Abstract
Background
Women infected with human immunodeficiency virus (HIV) increasingly demonstrate abnormalities in fat distribution and metabolism; however, the effects of a home-based exercise regimen in this group have not been investigated.
Methods
We conducted a 16-week randomized intervention study of a supervised home-based progressive resistance training and aerobic exercise program in 40 HIV-infected women with increased waist-hip ratio and self-reported fat redistribution. Cross-sectional muscle area and muscle attenuation were measured by computed tomography. Cardiorespiratory fitness was determined by calculated maximum oxygen consumption (V̇O2max) and strength by 1-repetition maximum.
Results
Cardiorespiratory fitness (V̇O2max) was markedly lower at baseline (median [95% confidence interval], 15.4 [8.3–25.2] mL · kg−1 · min−1) than reported values for healthy female subjects (26–35 mL · kg−1 · min−1). Subjects randomized to exercise had significant improvement in mean ± SEM V̇O2max (1.5 ± 0.8 vs −2.5 ± 1.6 mL · kg−1 · min−1; P<.001) and endurance (1.0±0.3 vs −0.6±0.3 minute; P<.001). Strength increased at the knee extensors, pectoralis, knee flexors, shoulder abductors, ankle plantar flexors, and elbow flexors (all P<.001). Total muscle area (6±1 vs 2±1 cm2; P=.02) and attenuation (2±1 vs −1±1 Hounsfield unit; P=.03) increased in the exercise group. No significant difference was seen in lipid levels, blood pressure, or abdominal visceral fat between the groups, but subjects randomized to exercise reported improved energy and appearance.
Conclusions
A 16-week, supervised, home-based exercise regimen improved measures of physical fitness in HIV-infected women. The effects on strength were most significant, but improvements in cardiorespiratory fitness, endurance, and body composition were also seen.
Trial Registration
clinicaltrials.gov Identifier: NCT00111332.
Metabolic abnormalities are increasingly reported among persons living with human immunodeficiency virus (HIV) infection,1,2 which may lead to increased cardiovascular risk among this population.1,3,4 The Adult Treatment Panel III guidelines place strong emphasis on primary prevention of coronary heart disease and highlight the importance of increased physical activity and therapeutic lifestyle modification in people with risk factors for cardiovascular disease5 To our knowledge, fitness levels and the effects of a combined aerobic and progressive resistance training program have not been investigated exclusively in the growing population of HIV-infected women.
Although exercise programs may be important as a nonpharmacologic strategy to improve cardiac risk factors,6–12 adherence to organized outpatient programs is often poor11 and may limit the efficacy of these strategies in HIV-infected women. Home-based fitness and aerobic programs may increase adherence but have not previously been used for the HIV population. To increase adherence, we designed a novel home-based, supervised aerobic and resistance training regimen.
METHODS
PATIENTS
A planned sample size of 40 HIV-infected women with self-report of changes in fat distribution were recruited through community advertisement and primary care provider referral from October 21, 2002, to March 2, 2005. Consecutive HIV-infected subjects between 18 and 60 years of age with a waist-hip ratio of 0.85 or more and self-report and physical evidence of fat redistribution were eligible for participation. Excluded from participation were subjects who used megestrol acetate, androgens, growth hormone, or glucocorticoid therapy; who had significant liver or kidney disease or severe anemia; who were receiving current therapy with insulin, had a history of diabetes mellitus, or had a fasting glucose level of 126 mg/dL (7.0 mmol/L) or more; who actively engaged in substance abuse; who were pregnant, actively seeking pregnancy, or breastfeeding; or who had had an acute infection or initiated a new antiretroviral therapy regimen within 1 month of the study.
All subjects gave written informed consent. The study was approved by the Human Research Committee at Massachusetts General Hospital.
DESIGN
Eligible subjects were seen at the general clinical research center after a 12-hour overnight fast for a baseline visit and again at 8 weeks (safety visit) and 16 weeks for an evaluation identical to the baseline visit. Subjects were asked regarding dates of all current and prior antiretroviral medications. Study subjects were characterized as eumenorrheic or not. Current smoking history as well as total duration and amount of smoking (in pack-years) was recorded. Fasting blood was drawn for determination of cholesterol, low-density lipoprotein cholesterol, triglycerides, high-density lipoprotein cholesterol, glucose, HIV viral load, and CD4 count. A standard 75-g glucose tolerance test was performed.
Randomization to the exercise program was performed by the General Clinical Research Center Biostatistics Center, using a permuted block algorithm. Subjects not randomized to the exercise program were encouraged to maintain their normal activities, without further proscription as to activity.
ASSESSMENT OF EXERCISE CAPACITY AND CARDIORESPIRATORY FITNESS
A submaximal exercise stress test was conducted on a cycle ergometer to measure endurance.13,14 Subjects cycled at 50 rpm and the workload was progressively increased in increments of 50 W, starting at 50 W, in stages lasting 3 minutes. At the end of each stage, separate readings of heart rate and blood pressure were taken, and rate of perceived exertion was measured with the Borg Rate of Perceived Exertion Scale. Once subjects became fatigued or reached their submaximal heart rate ([220 − age]×0.85), the test was stopped and separate readings of heart rate and blood pressure were taken at 1, 3, and 5 minutes of recovery. Exercise time was calculated on the basis of duration (in minutes) that each subject rode during the sub-maximal bicycle test. Weight-adjusted maximum oxygen consumption (V̇O2max) for measurement of cardiorespiratory fitness was calculated with the following formula: V̇O2max in milliliters per kilogram per minute=[(watts in kilograms times meters per minute × 2.0 mL · min−1)+(3.5 mL · kg−1 · min−1×mass in kilograms)]kg−1 based on the American College of Sports Medicine equation for estimation of V̇O2max during cycle ergometry.13,15 Submaximal exercise testing was performed at baseline and 16 weeks for all enrolled subjects.
ASSESSMENT OF FUNCTIONAL STATUS
Performance (distance walked) on the 6-minute walk test was assessed at baseline and end of study.
BODY COMPOSITION
Height, weight, waist circumference (at the umbilicus), waist-hip ratio, and body mass index (calculated as weight in kilograms divided by the square of height in meters) were determined at baseline and 16 weeks. Cross-sectional muscle area of the left thigh and muscle attenuation in Hounsfield units were determined as previously described.16,17 The coefficient of variation for the measurement of muscle attenuation in our laboratory is 2.4%. The precision for measurement of muscle area is ±1%.16 To assess abdominal visceral and subcutaneous adipose tissue area, a cross-sectional abdominal computed tomographic scan at the level of the L4 pedicle was performed.18 Total fat was measured by dual-energy x-ray absorptiometry (Hologic Inc, Waltham, Mass) with a precision error of 3.0% for fat.19
BIOASSAYS
Total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and glucose were measured by standard techniques. A CD4 count was determined by flow cytometry (Becton Dickinson Biosciences, San Jose, Calif), and HIV viral load was determined by ultrasensitive assay (Amplicor HIV-1 Monitor Assay; Roche Molecular Systems, Branchburg, NJ) with limits of detection of 50 000 to 100 000 RNA copies per milliliter.
NUTRITION EVALUATION
Participants completed a 4-day food record before their baseline and final study visits. These records were reviewed with each patient by a registered dietitian and analyzed (NDS Versions 4.01 and 4.02-NDS-R; Regents of the University of Minnesota, Minneapolis) to quantify total caloric, protein, carbohydrate, and fat intake.
HOME TRAINING EXERCISE PROTOCOL
Subjects randomized to exercise training were seen in their homes by a member of the study staff (S.E.D., J.L., K.L., or S.J.) 3 times a week, on alternating days, for 16 weeks (48 sessions, 2 hours per session, with makeup sessions scheduled for missed sessions). Subjects completed 96% of the total exercise sessions. Required exercise equipment for home use was provided to each subject randomized to the exercise regimen. Equipment included an upright stationary bicycle, a flexion-extension bench, a free-standing squat stand, weight sets, and a heart monitor (Polar A1 Heart Monitor; HRM USA, Levittown, Pa).
Each training session began with a 5-minute warm-up on a stationary bicycle at 50% of estimated maximal heart rate (maximal heart rate=220 - age), followed by a standard flexibility routine to minimize the risk of injury.20 Subsequently, the supervised aerobic and strength training protocol was performed, followed by a cool-down period.
The aerobic training program followed the general guidelines established by the American College of Sports Medicine.13,14 The duration of the aerobic component was 20 minutes during the first 2 weeks and 30 minutes for the remaining 14 weeks. The intensity of the exercise was set at 60% of maximal heart rate during the first 2 weeks and 75% thereafter. Each participant wore a heart monitor, and heart rate and rate of perceived exertion were assessed by a member of the study staff (S.E.D., J.L., K.L., or S.J.) at rest, 10 minutes, 20 minutes, and 30 minutes, and after 5 minutes of recovery.
Resistance training was based on the progressive resistance exercise concept21 and was performed with the equipment provided. Selected muscle groups were trained by alternating lower-and upper-body exercises in the following order: (1) knee-hip extension, (2) pectoralis (bench press), (3) knee flexors, (4) shoulder abduction (lateral raises), (5) ankle plantar flexors (standing calf raises), and (6) elbow flexors (arm curls). Each repetition included concentric and eccentric phases, with the total duration of each contraction being approximately 6 to 10 seconds. Combining concentric and eccentric muscle actions maximizes strength gains and muscle hypertrophy resulting from a strength training program.22 The initial intensity of exercise was set at 60% of the 1-repetition maximum (1 RM) or the maximal amount of weight that can be lifted throughout the full range of motion only once. After 2 weeks, the relative intensity was increased to 70% of the 1 RM and, after an additional 2 weeks, to 80% of the 1 RM. The 1 RM was measured at the first home exercise session and every other week, and the absolute load was adjusted accordingly to maintain the relative intensity of the work-out and to determine training effect. During the first 2 weeks, subjects performed 3 sets of 10 repetitions each for every muscle group, resting 3 to 5 seconds between repetitions, 2 minutes between sets, and 4 minutes between muscle groups. During weeks 3 to 16, subjects performed 4 sets of 8 repetitions for each muscle group, 2 to 3 seconds between repetitions, 1 minute between sets, and 2 minutes between muscle groups. Each home training session was supervised by a trained member of the study staff (S.E.D., J.L., K.L., or S.J.). Subjects not randomized to exercise received no intervention and had a determination of 1 RM made at baseline and the 8- and 16-week visits.
PATIENT SELF-ASSESSMENT OF BENEFIT
Patients were asked an exit question at the end of the study: “In the past 4 months, have you noticed any change in your cardiovascular health?” Patients were also asked to rate changes in fat distribution (more or less on a scale of 0–2) in their arms, neck, face, and abdomen at the beginning of the study as well as at the final visit. Lower scores indicate improvement over time.
STATISTICAL ANALYSIS
Demographic variables were compared by unpaired, 2-tailed t test for continuous variables and the χ2 test for noncontinuous variables. Treatment effects over time were compared between exercise and nonexercise study groups by analysis of covariance with the baseline value as a covariate. Use of HIV medication, including protease inhibitors, nucleoside reverse transcriptase inhibitors, and nonnucleoside reverse transcriptase inhibitors, was also tested for significance in the analyses of covariance and did not affect the results. The primary analysis was per protocol, but a confirmatory intent-to-treat analysis was performed carrying forward last available data on the 2 dropouts, with similar results (data not shown). Statistical analyses were performed with SAS JMP software (version 4.04; SAS Institute Inc, Cary, NC). All values are expressed as mean ± SEM unless otherwise indicated. With 40 patients, the study was powered at 85% to detect a treatment effect of 1.0 SD on clinical end points between the exercise and nonexercise groups. The study was powered to detect a difference of 3.4 mL · kg−1 · min−1 in V̇O2max between the groups. Six-minute walk data excluded 1 patient unable to perform the test adequately. The analysis of waist circumference excluded data from a single patient that did not fulfill the Dixon and Massey criteria.23
RESULTS
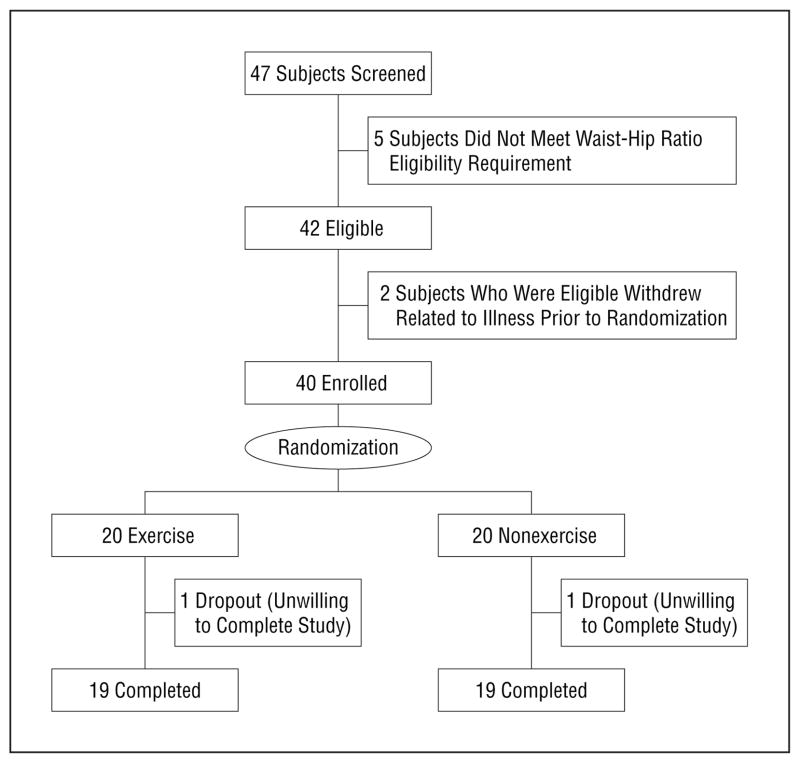
Flow of participants through the study is shown in the Figure. Table 1 presents the demographic characteristics of the study subjects. Randomization groups were similar in all demographic characteristics.
Figure.
Flow of participants through the study.
Table 1.
Baseline Demographic Characteristics
| Variable | Exercise Group (n = 20) | Control Group (n = 20) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SEM, y | 43 ± 2 | 40 ± 2 | .21 |
| Waist-hip ratio, mean ± SEM | 0.98 ± 0.02 | 0.94 ± 0.02 | .09 |
| Race | |||
| Ratio of white to nonwhite No. | 7:13 | 4:16 | .45 |
| African American | 10 | 12 | |
| Hispanic | 2 | 4 | |
| Other | 1 | 0 | |
| Current smoking, No. (%) | 7 (35) | 11 (55) | .20 |
| Eumenorrheic, No. (%) | 16 (80) | 13 (65) | .11 |
| Medication exposure | |||
| Duration of HIV, mean ± SEM, y | 11 ± 1 | 9 ± 1 | .29 |
| Current antiretrovirals, No. (%) | 17 (85) | 16 (80) | .68 |
| PI | 11 (55) | 10 (50) | .93 |
| NRTI | 17 (85) | 12 (60) | .07 |
| NNRTI | 6 (30) | 8 (40) | .73 |
| Antihypertensive agent, No. (%) | 3 (15) | 3 (15) | >.99 |
| Lipid-lowering agent, No. (%) | 2 (10) | 0 | .15 |
| Estrogen use, No. (%) | 2 (10) | 0 | .16 |
Abbreviations: HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The fitness characteristics of the study subjects are shown in Table 2. There was no difference at baseline in V̇O2max between the groups. Among the HIV-infected subjects, V̇O2max was markedly lower at baseline (median [95% confidence interval], 15.4 [8.3–25.2] mL · kg−1 · min−1) than reported values for healthy female subjects (26–35 mL · kg−1 · min−1).24 Other fitness and strength measures were similar between treatment groups, but endurance and knee flexor strength differed at baseline. Body composition, biochemical, immune, and dietary characteristics were similar between the study groups at baseline (Table 3).
Table 2.
Fitness and Strength Measures
| Variable | Exercise Group (Mean ± SEM)
|
Control Group (Mean ± SEM)
|
P Value for Baseline Comparison* | P Value for Treatment Effect† | ||
|---|---|---|---|---|---|---|
| Baseline (n = 20) | Change at 16 wk (n = 19) | Baseline (n = 20) | Change at 16 wk (n = 19) | |||
| Fitness measures | ||||||
| V̇O2max, mL · kg−1 · min−1 | 16.9 ± 1.0 | 1.5 ± 0.8 | 15.3 ± 1.1 | −2.5 ± 1.6 | .30 | <.001 |
| Submaximal bike exercise time, min | 6.1 ± 0.4 | 1.0 ± 0.3 | 5.0 ± 0.3 | −0.6 ± 0.3 | .02 | <.001 |
| 6-min walk distance, m | 489 ± 20 | 34 ± 11 | 474 ± 14 | −6 ± 15 | .53 | .009 |
| Strength measures, kg | ||||||
| Knee extensors | 22.1 ± 1.8 | 33.2 ± 4.4 | 29.5 ± 3.5 | 0.8 ± 1.5 | .07 | <.001 |
| Pectoralis | 19.1 ± 1.0 | 13.9 ± 1.2 | 18.0 ± 1.2 | 0.4 ± 0.7 | .47 | <.001 |
| Knee flexors | 9.6 ± 1.2 | 8.4 ± 1.0 | 6.0 ± 1.0 | −0.3 ± 0.5 | .03 | <.001 |
| Shoulder abductors | 3.7 ± 0.2 | 2.4 ± 0.3 | 3.3 ± 0.3 | 0.3 ± 0.1 | .30 | <.001 |
| Ankle plantar flexors | 22.4 ± 2.4 | 31.5 ± 4.0 | 22.8 ± 2.2 | 1.9 ± 1.2 | .89 | <.001 |
| Elbow flexors, right arm | 6.0 ± 0.2 | 3.5 ± 0.6 | 5.8 ± 0.5 | 0.5 ± 0.4 | .83 | <.001 |
| Elbow flexors, left arm | 5.9 ± 0.2 | 3.6 ± 0.6 | 5.2 ± 0.4 | 0.9 ± 0.3 | .14 | <.001 |
Abbreviation: V̇O2max, maximum oxygen consumption.
By unpaired, 2-tailed t test.
By analysis of covariance.
Table 3.
Biochemical, Body Composition, and Clinical Measures
| Variable | Exercise Group, Mean ± SEM
|
Control Group, Mean ± SEM
|
P Value for Baseline Comparison* | P Value for Treatment Effect† | ||
|---|---|---|---|---|---|---|
| Baseline (n = 20) | Change at 16 wk (n = 19) | Baseline (n = 20) | Change at 16 wk (n = 19) | |||
| Body composition | ||||||
| BMI | 29.3 ± 1.4 | 0.4 ± 0.2 | 28.6 ± 1.6 | −0.1 ± 0.4 | .71 | .34 |
| Waist circumference, cm | 103.8 ± 3.3 | −1.0 ± 0.6 | 98.5 ± 15.4 | 1.5 ± 1.0 | .27 | .03 |
| Total muscle area, cm2 | 134 ± 5 | 6 ± 1 | 123 ± 7 | 2 ± 1 | .23 | .02 |
| Total muscle attenuation, HU | 44 ± 2 | 2 ± 1 | 46 ± 2 | −1 ± 1 | .44 | .03 |
| Total fat, kg | 28.7 ± 2.5 | −0.2 ± 0.4 | 27.8 ± 2.7 | −0.7 ± 0.7 | .81 | .49 |
| SAT, cm2 | 354 ± 33 | 3 ± 8 | 314 ± 37 | −14 ± 13 | .43 | .16 |
| VAT, cm2 | 142 ± 18 | −2 ± 7 | 111 ± 13 | 8 ± 7 | .16 | .54 |
| Biochemical indexes, mg/dL | ||||||
| Total cholesterol | 191 ± 9 | −2 ± 6 | 162 ± 10 | 0 ± 6 | .04 | .69 |
| Triglycerides | 141 ± 17 | 6 ± 13 | 107 ± 10 | 0 ± 8 | .09 | .65 |
| LDL cholesterol | 112 ± 6 | −3 ± 5 | 94 ± 9 | 2 ± 5 | .11 | .90 |
| HDL cholesterol | 52 ± 4 | −1 ± 2 | 47 ± 3 | −3 ± 2 | .38 | .19 |
| Fasting glucose | 85 ± 2 | −1 ± 2 | 87 ± 2 | 1 ± 2 | .32 | .12 |
| 2-h glucose | 128 ± 9 | 3 ± 7 | 123 ± 8 | −9 ± 4 | .67 | .14 |
| Clinical measures | ||||||
| SBP, mm Hg | 118 ± 4 | 1 ± 4 | 122 ± 3 | 3 ± 4 | .43 | .44 |
| DBP, mm Hg | 76 ± 2 | −3 ± 2 | 78 ± 3 | −2 ± 2 | .46 | .21 |
| Immune function | ||||||
| Log HIV viral load, copies/μL | 2.3 ± 0.2 | 0.1 ± 0.1 | 3.0 ± 0.3 | −0.2 ± 0.2 | .06 | .28 |
| CD4 count, cells/μL | 539 ± 78 | 8 ± 37 | 419 ± 63 | 11 ± 38 | .24 | .94 |
| Dietary measures‡ | ||||||
| Total caloric intake, kcal/d | 1708 ± 109 | −17 ± 133 | 1547 ± 130 | −170 ± 115 | .35 | .28 |
| Total fat intake, g/d | 65.6 ± 5.1 | 1.7 ± 6.1 | 57.0 ± 7.0 | 0.5 ± 6.3 | .33 | .68 |
| Total carbohydrate intake, g/d | 216.6 ± 16.1 | −14.0 ± 17.8 | 199.1 ± 15.9 | −41.6 ± 18.6 | .44 | .21 |
| Total protein intake, g/d | 66.6 ± 4.2 | 4.7 ± 7.4 | 65.0 ± 5.6 | −5.3 ± 5.1 | .82 | .32 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); DBP, diastolic blood pressure; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HU, Hounsfield units; LDL, low-density lipoprotein; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; VAT, visceral adipose tissue.
SI conversion factors: To convert total, LDL, and HDL cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; triglyceride to millimoles per liter, multiply by 0.0113.
By unpaired, 2-tailed t test.
By analysis of covariance.
For dietary measures, change was determined in 14 patients in the exercise group and 14 in the control group.
FITNESS AND STRENGTH
At 16 weeks, subjects randomized to the home-based exercise regimen had significant improvement in aerobic capacity by V̇O2max relative to the control group (1.5±0.8 vs −2.5±1.6 mL · kg−1 · min−1; P<.001) and endurance–exercise time measured by the submaximal bicycle test (1.0±0.3 vs −0.6±0.3 minute; P<.001). Strength measured by change in 1 RM increased significantly at the knee extensors, pectoralis, knee flexors, shoulder abductors, ankle plantar flexors, elbow flexors (right), and elbow flexors (left) (Table 2). Distance on the 6-minute walk test increased to a significantly greater degree among patients assigned to exercise (34 ± 11 vs −6 ± 15 m; P=.009). Subjects randomized to the exercise regimen completed 96% of sessions.
BODY COMPOSITION
Total muscle area (6±1 vs 2±1 cm2; P=.02) and total muscle attenuation (2±1 vs −1±1 Hounsfield unit; P=.03) increased compared with the control group. Body mass index, abdominal fat, and total fat did not change between the groups, but waist circumference decreased more in the exercise-treated group than in the control group (−1.0±0.6 vs 1.5±1.0 cm; P=.03) (Table 3).
BIOCHEMICAL INDEXES, IMMUNE FUNCTION, AND BLOOD PRESSURE
No significant difference was seen in lipid levels, glucose, CD4 count, viral load, or blood pressure.
PATIENT SELF-ASSESSMENT
In response to the exit question, “In the past 4 months, have you noticed any change in your cardiovascular health?” 89% of the exercise group answered yes vs 16% of the control group (P<.001). Of the patients in the exercise group answering affirmatively, 76% reported increased energy, 35% reported increased strength, and 41% reported increased endurance. The total score for self-assessment of fat distribution improved in the exercise group (−1.4±0.3 vs 0.3±0.1; P<.001). The most significant effect seen was in the self-reported appearance of the abdomen (−0.8±0.2 vs 0.0±0.0; P<.001). A significant effect was also reported for the extremities (−0.7±0.2 vs 0.2±0.1; P<.001).
DIETARY INTAKE
Dietary intake of total calories, fat, carbohydrate, and protein did not differ over time between the treatment groups.
ADVERSE EVENTS
The exercise program was well tolerated. Few adverse events occurred, and none was related to the exercise protocol or study participation. One patient in the exercise protocol had exacerbation of asthma related to the development of bronchitis. One patient in the nonexercise group experienced chest pain at the baseline visit, but myocardial infarction and ischemia were ruled out. No changes in menstrual function were seen.
COMMENT
In this study, we investigated the effects of a home-based fitness program on HIV-infected women who reported changes in fat distribution and had an increased waist-hip ratio. We hypothesized that this group would be at increased risk for cardiovascular disease and would benefit from an exercise program. Previous studies of exercise training among HIV-infected patients are disproportionate with respect to sex6,9,12,25 and are not exclusively home-based. The great majority of subjects were receiving antiretroviral therapy, and antiretroviral treatment did not differ significantly between the groups. The study population was racially diverse and relatively young, reflecting the demographics of the growing population of HIV-infected women in the United States.26
Estimates of V̇O2max using submaximal exercise test data have been used as a surrogate measure of cardiorespiratory fitness and aerobic capacity.27 Among women asymptomatic for heart disease, exercise capacity less than 85% of age-predicted value was associated with a markedly increased hazard ratio for death from cardiac causes.28 Using the nomogram of Gulati et al,28 subjects in our study exercised at 50% of predicted exercise capacity for age, and thus would be expected to have increased cardiovascular disease on this basis. Decreased peak oxygen uptake has been observed in HIV-infected adults during treadmill exercise,29 and previous studies have demonstrated positive effects of aerobic exercise on V̇O2max among those with HIV.6,12,30 Our baseline data demonstrate that women with HIV are dramatically deconditioned with respect to cardiorespiratory fitness compared with HIV-negative women of equal age.24,31 Increased smoking rates, commonly reported in HIV-infected women, may have contributed to this observation, but V̇O2max did not differ by smoking status or pack-year history (data not shown).
Women who were randomized to exercise had improvement in V̇O2max after 16 weeks of aerobic training relative to the control group. The increase in V̇O2max within the exercise group was a relatively modest 10%. In contrast, the nonexercise group showed further decline in cardiorespiratory fitness over the duration of the study, which may have contributed to the observed differences with the treatment group. The mechanism of further decline in V̇O2max in the control group is not clear and does not relate to antiretroviral use or smoking status. To determine V̇O2max, we used a standardized sub-maximal cycle ergometry regimen.15 We did not measure oxygen consumption directly, but instead used a prediction equation not previously validated in a population with HIV infection, which may be a limitation of the study.
Our study used a combination of aerobic and progressive resistance training. Previous studies of resistance training alone10 or resistance training combined with aerobic exercise8,11,32 demonstrated increased strength and increased muscle mass among HIV-infected men and women. Our data show a significant improvement in muscle size and quality measured by total muscle area and attenuation among those who received exercise. Improvement in muscle attenuation indicates a significant decrease in muscle adiposity, which may help to improve metabolic indexes over the long-term in HIV-infected patients. A significant improvement in muscular strength among all measured muscle groups was also seen. In contrast, although subcutaneous fat decreased and visceral fat increased in the control group and opposite effects were seen among the group receiving exercise, these changes were not statistically significant. The data suggest that a rigorous exercise program does not further decrease subcutaneous fat in this population. In contrast, waist circumference improved in the exercise group relative to control group, potentially explaining the observation of a significant improvement in appearance reported by the patients assigned to exercise. Such changes may make patients more compliant with anti-retroviral medications associated with negative effects on body image.
Improvement in aerobic endurance as measured by exercise time has been previously reported in a study30 of 19 subjects with HIV randomized to aerobic exercise and a small study32 of 6 HIV-infected subjects completing an aerobic and resistance training regimen. Among our cohort, improvement in endurance was seen among those who were randomized to the exercise component. In addition, a significant effect was seen in the 6-minute walk test, a measure of functional status,33 among patients in the exercise group. Patients assigned to exercise also reported a subjective improvement in energy and endurance on self-assessment. We also performed modeling for all end points including duration of protease inhibitor, nucleoside reverse transcriptase inhibitor, and non-nucleoside reverse transcriptase inhibitor use, without significant effects on the results (data not shown).
Women with HIV infection have identified barriers to appointment adherence and research participation, including lack of childcare and/or transportation.34,35 The use of a home-based exercise regimen was selected to help curtail these barriers and to improve adherence. In addition, the use of a dedicated physical therapist provided a consistent and supportive exercise environment and contributed to the high (96%) compliance rate in the study. The patient population studied is representative of the general population of HIV-infected women, who are often burdened by socioeconomic challenges. We were able to show that this population has a significant reduction in cardiorespiratory fitness but can be motivated to perform a standardized fitness program. In this study, we achieved significant improvements in cardiorespiratory fitness with relatively limited resources and equipment (2 trainers covered all of the patients assigned to exercise, and the total cost of the exercise equipment provided by the study investigators was approximately $400 per patient). Therefore, this program might be considered cost-effective and associated with little risk compared with medication strategies, which do not improve fitness.
Significant effects on traditional markers of cardiovascular risk, including lipid and clinical measures, were not seen. The absence of an effect on lipid measures may relate to the pathophysiology of lipid abnormalities in HIV-infected patients36 or an ongoing effect of antiretroviral treatment that may limit the known effects of exercise training on lipid metabolism in this population. As demonstrated in the study by Katzmarzyk et al,37 changes in lipid levels may best reflect fat loss, not improvement in fitness in response to exercise. No effect on glucose level was seen, in agreement with some previous studies of exercise in this population.38 The lack of effects on lipid levels and glucose may also have been due to small sample size and degree and limited aerobic stimulus. No effects on dietary intake were seen. Immune function remained stable and did not change between the groups.
In summary, our data demonstrate the positive effects of a 16-week supervised home-based exercise regimen on measures of physical fitness among women with HIV. The most significant and robust results from this study relate to the effects of resistance training on strength, but positive effects of the combined resistance aerobic program on other measures, including cardiorespiratory fitness, body composition (muscle area and attenuation, and waist circumference), and endurance, were also seen.
Acknowledgments
Funding/Support: This study was supported in part by grants R01 DK-49302 and RR-01066 from the National Institutes of Health.
We would like to acknowledge the nursing and bionutrition staff of the Massachusetts General Hospital General Clinical Research Center and the Massachusetts Institute of Technology Clinical Research Center, the Massachusetts General Hospital Physical Therapy Department, and Partners Home Health Care, Boston, for their contributions to the study.
Footnotes
Financial Disclosure: None.
References
- 1.Grinspoon S, Carr A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 3.Hadigan C, Meigs JB, Wilson PW, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–916. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 4.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.MacArthur RD, Levine SD, Birk TJ. Supervised exercise training improves cardiopulmonary fitness in HIV infected persons. Med Sci Sports Exerc. 1993;25:684–688. [PubMed] [Google Scholar]
- 7.Roubenoff R, Skolnik PR, Shevitz A, et al. Effect of a single bout of acute exercise on plasma human immunodeficiency virus RNA levels. J Appl Physiol. 1999;86:1197–1201. doi: 10.1152/jappl.1999.86.4.1197. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R, Weiss L, McDermott A, et al. A pilot study of exercise training to reduce trunk fat in adults with HIV-associated fat redistribution. AIDS. 1999;13:1373–1375. doi: 10.1097/00002030-199907300-00015. [DOI] [PubMed] [Google Scholar]
- 9.Roubenoff R, Wilson IB. Effect of resistance training on self-reported physical functioning in HIV infection. Med Sci Sports Exerc. 2001;33:1811–1817. doi: 10.1097/00005768-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Roubenoff R, McDermott A, Weiss L, et al. Short-term progressive resistance training increases strength and lean body mass in adults infected with human immunodeficiency virus. AIDS. 1999;13:231–239. doi: 10.1097/00002030-199902040-00011. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV infected patients. AIDS. 2004;18:465–473. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 12.Stringer WW, Berezovska M, O’Brien WA, Beck CK, Casaburi R. The effect of exercise training on aerobic fitness, immune indices and quality of life in HIV + patients. Med Sci Sports Exerc. 1998;30:11–16. doi: 10.1097/00005768-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 13.American College of Sports Medicine. American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. 5. Baltimore, Md: Williams & Wilkins; 1995. [Google Scholar]
- 14.American College of Sports Medicine. American College of Sports Medicine’s Exercise Management for Person With Chronic Diseases and Disabilities. Champaign, Ill: Human Kinetics; 1997. [Google Scholar]
- 15.Stanforth PR, Ruthven MD, Gagnon J, et al. Accuracy of prediction equations to estimate submaximal VO2 during cycle ergometry: the HERITAGE Family Study. Med Sci Sports Exerc. 1999;31:183–188. doi: 10.1097/00005768-199901000-00028. [DOI] [PubMed] [Google Scholar]
- 16.Fairfield WP, Treat M, Rosenthal DI, et al. Effects of testosterone and exercise on muscle leanness in eugonadal men with AIDS wasting. J Appl Physiol. 2001;90:2166–2171. doi: 10.1152/jappl.2001.90.6.2166. [DOI] [PubMed] [Google Scholar]
- 17.Driscoll SD, Meininger GE, Ljunquist K, et al. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus–infected patients. J Clin Endocrinol Metab. 2004;89:2171–2178. doi: 10.1210/jc.2003-031858. [DOI] [PubMed] [Google Scholar]
- 18.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 19.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 20.Hartig DE, Henderson JM. Increasing hamstring flexibility decreases lower extremity overuse injuries in military basic trainees. Am J Sports Med. 1999;27:173–176. doi: 10.1177/03635465990270021001. [DOI] [PubMed] [Google Scholar]
- 21.De Lorme TL, Watkins AL. Techniques of progressive resistance exercise. Arch Phys Med. 1948;29:263–273. [PubMed] [Google Scholar]
- 22.LaStayo PC, Pierotti DJ, Pifer J, Hoppeler H, Lindstedt SL. Eccentric ergometry: increases in locomotor muscle size and strength at low training intensities. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1282–R1288. doi: 10.1152/ajpregu.2000.278.5.R1282. [DOI] [PubMed] [Google Scholar]
- 23.Dixon WJ, Massey FJ. Introduction to Statistical Analysis. 2. New York, NY: McGraw-Hill Book Co; 1957. [Google Scholar]
- 24.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11. [PubMed] [Google Scholar]
- 25.Strawford A, Barbieri T, Van Loan M, et al. Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV-related weight loss. JAMA. 1999;281:1282–1290. doi: 10.1001/jama.281.14.1282. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2003. Atlanta, Ga: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2004. p. 15. [Google Scholar]
- 27.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 28.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 29.Cade WT, Fantry LE, Nabar SR, Shaw DK, Keyser RE. Impaired oxygen on-kinetics in persons with human immunodeficiency virus are not due to highly active antiretroviral therapy. Arch Phys Med Rehabil. 2003;84:1831–1838. doi: 10.1016/j.apmr.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Smith BA, Neidig JL, Nickel JT, Mitchell GL, Para MF, Fass RJ. Aerobic exercise: effects on parameters related to fatigue, dyspnea, weight and body composition in HIV-infected adults. AIDS. 2001;15:693–701. doi: 10.1097/00002030-200104130-00004. [DOI] [PubMed] [Google Scholar]
- 31.Wilmore JH, Costill DL. Physiology of Sport and Exercise. 2. Champaign, Ill: Human Kinetics; 1999. [Google Scholar]
- 32.Jones SP, Doran DA, Leatt PB, Maher B, Pirmohamed M. Short-term exercise training improves body composition and hyperlipidaemia in HIV-positive individuals with lipodystrophy. AIDS. 2001;15:2049–2051. doi: 10.1097/00002030-200110190-00021. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Thompson PJ, Berman LB, et al. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38:517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 34.Meredith K, Delaney J, Horgan E, Fisher JR, Fraser V. A survey of women with HIV about their expectations for care. AIDS Care. 1997;9:513–522. doi: 10.1080/713613195. [DOI] [PubMed] [Google Scholar]
- 35.Kelly PJ, Cordell JR. Recruitment of women into research studies: a nursing perspective. Clin Nurse Spec. 1996;10:25–28. doi: 10.1097/00002800-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 37.Katzmarzyk PT, Leon AS, Rankinen T, et al. Changes in blood lipids consequent to aerobic exercise training related to changes in body fatness and aerobic fitness. Metabolism. 2001;50:841–848. doi: 10.1053/meta.2001.24190. [DOI] [PubMed] [Google Scholar]
- 38.Thoni GJ, Fedou C, Brun JF, et al. Reduction of fat accumulation and lipid disorders by individualized light aerobic training in human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidemia. Diabetes Metab. 2002;28:397–404. [PubMed] [Google Scholar]