Abstract
Variation in disease severity among E. coli O157:H7 infections may result from differential expression of Shiga toxin 2 (Stx2). Eleven strains belonging to four prominent phylogenetic clades, including clade 8 strains representative of the 2006 U.S. spinach outbreak, were examined for stx2 expression by real-time PCR and western blot analysis. Clade 8 strains were shown to overexpress stx2 basally, and following induction with ciprofloxacin when compared to strains from clades 1-3. Differences in stx2 expression generally correlated with Stx2 protein levels. Single-nucleotide polymorphisms identified in regions upstream of stx2AB in clade 8 strains were largely absent in non-clade 8 strains. This study concludes that stx2 overexpression is common to strains from clade 8 associated with hemolytic uremic syndrome, and describes SNPs which may affect stx2 expression and which could be useful in the genetic differentiation of highly-virulent strains.
Keywords: Shiga toxin, E. coli O157:H7, Clade 8, Stx2, hemolytic uremic syndrome
1. Introduction
Escherichia coli O157:H7 is an enteric human pathogen which produces a cytotoxin (Shiga toxin) responsible for the life-threatening illness hemolytic uremic syndrome (HUS). Three species of Shiga toxin (Stx1, Stx2 and Stx2c) have been described in association with human disease, each of which is encoded on a distinct lambdoid bacteriophage that forms stable lysogens in O157:H7 [1-4]. Production of Stx is linked to induction of the lytic cycle of Stx phage replication, which occurs spontaneously and in response to stressors [5-10].
Variation in disease severity has been reported among O157:H7 phylogenetic lineages (clades) distinguished by single nucleotide polymorphism (SNP) genotyping [11], however, the factors which contribute to this variation are unknown. There is evidence that differential stx2 expression may account for differences in virulence. For example, studies have shown the presence of stx2 to be correlated with a higher frequency of HUS [12-14], and that lysogeny with multiple Stx-phage results in less Stx2 production and reduced rates of HUS relative to strains lysogenized with Stx2-phage alone [15], [16, 17],[18]. In addition, polymorphisms and rearrangements in Stx2-phage DNA have been observed in O157:H7 strains with reduced levels of Stx2 [19-22].
Recently, strains belonging to the clade 8 lineage of O157:H7 have been reported to be associated with HUS [11], cause severe disease in mice [23], and express stx2 at increased levels following exposure to epithelial cells [24, 25] relative to strains from other clades. This study quantified transcript and protein levels for stx2 among eleven O157:H7 strains representing four prominent clades, and lysogenized with various Stx-phages.
2. Results and discussion
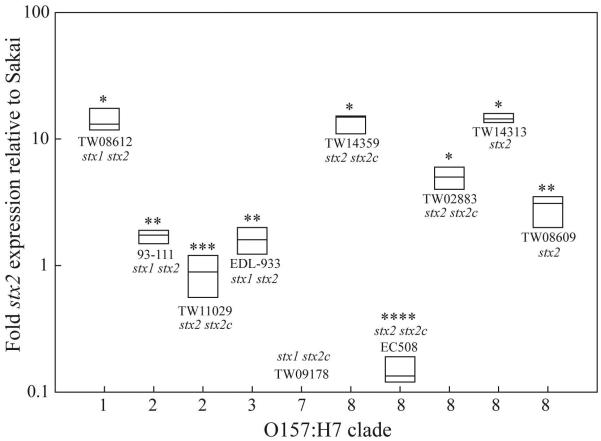
Among the eleven O157:H7 strains, five demonstrated a 2.9- to 14.6-fold increase in basal stx2 transcript levels relative to Sakai by quantitative real-time PCR (qRT-PCR), four of which belonged to clade 8 (Fig. 1). Three of four strains from clades 1-3 expressed only a 0.88- to 1.7-fold increase in stx2 relative to Sakai. Levels of stx2 were significantly higher in clade 8 strains TW14359, TW14313 and TW02883, as well as in clade 1 strain TW08612, than all other strains (P<0.05). Clade 8 strain EC508 was observed, however, to significantly underexpress stx2 relative to all other strains (P<0.05). These results reveal that although increased stx2 expression is characteristic of clade 8 strains, it is not exclusive to this clade, and not all strains within clade 8 express stx2.
FIG. 1. Basal stx2 transcript levels.
Boxplots of mean fold-change in stx2 expression relative to strain Sakai for 10 strains representing 5 O157:H7 clades as determined by qRT-PCR. Plot boundaries represent the 25th and 75th percentiles; the median is given by the line. Plots which differ in the number of asterisks, differ significantly by Tukey’s HSD following a significant F-test (P<0.05). Strain names and stx complement are provided in proximity to each respective plot. As expected, no stx2 transcript was detectable for stx2-stx2c+ control strain TW09178.
Lysogeny with multiple Stx-phages has been observed to both increase and decrease Stx2 production [18, 26]. In the present study however, there was no apparent correlation between the complement of Stx-phage and actual stx2 expression. Although the reason for this difference in results is unknown, the former studies measured the influence of multiple lysogeny on Stx2 production among EHEC O157:NM strains [26], or in Stx2 lysogens of E. coli K-12 [18], which may produce substantially different results when compared to clinical O157:H7 strains. Furthermore, these studies did not measure Stx2 production in strains bearing Stx2-phage in combination with Stx1- or Stx2c-phage.
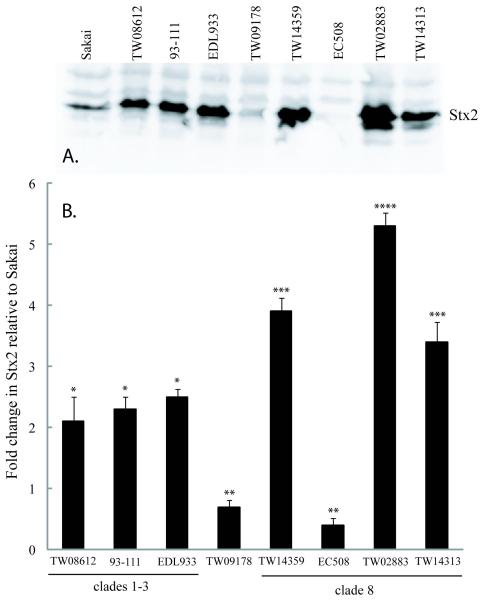
Differences in basal stx2 expression were modestly correlated (r2=0.70) to actual Stx2 levels by western blots for proteins extracted from OD600=0.65 cultures (Fig. 2). For example, in strains TW08612, TW14359, and TW14313, which were determined to express stx2 at 14.1-, 13.7-, and 14.6-fold higher than Sakai, corresponding Stx2 protein levels were 2.1-, 3.9-, and 3.4-fold higher, respectively (Figs. 1 and 2). The reason for this difference in fold-change is unknown, but is consistent with studies reporting increased stx2 expression without an equivalent increase in Stx2 product [9], and disparities between mRNA and protein levels in E. coli [27]. Importantly, western blot analysis statistically confirmed stx2 expression levels by qRT-PCR for 7/8 strains analyzed relative to Sakai, including the overexpression of stx2 in clade 8 strains TW14359, TW02883 and TW14313 (P<0.05). This also included the underexpression of stx2 in clade 8 strain EC508. This strain originates from a patient with HUS in 1984 (N. Carolina, U.S.), and has been shown to be cytotoxic for HeLa cells [28]. Perhaps significantly, EC508 is a stx2+ stx2c+ strain (Table 1), and the stx2c product is also cytotoxic for HeLa cells [29]. Finally, strains bearing stx2c only have also been determined to cause HUS [12]. Only limited cross-reactivity was observed for strain TW09178 (stx2-stx2c+) using anti-Stx2 mAbs.
FIG. 2. Basal Stx2 protein levels.
(A): Representative western blot measuring Stx2 levels among 9 strains encompassing 5 O157:H7 clades using anti-Stx2 mAbs and visualized with enhanced chemioluminescence. The location of Stx2 is indicated (inset right); (B): Graphical representation of fold-change in Stx2 levels relative to strain Sakai for strains in panel A. Bars represent mean Stx2 levels as determined using Image J. Error bars indicate standard deviation (N=3). Bars which differ in the number of asterisks, differ significantly by Tukey’s HSD following a significant F-test (P<0.05). Strain TW09178 belongs to clade 7, and is included as a stx2-stx2c+ control for cross reactivity of anti-Stx2 mAb with Stx2c.
Table 1. List of O157:H7 strains used in this study.
| Straina | Alias | O157:H7 Cladeb |
SGb | Shiga toxin Complement |
Clinical presentation, origin and reference |
|---|---|---|---|---|---|
| TW08264 | Sakai | 1 | 1 | stx1, stx2 | Unknown, 1996 outbreak, Sakai, Japan [40] |
| TW08612 | EK4 | 1 | 1 | stx1, stx2 | HUSc, 2001, WA, USA [41] |
| TW04863 | 93-111 | 2 | 9 | stx1, stx2 | Diarrhea, 1993 outbreak, northwest USA [42] |
| TW11029 | 2 | NDd | stx2 | HCe, 2002, MI, USA | |
| TW02302 | EDL933 | 3 | 12 | stx1, stx2 | HC, 1982 outbreak, MI and OR, USA [43] |
| TW09178 | MI03-9 | 7 | 29 | stx1, stx2c | |
| TW14359 | MI06-63 | 8 | 30 | stx2, stx2c | HC, 2006 outbreak, western USA [11] |
| TW00885 | EC508 | 8 | 31 | stx2, stx2c | HUS, 1984, NC, USA [28] |
| TW02883 | E32511 | 8 | 31 | stx2, stx2c | HUS, CDC [29, 44] |
| TW14313 | MI06-31 | 8 | 33 | stx2 | HUS, 2006, MI, USA |
| TW08609 | EK1 | 8 | 33 | stx2 | Diarrhea, 1999, WA, USA [41] |
All strains were acquired from the STEC Center at Michigan State University.
Based on the work of Manning et al. [11]. SG is single nucleotide polymorphism (SNP) genotype.
Hemolytic uremic syndrome (HUS).
Not determined (ND).
Hemorrhagic colitis (HC).
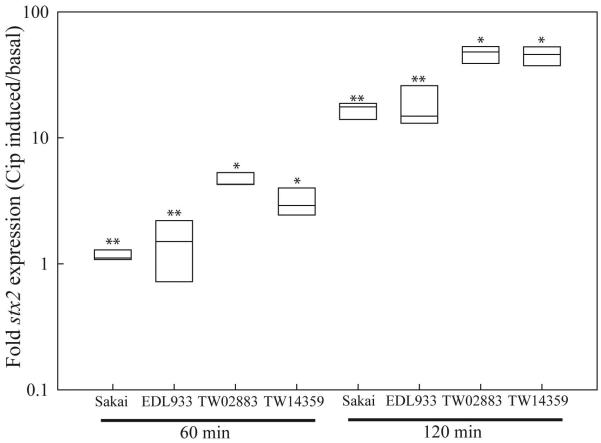
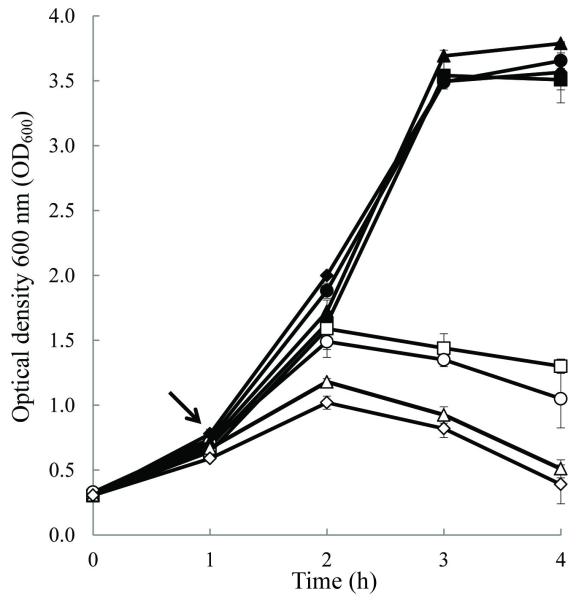
Differences in stx2 induction following 60- and 120-min exposure to ciprofloxacin (Cip) were observed for a subset of strains shown in this study to differentially express stx2 at the basal level. Following 60-min, Cip was observed to induce stx2 in clade 8 strains TW02883 and TW14359, but not in Sakai (clade 1) or EDL933 (clade 3) (Fig. 3) (P<0.05). This is an interesting observation in light of evidence that the EDL933 Stx2-phage (933W) is sensitive to lytic induction [30]. After 120-min, stx2 expression increased substantially for all strains, however the fold increase in stx2 expression remained significantly higher in clade 8 strains relative to both Sakai and EDL933 (Fig. 3) (P<0.05). Moreover, the addition of Cip to cultures at OD600=0.65 reduced growth earlier, and in a more dramatic manner for TW02883 and TW14359 when compared to Sakai and EDL933 (Fig. 4). Following 18 h of growth, cell pellets were obtained from Sakai and EDL933 by centrifugation (10,000 × g, 2-min), but could not be obtained for clade 8 strains (data not shown). Although strictly correlative, this observation suggests an increased lytic activity in clade 8 strains relative to Sakai and EDL933 when exposed to Cip. Collectively, these results reveal that an inherent difference exists among clade 8 strains in a common regulatory pathway of basal (spontaneous) and inducible stx2 expression.
FIG. 3. Ciprofloxacin-induced stx2 transcript levels.
Boxplots of mean fold-change in stx2 expression following 60- and 120-min exposure to ciprofloxacin (Cip) relative to basal expression levels as determined by qRT-PCR. Plot boundaries represent the 25th and 75th percentiles; the median is given by the line. Plots which differ in the number of asterisks at each time of exposure, differ significantly by Tukey’s HSD following a significant F-test (P<0.05). Clade designations are: Sakai (clade 1), EDL933 (clade 3), TW02883 (clade 8), and TW14359 (clade 8).
FIG. 4. Bacteriophage induction during growth with ciprofloxacin.
Plot of optical density at 600 nm (OD600) as a function of time for uninduced control (filled plots) and ½X MIC ciprofloxacin induced (empty plots) cultures. Arrow indicates point of ciprofloxacin addition. Strains included Sakai (squares), EDL933 (circles), TW02883 (diamonds), and TW14359 (triangles). Measurements represent the mean (N=3) OD600 and error bars indicate standard deviation.
Support for this hypothesis comes from the observation that stx2 expression can be influenced by alterations in upstream regulatory regions [20, 22, 31]. To explore this possibility using the strain set in this study, a DNA region containing stx2P, the phage late promoter pR’, the Q utilization site (qut), and q were examined for each strain by PCR and sequencing, and through analysis of DNA sequences available through GenBank (NCBI). PCR products were obtained for all test strains except TW11029, a stx2+ strain that expressed low stx2 levels (Table 1, Fig. 1), despite repeated attempts using different primers, suggesting that sequence variation in the priming sites is preventing amplification. Sequencing revealed a number of SNPs in clade 8 strains which were largely absent in non-clade 8 strains (Supplemental Fig. 1). Ten SNPs were identified within the q gene of clade 8 strains TW14313 and TW08609, which were also present in strains EC508 (clade 8) and TW14588 (clade 2). Two of the 10 SNPs in q result in non-synonymous mutations: R10→C and Y19→H. In addition, 23 SNPs were identified between q and the start codon of stx2A in TW14313 and TW08609, which were also present in EC508. Eight of these SNPs were within gene ECs1204, encoding a hypothetical protein, and five of these conferred non-synonymous mutations: A20→S, S32→T, G33→E, N44→D, and V45→L. These 33 SNPs were therefore present in strains TW08609 and TW14313 which overexpressed stx2, but also in EC508, in which stx2 expression was barely detectable (Figs. 1 and 2). Furthermore, the q-stx2 region of TW08612 (clade 1), which overexpressed stx2 transcript but not Stx2 product, was identical to 93-111 and EC4501 (clade 2), Sakai (clade 1), and EDL933 (clade 3), together suggesting that these SNPs may partly contribute to differential expression, but are not solely responsible. Two SNPs (one in argN2) and a 2-bp deletion were also identified exclusively in clade 8 strains TW02883, TW14359, EC4042, EC4045, EC4076, EC4113, EC4115, EC4196, and EC4206. In concordance with previous studies [24, 32], no difference was identified in the pR’, qut, or stx2P sequences. As toxin production is intimately linked to Stx2-phage lytic development [33], it is likely that other determinants of phage replication, not investigated in this study, also contribute to differential stx2 expression.
To conclude, this study reveals elevated basal and inducible stx2 transcript and protein levels among O157:H7 clade 8 strains relative to strains of clades 1-3, and describes genetic polymorphisms upstream of stx2 in regions which may be important for stx2 expression. The results are concordant with studies that show stx2 upregulation in clade 8 strains exposed to MAC-T bovine epithelial cells in DMEM [24, 25], and suggests that Stx2 production is increased in these strains under a variety of in vitro growth conditions. Furthermore, the findings of this research support the hypothesis that differences in disease severity observed between O157:H7 clades can be partly explained by differential Stx2 production. Clade 8 strains have also been observed to overexpress genes of the locus of enterocyte effacement (LEE), a 35.6-kb pathogenicity island encoding structural and regulatory proteins of a type III secretion apparatus [24, 25]. Thus the virulence of clade 8 strains likely reflects the upregulation of several discrete virulence systems. Further research is required to understand the genetic basis and biological significance of differential stx2 expression.
3. Materials and methods
3.1 Quantitative real-time PCR (qRT-PCR) analysis of stx2 expression
Strains (Table 1) were grown in MOPS media [34] with 4 g/l glucose as described [35]. For basal stx2 expression, cultures were grown to OD600=0.65 before RNA extraction (RNeasy™ kit, Qiagen, Valencia, CA) and cDNA synthesis (iScript, Bio-Rad, Carlsbad, CA). For inducible stx2 expression, cultures were grown to OD600=0.4 before induction with ½X MIC ciprofloxacin (Cip, 0.025 μg/ml) for 60-min and 120-min before RNA extraction. Quantitative real-time PCR (qRT-PCR) was performed using primers for rrsH (16S rRNA gene) to normalize cDNA levels, and primers Stx2b-6/Stx2b-120 for stx2B as described [36]. Reaction conditions included 40 cycles of denaturing at 94°C for 15-sec, followed by annealing at 55°C for 20-sec.
3.2 Growth experiments
MOPS cultures (N=3) were grown to OD600=0.65 before the addition of ½X MIC ciprofloxacin (0.025 μg/ml) or an equal volume of water (controls), and OD was used to measure growth for 4 h at 1 h intervals.
3.3 Western blots and immunodetection of Shiga toxin 2
Strains were grown in MOPS as described for qRT-PCR (above) to OD600=0.65, and following the method of Uzzau et al. [37], protein was extracted and resolved by 12% SDS-PAGE, and then transferred to PVDF membranes (Sigma). Mouse anti-Stx2 mAb (Santa Cruz Inc., Santa Cruz, CA), diluted to 1:3000 (v/v) were added to probe these membranes for Stx2, followed by a 1:2000 (v/v) dilution of HRP-conjugated goat anti-mouse IgG (Santa Cruz). Detection was performed using ECL Plus (Amersham Pharmacia, Piscataway, NJ). Relative quantitation of blots representative of three independent experiments was performed using Image J [38].
3.3 DNA sequencing and sequence analysis
For sequencing, purified DNA (Puregene® kits, Gentra, Minneapolis, MN) was used for PCR with primers Q-54 (ACGCTATCGTCAACGGTGTT)/Q+741 (CATTGCTGCTTTGACGCTAC) to amplify a 754-bp product (positions 1265693-1266486 in Sakai, GenBank BA000007) using LA Taq polymerase (TaKaRa Inc, Shiga, Japan). Primers Stx2-569 (CGAAGTTTGCGTAACAGCAT)/Stx2+68 (CGGGAATAGGATACCGAAGAA) were used to amplify a 636-bp product (positions 1266396-1267032). PCR products were purified (QIAquick, Qiagen) and sequencing was performed in duplicate using Q-54 and Stx2+68 primers (Eurofins MWG Operon, Huntsville, AL). DNA sequence data was concatenated into a 1272-bp read representing the q-stx2 region and aligned in MEGA [39]. Because this q-stx2 region shares only 33% nucleotide identity with the q-stx2c region in Stx2c-phage 2851 (GenBank AJ605767) [4], no PCR products were generated from the stx2-stx2c+ control strain TW09178.
Supplementary Material
Supplemental FIG. 1. Linear map of sequence polymorphisms in the q-stx2 region of E. coli O157:H7. Map of the Sakai reference sequence (top sequence) corresponding to nucleotide positions 1265693-1267032 (GenBank BA000007) with genes (q, ECs1204, stx2A) and tRNAs (ile72, argN2, and argO2) indicated by boxed arrows. Positions of non-coding regulatory sequences (pR’, qut, and stx2P) are shown and annealing sites for PCR and sequencing primers are indicated by arrows. All positions are approximate and not to scale. Numbers on the Sakai reference sequence show the distance in nucleotides relative to the translational start (+1) for stx2A. Nucleotides which differ from the Sakai reference sequence are presented on separate maps aligned and positioned below the reference sequence. Nucleotide polymorphisms are indicated atop each map, and their position relative to the translational start for stx2A is indicated below each map sequentially. Regions of identity with the reference sequence are unmarked. Underlined nucleotides indicate a non-synonymous mutation in the ORF of the respective gene in which they are found. The hatched box indicates a deletion. O157:H7 strains which share identical q-stx2 regions are grouped and listed to the right of each map. For strains with asterisks, the q-stx2 region was sequenced in this study; all other sequence data was acquired through GenBank. Strain TW14588 contains two distinct q-stx2 regions indicated by (¥) (positions 991647-992918) and (Φ) (positions 505342-506613) GenBank ABKY00000000.
Acknowledgements
We thank Cesar Taborta for assistance with DNA sequencing, and acknowledge that a significant portion of this research was supported by funds awarded to the late Thomas S. Whittam (Michigan State University) from NIAID under NIH research contract N01-AI-30058. This manuscript is dedicated to his memory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report that there are no conflicts of interest.
REFERENCES
- 1.Huang A, et al. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986;166(2):375–9. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newland JW, et al. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985;230(4722):179–81. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien AD, et al. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226(4675):694–6. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 4.Strauch E, Schaudinn C, Beutin L. First-time isolation and characterization of a bacteriophage encoding the Shiga toxin 2c variant, which is globally spread in strains of Escherichia coli O157. Infect Immun. 2004;72(12):7030–9. doi: 10.1128/IAI.72.12.7030-7039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner PL, et al. Bacteriophage control of Shiga toxin 1 production and release by Escherichia coli. Mol Microbiol. 2002;44(4):957–70. doi: 10.1046/j.1365-2958.2002.02950.x. [DOI] [PubMed] [Google Scholar]
- 6.Grif K, et al. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1998;17(11):761–6. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]
- 7.de Mena MF, et al. Differential kinetic patterns for Shiga toxin production by Escherichia coli. Rev Argent Microbiol. 1997;29(4):167–75. [PubMed] [Google Scholar]
- 8.Wagner PL, Acheson DW, Waldor MK. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect Immun. 2001;69(3):1934–7. doi: 10.1128/IAI.69.3.1934-1937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenanon B, Elhanafi D, Drake MA. Acid adaptation and starvation effects on Shiga toxin production by Escherichia coli O157:H7. J Food Prot. 2003;66(6):970–7. doi: 10.4315/0362-028x-66.6.970. [DOI] [PubMed] [Google Scholar]
- 10.Livny J, Friedman DI. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol Microbiol. 2004;51(6):1691–704. doi: 10.1111/j.1365-2958.2003.03934.x. [DOI] [PubMed] [Google Scholar]
- 11.Manning SD, et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A. 2008;105(12):4868–73. doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich AW, et al. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis. 2002;185(1):74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 13.Boerlin P, et al. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37(3):497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beutin L, et al. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J Clin Microbiol. 2004;42(3):1099–108. doi: 10.1128/JCM.42.3.1099-1108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muniesa M, et al. Shiga toxin 2-converting bacteriophages associated with clonal variability in Escherichia coli O157:H7 strains of human origin isolated from a single outbreak. Infect Immun. 2003;71(8):4554–62. doi: 10.1128/IAI.71.8.4554-4562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson S, et al. Subtyping method for Escherichia coli shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J Clin Microbiol. 2007;45(6):2020–4. doi: 10.1128/JCM.02591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostroff SM, et al. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis. 1989;160(6):994–8. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 18.Serra-Moreno R, Jofre J, Muniesa M. The CI repressors of Shiga toxin-converting prophages are involved in coinfection of Escherichia coli strains, which causes a down regulation in the production of Shiga toxin 2. J Bacteriol. 2008;190(13):4722–35. doi: 10.1128/JB.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koitabashi T, et al. Isolation and characterization of the Shiga toxin gene (stx)-bearing Escherichia coli O157 and non-O157 from retail meats in Shandong Province, China, and characterization of the O157-derived stx2 phages. J Food Prot. 2008;71(4):706–13. doi: 10.4315/0362-028x-71.4.706. [DOI] [PubMed] [Google Scholar]
- 20.Koitabashi T, et al. Genetic characterization of Escherichia coli O157: H7/-strains carrying the stx2 gene but not producing Shiga toxin 2. Microbiol Immunol. 2006;50(2):135–48. doi: 10.1111/j.1348-0421.2006.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 21.Lejeune JT, et al. Human Escherichia coli O157:H7 genetic marker in isolates of bovine origin. Emerg Infect Dis. 2004;10(8):1482–5. doi: 10.3201/eid1008.030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowd SE, et al. Microarray Analysis and Draft Genomes of Two Escherichia coli O157:H7 Lineage II Cattle Isolates FRIK966 and FRIK2000 Investigating Lack of Shiga Toxin Expression. Foodborne Pathog Dis. doi: 10.1089/fpd.2009.0482. [DOI] [PubMed] [Google Scholar]
- 23.Eaton KA, et al. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect Immun. 2008;76(7):3054–63. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Ali GS, et al. Increased adherence and expression of virulence genes in a lineage of Escherichia coli O157:H7 commonly associated with human infections. PLoS One. 5(4):e10167. doi: 10.1371/journal.pone.0010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Ali GS, et al. Differences in adherence and virulence gene expression between two outbreak strains of enterohaemorrhagic Escherichia coli O157: H7. Microbiology. 156(Pt 2):408–19. doi: 10.1099/mic.0.033126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielaszewska M, et al. Chromosomal dynamism in progeny of outbreak-related sorbitol-fermenting enterohemorrhagic Escherichia coli O157:NM. Appl Environ Microbiol. 2006;72(3):1900–9. doi: 10.1128/AEM.72.3.1900-1909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi Y, et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 329(5991):533–8. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marques LR, et al. Production of Shiga-like toxin by Escherichia coli. J Infect Dis. 1986;154(2):338–41. doi: 10.1093/infdis/154.2.338. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt CK, McKee ML, O’Brien AD. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H-strain E32511. Infect Immun. 1991;59(3):1065–73. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy KC, et al. Dam methyltransferase is required for stable lysogeny of the Shiga toxin (Stx2)-encoding bacteriophage 933W of enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2008;190(1):438–41. doi: 10.1128/JB.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner PL, et al. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J Bacteriol. 2001;183(6):2081–5. doi: 10.1128/JB.183.6.2081-2085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Los JM, et al. Differential efficiency of induction of various lambdoid prophages responsible for production of Shiga toxins in response to different induction agents. Microb Pathog. 2009;47(6):289–98. doi: 10.1016/j.micpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H. Shiga-toxin-converting bacteriophages. Res Microbiol. 2001;152(8):687–95. doi: 10.1016/s0923-2508(01)01249-9. [DOI] [PubMed] [Google Scholar]
- 34.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119(3):736–47. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergholz TM, et al. Global transcriptional response of Escherichia coli O157:H7 to growth transitions in glucose minimal medium. BMC Microbiol. 2007;7(1):97. doi: 10.1186/1471-2180-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riordan JT, et al. Inactivation of alternative sigma factor 54 (RpoN) leads to increased acid resistance, and alters locus of enterocyte effacement (LEE) expression in Escherichia coli O157: H7. Microbiology. 156(Pt 3):719–30. doi: 10.1099/mic.0.032631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uzzau S, et al. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A. 2001;98(26):15264–9. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 39.Kumar S, et al. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9(4):299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michino H, et al. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am J Epidemiol. 1999;150(8):787–96. doi: 10.1093/oxfordjournals.aje.a010082. [DOI] [PubMed] [Google Scholar]
- 41.Shaikh N, Tarr PI. Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolutionary implications. J Bacteriol. 2003;185(12):3596–605. doi: 10.1128/JB.185.12.3596-3605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers--western United States, 1992-1993. Morb. Mortal. Wkly. Rep. 1993;42:258–263. [PubMed] [Google Scholar]
- 43.Riley LW, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308(12):681–5. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 44.Unkmeir A, Schmidt H. Structural analysis of phage-borne stx genes and their flanking sequences in shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect Immun. 2000;68(9):4856–64. doi: 10.1128/iai.68.9.4856-4864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental FIG. 1. Linear map of sequence polymorphisms in the q-stx2 region of E. coli O157:H7. Map of the Sakai reference sequence (top sequence) corresponding to nucleotide positions 1265693-1267032 (GenBank BA000007) with genes (q, ECs1204, stx2A) and tRNAs (ile72, argN2, and argO2) indicated by boxed arrows. Positions of non-coding regulatory sequences (pR’, qut, and stx2P) are shown and annealing sites for PCR and sequencing primers are indicated by arrows. All positions are approximate and not to scale. Numbers on the Sakai reference sequence show the distance in nucleotides relative to the translational start (+1) for stx2A. Nucleotides which differ from the Sakai reference sequence are presented on separate maps aligned and positioned below the reference sequence. Nucleotide polymorphisms are indicated atop each map, and their position relative to the translational start for stx2A is indicated below each map sequentially. Regions of identity with the reference sequence are unmarked. Underlined nucleotides indicate a non-synonymous mutation in the ORF of the respective gene in which they are found. The hatched box indicates a deletion. O157:H7 strains which share identical q-stx2 regions are grouped and listed to the right of each map. For strains with asterisks, the q-stx2 region was sequenced in this study; all other sequence data was acquired through GenBank. Strain TW14588 contains two distinct q-stx2 regions indicated by (¥) (positions 991647-992918) and (Φ) (positions 505342-506613) GenBank ABKY00000000.