research connecting BBB disruption in stroke 40, 41. Consistent with this, we found decreased MMP-9 expression by NOX2 inhibition or deficiency, and prior work has linked NOX2’s gp91 subunit to MMP-9 expression 42.
We show the importance of NOX2 in potentiating ischemic brain injury, along with BBB disruption and hemorrhage. NOX2 and resultant reactive oxygen species originating from circulating cells seems to contribute more to ischemic damage than that from brain resident cells, and emphasizes the importance and relevance of targeting the peripheral circulation for stroke treatment.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 NS40516 (MAY), P50 NS014543 (MAY, RGG), and GM 49831 (RGG), and an American Heart Association Established Investigator Award (MAY). We wish to thank Drs. Liping Liu, Dennis Deen and Judith Shizuru and Ms. Lily Hu for technical assistance and Dr. Hualong Ma for maintaining the mutant mouse colonies.
References
- 1.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veldhuis WB, Floris S, van der Meide PH, et al. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J Cereb Blood Flow Metab. 2003;23:1060–1069. doi: 10.1097/01.WCB.0000080701.47016.24. [DOI] [PubMed] [Google Scholar]
- 3.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, et al. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–1120. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- 4.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 6.Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005;50:340–350. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- 7.Yenari MA, Xu L, Tang XN, et al. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 8.Tang XN, Yenari MA. Inflammation in stroke. In: Yenari MA, Giffard RG, editors. Glia and inflammation in neurodegenerative disease. Hauppauge: Nova Science Publishers, Inc.; 2006. pp. 85–115. [Google Scholar]
- 9.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 12.Siesjo BK, Siesjo P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- 13.Saver JLS. J. L. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–2283. doi: 10.1161/STROKEAHA.107.487009. [DOI] [PubMed] [Google Scholar]
- 14.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 15.Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. Faseb J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 16.Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 18.Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Tompkins KD, Simonyi A, et al. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackman KA, Miller AA, De Silva TM, et al. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 2009;156:680–688. doi: 10.1111/j.1476-5381.2008.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walder CE, Green SP, Darbonne WC, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 23.Wang GJ, Deng HY, Maier CM, et al. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 24.Maier CM, Sun GH, Kunis D, et al. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 25.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 26.Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 27.Kawase M, Murakami K, Fujimura M, et al. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke. 1999;30:1962–1968. doi: 10.1161/01.str.30.9.1962. [DOI] [PubMed] [Google Scholar]
- 28.Beilhack GF, Scheffold YC, Weissman IL, et al. Purified allogeneic hematopoietic stem cell transplantation blocks diabetes pathogenesis in NOD mice. Diabetes. 2003;52:59–68. doi: 10.2337/diabetes.52.1.59. [DOI] [PubMed] [Google Scholar]
- 29.Yenari MA, Minami M, Sun GH, et al. Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke. 2001;32:1028–1035. doi: 10.1161/01.str.32.4.1028. [DOI] [PubMed] [Google Scholar]
- 30.Morgan D, Cherny VV, Finnegan A, et al. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2 alpha activity. J Physiol. 2007;579:327–344. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellinzona M, Gobbel GT, Shinohara C, Fike JR. Apoptosis is induced in the subependyma of young adult rats by ionizing irradiation. Neurosci Lett. 1996;208:163–166. doi: 10.1016/0304-3940(96)12572-6. [DOI] [PubMed] [Google Scholar]
- 32.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 33.Diserbo M, Agin A, Lamproglou I, et al. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can J Physiol Pharmacol. 2002;80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 34.Ajmo CT, Jr, Collier LA, Leonardo CC, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 36.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 37.Chou WH, Choi DS, Zhang H, et al. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldieri E, Riganti C, Polimeni M, et al. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 39.Heumuller S, Wind S, Barbosa-Sicard E, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 41.Nagel S, Su Y, Horstmann S, et al. Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: effects on BBB breakdown and MMP expression in the acute and subacute phase. Brain Res. 2008;1188:198–206. doi: 10.1016/j.brainres.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Sood R, Chen Q, et al. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.