Abstract
Half a century after the first formulation of the monoamine hypothesis, compelling evidence implies that long-term changes in an array of brain areas and circuits mediating complex cognitive-emotional behaviors represent the biological underpinnings of mood/anxiety disorders. A large number of clinical studies suggest that pathophysiology is associated with dysfunction of the predominant glutamatergic system, malfunction in the mechanisms regulating clearance and metabolism of glutamate, and cytoarchitectural/morphological maladaptive changes in a number of brain areas mediating cognitive-emotional behaviors. Concurrently, a wealth of data from animal models have shown that different types of environmental stress enhance glutamate release/transmission in limbic/cortical areas and exert powerful structural effects, inducing dendritic remodeling, reduction of synapses and possibly volumetric reductions resembling those observed in depressed patients. Because a vast majority of neurons and synapses in these areas and circuits use glutamate as neurotransmitter, it would be limiting to maintain that glutamate is in some way ‘involved’ in mood/anxiety disorders; rather it should be recognized that the glutamatergic system is a primary mediator of psychiatric pathology and, potentially, also a final common pathway for the therapeutic action of antidepressant agents.
A paradigm shift from a monoamine hypothesis of depression to a neuroplasticity hypothesis focused on glutamate may represent a substantial advancement in the working hypothesis that drives research for new drugs and therapies. Importantly, despite the availability of multiple classes of drugs with monoamine-based mechanisms of action, there remains a large percentage of patients who fail to achieve a sustained remission of depressive symptoms. The unmet need for improved pharmacotherapies for treatment-resistant depression means there is a large space for the development of new compounds with novel mechanisms of action such as glutamate transmission and related pathways.
Keywords: Glutamate, major depression, mood disorder, antidepressant, stress, NMDA receptor
1. Introduction. Do we need a glutamate hypothesis of depression?
The fields of neuropsychopharmacology and biological psychiatry have been dominated for over half a century by the monoamine hypothesis, which has driven the research on pathophysiology of neuropsychiatric disorders, in particular mood/anxiety disorders, as well as the development of therapeutic drugs. The basic version of the hypothesis, with regard to depression, speculated that pathology was due to (or accompanied by) reduced availability of monoamines, particularly serotonin and noradrenaline, and that antidepressants exerted their therapeutic action by increasing extracellular availability of monoamines, particularly at synaptic level (Bunney and Davis, 1965; Schildkraut, 1965). The early hypothesis was intrinsically tautological, in that the main evidence was based on the mechanism itself of monoamine oxidase inhibitors and tricyclic antidepressants, drugs that acutely increase the availability of monoamines.
In subsequent years and decades the hypothesis has registered several modifications in the attempt to solve its inherent inconsistencies, the main one being the temporal discrepancy between the immediate effects of drugs on monoamines availabilty (minutes, hours) and their therapeutic effects (several weeks) (Heninger et al., 1996; Hyman and Nestler, 1996; for a historical perspective, see: Racagni and Popoli 2008). The roots of a ‘glutamate hypothesis’ can be traced back to the early 1990s, when early findings showed that N-methyl-D-aspartate receptor (NMDA-R) antagonists possess antidepressant-like action (Trullas and Skolnick, 1990). More recently, the hypothesis has again evolved, integrating research results from many different fields, including intracellular signaling/mechanisms of gene expression, neurotrophic mechanisms, neurogenesis, synaptic function/plasticity, remodeling of neuronal cells/circuitry, into what has been referred to as ‘neuroplasticity hypothesis’ (Pittenger and Duman, 2008; Racagni and Popoli, 2008; Sanacora et al., 2008). Incidentally, a corollary of this hypothesis is that neuroplasticity can be biphasic: adaptive and beneficial, such as that induced by physical exercise and antidepressants, vs maladaptive, such as that observed in neuroimaging brain studies of depressed patients or in animal models of stress and mood disorders (see sections 5–6). An additional corollary, as evidenced by a number of studies, is that maladaptive plasticity in animal models can at least partly be reversed by therapeutic treatments, including antidepressants (Norrholm and Ouimet, 2001; Hajszan et al., 2009; Bessa et al., 2009).
Curiously, although most of the neuroplastic changes have been detected in the neurons and circuitry of the predominant glutamatergic neurotransmitter system (see section 2), we still by default refer to a ‘monoamine hypothesis’, not a ‘glutamate hypothesis’ of depression. As we will briefly summarize in the next few sections, a vast majority of brain neurons and synapses are glutamatergic, and glutamate synaptic transmission largely mediates both cognition and emotion, two brain functions that seem to be inextricably linked (Pessoa, 2008). Moreover, compelling evidence from clinical studies suggest that glutamate transmission is abnormally regulated in a number of limbic/cortical areas in the brains of depressed individuals. Furthermore, increasing evidence suggests the aberrant glutamatergic signaling is associated with maladaptive changes in the structure and function of excitatory circuitry, e.g., the cytoarchitectural and volumetric changes measured by postmortem histopathology and magnetic resonance imaging (MRI) (Campbell and MacQueen, 2006; Konarski et al., 2008; Koolschijn, 2009; Lorenzetti et al., 2009). Concurrently, a wealth of data from animal models of stress have shown that different types of environmental stress enhance glutamate release, reduce glial mediated glutamate cycling, and alter synaptic transmission in limbic/cortical areas. Stress also induces powerful effects on structure and morphology in the rodent brain, inducing dendritic remodeling, synaptic spine reductions, glial loss, and possibly volumetric reductions resembling those observed in depressed patients (Gorman and Docherty, 2010; Holmes and Wellmann, 2009; McEwen, 2005; Musazzi et al., 2011; Pittenger and Duman, 2008; Shansky and Morrison, 2009).
Therefore, while it is well-established that monoaminergic transmission has a primary role in the modulation of emotion and cognition (Pralong et al., 2002; Robbins and Arnsten, 2009), the time has come to recognize and posit that excitatory transmission plays a central role in mediating the complex emotional/cognitive changes associated with depression, and also likely represents the actual final common pathway of therapeutic treatments for depression and other mood/anxiety disorders. It should be noted that while all antidepressant agents available on the market have a monoamine-based mechanism or at least a monoamine-based component in their mechanism (Racagni and Popoli, 2008; Sanacora et al., 2008), there are no approved antidepressant agents directly targeting the glutamatergic system. Currently only 50–60% of depressed patients respond to first antidepressant treatment (less than one third of the subjects achieved remission in the largest open-label study conducted so far; Trivedi et al., 2006). This means there is a large space for improvement in antidepressant treatments if non-monoamine targets are taken into account and new compounds are developed that target directly glutamate transmission or related pathways.
2. Brain is in good part a glutamatergic/GABAergic machine
Although it was not readily recognized as a neurotransmitter until the early 1980s, much later than the monoaminergic transmitters, the amino acid glutamate is now accepted as the major excitatory neurotransmitter in the nervous system (Orrego and Villanueva, 1993). Glutamate mediates the vast majority of fast excitatory transmission in the brain, while γ-aminobutyric acid (GABA), another amino acid neurotrasmitter, mediates the vast majority of fast inhibitory transmission. As an example, it has been estimated that, in the whole brain, there are approximately two to three hundred thousand serotonergic neurons out of a hundred billion total neurons (Baker et al., 1991). In humans, neocortex represents about 85% of the total brain mass. Modern stereological methods have estimated that about 80% of neurons in neocortex are spiny and excitatory and form 85% of all synapses, while about 20% of the neurons are smooth and inhibitory, forming 15% of the synapses (Douglas and Martin, 2007). These data indicate that glutamate neurons and synapses by far outnumber all other neurotransmitter systems in the brain, with the only exception of GABAergic system. In other words the brain, under the point of view of chemical neurotransmission, is largely a glutamatergic excitatory machine, regulated by a relatively smaller GABAergic inhibitory component and modulated by a much smaller number of neurons releasing a variety of other neurotransmitters (including monoamines). It is well-known that monoamines, by regulating fast chemical neurotransmission, modulate all forms of brain function, including sleep/wakefulness, biological drive-related activities mediated by the hypothalamus, emotional/motivational activities mediated by limbic circuitry and value-based behaviors in the neocortex (Othmer et al., 1998; Pralong et al., 2002). However, it is ultimately the changes in excitatory transmission, counterbalanced by the inhibitory component, that actually mediate these functions, in particular cognition and emotion which, as we discuss in the following section, are inextricably intermingled.
3. Emotion vs cognition. The role of glutamate transmission
The last three decades of neurobiological research have clearly associated cognitive processes in the brain with functional and structural changes in glutamate neurotransmission. A wealth of studies (particularly in the hippocampus) have shown that activity-dependent changes in the synaptic strength of central glutamate synapses correlate with the processes of learning and memory, and that interfering with these changes impairs the fixation of memories (Citri and Malenka, 2008; Diamond et al., 2004; Neves et al., 2008; Wong and Ghosh, 2002). The most extensively studied and prototypic forms of synaptic plasticity at glutamate synapses are long-term potentiation (LTP) and depression (LTD), described in different cell fields of hippocampus and in other areas, including amygdala and prefrontal cortex. Therefore, it is widely accepted that plasticity occuring at glutamate synapses allows the encoding of new information, a hypothesis further validated by the findings that both sensory stimulation and learning processes in vivo induce LTP in the hippocampus (Holtmaat and Svoboda, 2009; Whitlock et al., 2006).
However, a similar linkage of emotional processes with glutamatergic transmission has been less firmly established. For a long time a popular view has been that a remarkable degree of functional and evolutionary specialization in the brain allows us to classify areas with predominant ‘affective’ vs ‘cognitive’ function. Early manifestations of this dualistic emotion/cognition view were the ‘circuit of emotion’ by Papez and the definition by McLean of limbic system, an evolutionary more ancient emotional part of the brain that is integrated and regulated in a top/down fashion by the more recent ‘cognitive’ brain (Papez, 1937; MacLean, 1949). As an example, in line with this scheme, it was found that subjects with lesions of the amygdala (a key area for emotional processing) do not show conditioned response in a classic fear conditioning paradigm, while being able to acquire explicit memory. Conversely, subjects with lesions to the hippocampus (a key area for the encoding of explicit memory) show a normal response to fear conditioning but impaired explicit memory of the events (Bechara et al., 1995; LaBar et al., 1995). Interestingly, as exemplified by the hippocampus (originally included in the limbic system), the same area has been with time assigned to either emotional or cognitive circuits, showing an inherent weakness of this dualistic scheme (Pessoa, 2008). This view was further elaborated in a popular top/down scheme stating that cognitive processes are subserved by a ventral neural system, mainly in charge of identification of salient emotional tags and production of affective responses, and a dorsal system that exerts control and regulation of affective states (Phillips et al., 2003a). According to this view, distinct patterns of structural and functional abnormalities in these neural systems are responsible for specific symptomatology of different neuropsychiatric disorders (schizophrenia, major depression, bipolar disorder) (Phillips et al., 2003b).
However, in the last two decades much evidence has accumulated that makes this kind of view increasingly problematic, including structural connectivity data that suggest a remarkable potential for integration of information among brain areas, and functional neuroimaging studies showing that large portions of both cortical and subcortical areas are engaged during emotional information analyses (Kober et al., 2008; Pessoa, 2008; Phelps, 2006; Taylor and Liberzon, 2007). A primer on this was the ‘somatic marker’ hypothesis by Damasio and coworkers, which proposed that bodily states work as marker signals that influence reasoning and participate to decision-making (Damasio, 1994; Bechara et al., 2000). Therefore, it has been proposed that complex cognitive-emotional behaviors have their basis in dynamic coalitions of networks of brain areas, none of which should be conceptualized as specifically affective or cognitive (Pessoa, 2008; Phelps, 2006; Taylor and Liberzon, 2007).
Again, most connections between and within these brain areas are glutamatergic, which suggest that both maladaptive changes that have been accurately described in depressed subjects and restorative changes induced by antidepressant treatments mainly affect glutamate neurotransmission. In the following sections we will briefly summarize the different clinical studies that showed changes in glutamate and glutamatergic circuitry in mood disorders.
4. Clinical evidence of glutamatergic dysfunction in mood disorders
4.1. Glutamate levels: plasma, cerebrospinal fluid, tissue studies
There is now rapidly growing evidence that pathophysiological changes within the amino acid neurotransmitter systems are associated with mood and cognitive dysfunction. Similar to reports of wide ranging abnormalities in GABA content in individuals with mood disorders (Brambilla et al., 2003; Tunnicliff and Malatynska, 2003; Sanacora and Saricicek, 2007), glutamatergic abnormalities have been demonstrated in the plasma, cerebrospinal fluid (CSF) and brain tissue of individuals afflicted with mood disorders. Multiple studies have reported findings of elevated glutamate content, and a trend for decreased plasma glutamine/glutamate ratios in the plasma of depressed patients compared to healthy comparison subjects (Kim et al., 1982; Altamura et al., 1993; Mauri et al., 1998; Altamura et al., 1995; Mitani et al., 2006; Kucukibrahimoglu et al., 2009). Other studies have provided evidence that treatment with antidepressant agents may decrease the plasma glutamate levels in depressed individuals (Altamura et al., 1995; Kucukibrahimoglu et al., 2009; Maes et al., 1998). The origins of the plasma glutamate and the pathophysiological mechanisms accounting for the different levels in the depressed patients have not yet been determined. However, platelets possess a high affinity glutamate uptake system and are known to express the same glutamate transporters that are present in the brain (Zoia et al., 2004; Begni et al., 2005). The fact that glutamate transport by human platelets appears to serve as a marker for a variety of neurovegetative diseases associated with impaired glutamate transport (Tremolizzo et al., 2004), suggests that altered platelet glutamate uptake in depressed individuals could contribute to the findings. Yet, seemingly inconsistent with this hypothesis, a study of bipolar subjects in a manic state showed the subjects to have increased rates of glutamate uptake into platelets (do Nascimento et al., 2006), and a single study of depressed subjects showed platelet glutamate levels to be numerically, but not significantly, higher in depressed subjects compared to healthy controls (Mauri et al., 1998). There have been few attempts to directly compare central nervous system tissue glutamate content between mood disordered patients and comparison subjects. Studies examining CSF from mood disordered patients are extremely limited. A single study reported reduced CSF glutamate content in a mixed sample of individuals with major depressive and bipolar disorder (Frye et al., 2007) and an earlier study of CSF samples was unable to effectively measure glutamate levels but did report significantly higher CSF glutamine concentrations in subjects with major depression (Levine et al., 2000). The sole study examining neurosurgical samples from chronically depressed subjects failed to find any significant difference in frontal cortex glutamate concentrations (Francis et al., 1989), yet a more recent postmortem study of the frontal cortex showed a significant increase in tissue glutamate levels in individuals with major depressive and bipolar disorders after controlling for postmortem interval (Hashimoto et al., 2007). Consistent with this finding, a recent analysis of postmortem dorsolateral prefrontal cortex tissue also revealed elevated glutamate levels in bipolar individuals (Lan et al., 2009).
4.2. Glutamate levels: magnetic resonance spectroscopy studies
In vivo proton Magnetic resonance spectroscopy (1H-MRS) measures of brain glutamate and glutamine can avoid many of the confounding variables such as loss of region specificity and post mortem changes, restricting the interpretation of the peripheral and postmortem tissue measures. Although this approach remains somewhat technically challenging, an increasing number of studies employing in vivo 1H-MRS to investigate potential abnormalities in brain glutamate content have been completed over the last decade. As it is difficult to assign unequivocal resonance peaks to individual resonances using standard 1H-MRS methods at lower field strengths, a combined measure termed Glx, which predominantly reflects glutamate content but also contains glutamine and GABA components, has been used in the majority of studies.
Cousins et al. were the first to report an association between mood and 1H-MRS determined Glx measures, demonstrating temporary decreases in Glx levels coinciding with a patient’s transient experience of suicidal depression (Cousins and Harper, 1996). Since the time of this initial study there have been more than 50 publications examining the relationship between glutamate related metabolites (Glx, glutamate or glutamine) and mood (for recent reviews, see Yuksel and Ongur, 2010; Kondo et al., 2011). While results of these studies varied, a few strong trends are emerging from the literature. A large majority of studies have provided evidence of reduced glutamate metabolite levels in the frontal cortex and cingulate regions of patients with major depressive disorder in the midst of a current depressive episode (Auer et al., 2000; Michael et al., 2003a; Michael et al., 2003b; Hasler et al., 2007). Reduced Glx content was also found in the anterior cingulate cortex in pediatric depression (Mirza et al., 2004; Rosenberg et al., 2004; Rosenberg et al., 2005). In contrast to the reductions in frontal and cingulate regions, glutamate metabolite measures in the occipital and parietal/occipital regions have been found to be elevated in medication-free major depression subjects in an active depressive episode (Sanacora et al., 2004), in remission (Bhagwagar et al., 2007), and most recently in young people with increased familiar risk for major depression (Taylor et al., 2011). In other related studies, higher glutamate metabolite measures were reported in the frontal cortex of patients experiencing late life depression and post-stroke depression (Binesh et al., 2004; Glodzik-Sobanska et al., 2006; Wang et al., 2011). In bipolar disorder the findings most consistently showed elevated Glx content in the various brain regions studied in adults (Bhagwagar et al., 2007; Michael et al., 2003c; Dager et al., 2004; Frye et al., 2007; Ongur et al., 2008) and in children (Castillo et al., 2000). However, there are other studies showing either decreased or no significant change in glutamate metabolites in similar populations of bipolar subjects (Port et al., 2008; Michael et al., 2003a; Davanzo et al., 2003). In summary, these studies seem to show glutamate metabolite MRS measures vary significantly by brain region, subtype of mood disorder, course of illness progression and state or phase of illness.
Before attempting to draw any pathophysiological meaning from the MRS findings it is important to clearly understand what is being measured in these studies. 1H-MRS provides measures of total tissue metabolite content. The overwhelming majority of the metabolites being measured are intracellular, with an exceedingly small fraction reflecting synaptic glutamate. Thus, it is very difficult to draw any conclusions about glutamatergic neurotransmitter function directly from these findings. However, the fact that tissue glutamate, glutamine and even GABA content appear to be abnormal in several brain regions, and likely even in plasma of individuals afflicted with various forms of mood disorder, suggests that dysregulation of amino acid neurotransmitter system metabolism in some form is likely to be associated with the disorders. As glial cells are central to the clearance and metabolism of these metabolites within the brain, they have increasingly grown in interest.
5. Cytoarchitectural and morphological correlates to mood disorders
Consistent with the idea that changes in glial cell function may be related to the reports of abnormal levels of glutamate and glutamine in patients with mood disorders, reduced numbers and density of glial cells have been reported in a number of postmortem studies (Rajkowska, 2000; Rajkowska et al., 1999; Bowley et al., 2002; Rajkowska et al., 2001; Miguel-Hidalgo et al., 2002; Webster et al., 2001; Cotter et al., 2001; Miguel-Hidalgo et al., 2000; Ongur et al., 1998; Hamidi et al., 2004) (see Rajkowska and Miguel-Hidalgo 2007 for review). Although there is some evidence suggesting the glial abnormalities are predominantly due to reduced numbers of oligodendrocytes (Hamidi et al., 2004), reports of reduced GFAP levels in several brain regions from mood disorder patients suggest that astrocytes are also involved in the glial pathology (Muller et al., 2001; Si et al., 2004; Fatemi et al., 2004; Toro et al., 2006; Miguel-Hidalgo et al., 2010; Altshuler et al., 2010). Studies demonstrating reduced expression of excitatory amino acid transporters (EAAT1, EAAT2) responsible for the majority of glutamate uptake and glutamine synthetase, the enzyme responsible for the conversion of glutamate to glutamine within glia, in several brain regions from depressed individuals, suggest the glial changes are associated with impaired glutamate uptake and metabolism (Altshuler et al., 2010; Bernard et al., 2010; Choudary et al., 2005; Miguel-Hidalgo et al., 2010; Sequeira et al., 2009). Reduced astrocytic function can result in impaired glutamate clearance from the extrasynaptic space and altered rates of glutamate/glutamine and GABA/glutamine cycling. Thus, many of the observed differences in amino acid neurotransmitter content between mood disorder and healthy comparison subjects could be related to glial pathology.
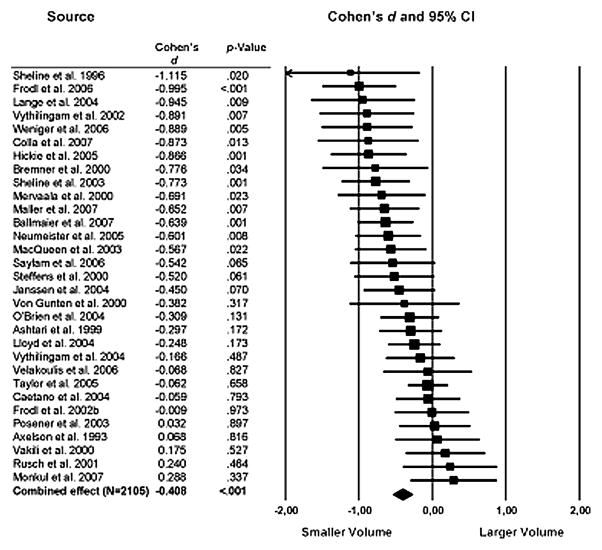
The last decade has also generated a large body of neuroimaging studies identifying regional volumetric changes in the brains of mood/anxiety disorder patients (Campbell and MacQueen, 2006; Konarski et al., 2008; Koolschijn, 2009; Lorenzetti et al., 2009). Interestingly, the results of a large comprehensive meta-analysis show in depressed subjects consistent structural abnormalities in brain regions closely associated with stress-responsiveness and emotional/cognitive processing in depressed subjects (Koolschijn, 2009) (Fig. 1). Specifically, depressed patients had large volume reductions in frontal regions including the anterior cingulate and orbitofrontal cortex with smaller reductions in the prefrontal cortex. Other brain regions such as the hippocampus and striatum also showed moderate volume reductions. Overall, no significant difference was detected in the amygdala, however the large degree of variability in the studies of this region highlight the technical difficulties and the fact that studies of this region may be complicated by other confounding variables. Interestingly, many of the regions showing volumetric reductions overlap with the regions demonstrated to have significant reductions in glial cell numbers and density, and neuronal atrophy (Rajkowska, 2002).
Figure 1.
Summary of MRI studies measuring volumetric changes in hippocampus in major depressive disorder. Values represent the calculated Cohen’s d effect size of changes from mean total hippocampal volume. Error bars indicate 95% confidence intervals. From Koolschijn et al., 2009.
Considering the evidence suggesting that increased activation of extrasynaptic relative to synaptic glutamate receptors results in cellular toxicity and neurodegeneration (Hardingham and Bading, 2010), it is possible that reduced extracellular glutamate clearance, especiallly in the face of excessive glutamate release, could provide a mechanism linking the observed glutamate abnormalities to the growing evidence of regional volume reductions in the brains of mood disordered patients (Koolschijn et al., 2009). A recent report demonstrating a strong correlation between extracellular hippocampal glutamate content measured by in vivo microdialysis, and reduced hippocampal volumes in individuals with seizure disorders (Cavus et al., 2008) provides support to this hypothesis. Additional work showing early evidence of a correlation between structural changes determined by diffusor tensor imaging measures and glutamate metabolite levels lends further support to this hypothesis (Gupta et al., 2010; Modrego et al., 2011). The hypothesis has also received support from the numerous studies reporting structural remodeling in animal models of stress (in the following section).
6. Dendritic remodeling in the brain. Evidence from preclinical stress models
The effects of stress on structural remodeling in the brain have been investigated by a number of studies (for a review see: Gorman and Docherty, 2010; Holmes and Wellmann, 2009; McEwen, 2005; Musazzi et al., 2011; Pittenger and Duman, 2008; Shansky and Morrison, 2009). The largest number of studies have been carried out in hippocampus, but prefrontal cortex and amygdala were also analyzed (Tab. 1). Different forms of stress have been shown to induce atrophy, retraction and consistently reproduced remodeling of dendrites in pyramidal neurons of CA3 hippocampal region (Magarinos and McEwen, 1995; Watanabe et al., 1992). Quite similar effects of stress have been described in medial prefrontal cortex (Radley et al., 2004). The remodeling of apical dendrites induced by stress in medial prefrontal cortex was found associated with a significant decrease in the density of dendritic spines (Michelsen et al., 2007; Radley et al., 2008; Silva-Gomez et al., 2003). Interestingly, a few studies analyzed the correlation between these effects of stress on dendritic morphology and concomitant impairment of cognitive functions. It was found that 6 days or 4 weeks of chronic unpredictable stress impaired spatial working memory (water maze), reduced plasticity in hippocampal-medial prefrontal cortex synapses (a glutamatergic pathway) and caused dendritic atrophy in medial prefrontal cortex (Cerqueira et al., 2007). In a different study it was found that chronic restraint stress (21 days) induced selective impairment in attentional set-shifting task (a function mediated by medial prefrontal cortex), accompanied by concomitant retraction of apical dendritic arbors in anterior cingulate cortex and extension in lateral orbitofrontal cortex (Liston et al., 2006).
Table 1.
Animal studies on the effects of stress, glucocorticoids or antidepressant agents on dendrites and synaptic spines
| Stressor/Glucocorticoid/Animal model | Drug treatments | Changes in dendrites/spines | Brain area | References |
|---|---|---|---|---|
| Chronic restraint stress (21 days) | -- | Atrophy of apical dendrites | Hippocampus | Watanabe et al., 1992 |
| Chronic restraint stress (21 days) | Cyanoketone, CGP 43487 | Atrophy of apical dendrites (prevented by drug treatment) | Hippocampus | Magarinos and McEwen, 1995 |
| Daily corticosterone treatment (21 days) | Phenytoin | |||
| Chronic restraint stress (21 days) | Tianeptine | Dendritic remodeling (reversible after cessation of stress and prevented by drug treatment) | Hippocampus | Conrad et al., 1999 |
| Olfactory bulbectomy | Amytriptiline Mianserin |
Reduction in spine density (reversed by drug, mianserin effect only in dentate gyrus) | Hippocampus and Dentate Gyrus | Norrhom and Ouimet, 2001 |
| Repeated neonatal handling | Elevation of spine density | Dentate Gyrus | ||
| Chronic immobilization stress | -- | Dendritic atrophy and debranching Enhancing arborization |
Hippocampus Basolateral Amygdala |
Vyas et al., 2002 |
| Chronic unpredictable stress | Dendritic atrophy | Basolateral Amygdala | ||
| Postweaning Social Isolation | -- | Decrease in dendritic length Decrease in density of dendritic spines |
Hippocampus Medial Prefrontal Cortex and Hippoampus |
Silva-Gomez et al., 2003 |
| Chronic restraint stress (21 days) | -- | Reduction on the total length and branch numbers of apical dendrites | Medial Prefrontal Cortex | Radley et al., 2004 |
| Chronic immobilization stress (21 days) | -- | Increased dendritic length and spine density (not reversed after cessation of stress) | Basolatera Amygdala | Vyas et al., 2004 |
| Chronic Restraint Stress | Lithium | Decrease in dendritic length (prevent by drug) | Hippocampus | Wood et al., 2004 |
| Acute immobilization stress Chronic immobilization stress |
-- | Increase in spine density | Basolateral amygdala | Mitra et al., 2005 |
| Chronic Restraint Stress (3 weeks or 6 weeks) | Reduction in apical dendritic length and branch number (reversible after cessation of stress) | Medial Prefrontal Cortex | Radley et al., 2005 | |
| Chronic restraint stress (21 days) | -- | Retraction of apical dendritic arbors Extension of apical dendrites arbors |
Medial Prefrontal Cortex Orbital Frontal Cortex |
Liston et al., 2006 |
| Dexametasone and Corticosterone treatment | -- | Reduction in the total length of apical dendrites Impoverishing arborizations in distal portions of apical dendrites | Medial Prefrontal Cortex | Cerqueira et al., 2007 |
| Corticosterone treatment | Increasing branching in middle portion of apical dendrites | |||
| Chronic Mild Stress | -- | Decrease in density of dendritic spines | Medial Prefrontal Cortex | Michelsen et al., 2007; |
| Prenatal Stress | Decrease the ratio of spines | |||
| Chronic Restraint Stress (3 weeks) | -- | Decrease dendritic spine volume and surface area | Medial Prefrontal Cortex | Radley et al., 2008; |
| Chronic mild stress | Fluoxetine, Imipramine, CP 156 CP 526, SSR 1494515 (alone or methylazoxymeth anol) | Reduction in dendritic arborization and spine density (prevented by drug) | Hippocampus and Prefrontal Cortex | Bessa et al., 2009 |
| Inescapable Footshock stress Corticosterone injection | Desipramine | Loss of synaptic spines (reversed by drug) | Hippocampus and Dentate Gyrus | Hajszan et al., 2009 |
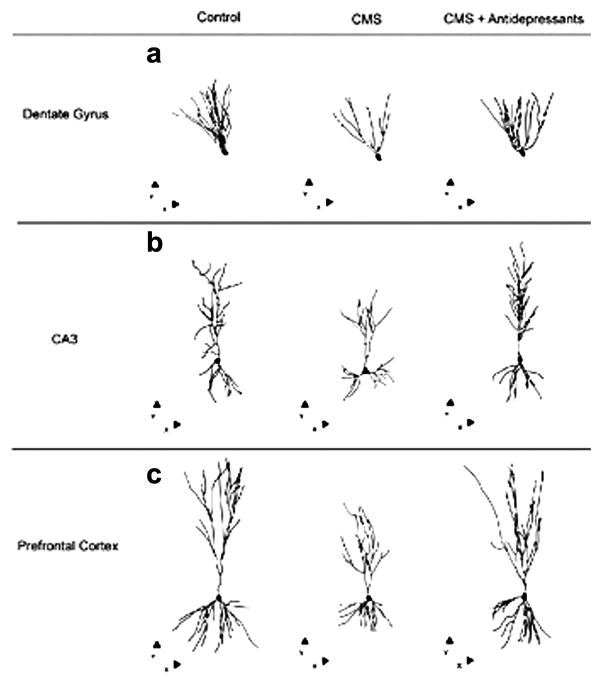
Interestingly, dendritic remodeling induced by stress has been shown to be reversible after cessation of stress in both hippocampus and medial prefrontal cortex, (Conrad et al., 1999; Radley et al., 2005). In addition, it has been shown that pharmacological treatments may reverse the structural changes induced by stress or present in animal models of depression. Lithium treatment was able to prevent stress-induced dendritic remodeling in hippocampus (Wood et al., 2004); amytriptiline reversed the reduction in spine density in an animal model of depression (Norrholm and Ouimet, 2001). Recently it was found that 6 days fluoxetine treatment at the same time rescued the loss of synaptic spines in hippocampus and the escape deficit in the stress-induced Learned Helplessness model of depression (Hajszan et al., 2009). Moreover, various monoaminergic antidepressants and non-monoaminergic putative antidepressants were shown to reverse the reduction in dendritic arborization and spine density induced by chronic mild stress (Fig. 2) (Bessa et al., 2009).
Figure 2.
Dendritic remodeling in hippocampus (Dentate Gyrus and CA3) and prefrontal cortex in a 6 weeks chronic mild stress (CMS) protocol. These effects were reversed by treatment with different antidepressant agents (imipramine; fluoxetine; CP 156,526; SSR 149415), administered during the last 2 weeks of CMS. Three-dimensional morphometric analysis of Golgi-impregnated neurons using computer-assisted reconstructions of hippocampal and medial prefrontal cortex neurons. Representative dentate granule (a), CA3 pyramidal (b), and layer II/III pyramidal neurons from prelimbic area of medial prefrontal cortex (c). Cells are depicted in the x-y orthogonal plan. Adapted from Bessa et al., 2009.
Opposite changes were observed in amygdala. The same stress paradigms, that induced reduction of dendrite arborization and spine density in hippocampus and medial prefrontal cortex, increased dendritic length and spine density in amygdala, at the same time enhancing behavioral measures of anxiety (Vyas et al., 2002; 2004). Different from other areas, these structural changes were not reversed several weeks after cessation of stress (Vyas et al., 2004). Interestingly, a single immobilization stress episode caused a gradual increase in spine density 10 days later, without any effect on dendritic arbors, in basolateral amygdala (Mitra et al., 2005). Therefore, somewhat in line with neuroimaging measures from clinical studies, the effects of stress on brain morphology and function in rodents seem to be area- and circuit-dependent.
It appears from rodent studies that different forms and lengths of stress have powerful effects on synaptic connections and circuits, mostly glutamatergic in nature, in the same brain areas that showed volumetric changes in depressed patients. Therefore, it has been suggested that changes in glutamate release and synaptic transmission are responsible for dendritic remodeling and, in turn, for tissue morphological changes. However, other mechanisms have also been involved, including loss of glial cells, particularly in PFC (Raikowska et al., 1999), which impacts on glutamate homeostasis (see below), and inhibition of neurogenesis in hippocampus (Duman, 2004). The nature of changes induced by stress and glucocorticoids at synapses, which may in turn be responsible for morphological and volumetric changes, will be analyzed in the following section.
7. The effects of stress and glucocorticoids on glutamate synapses and neurotransmission
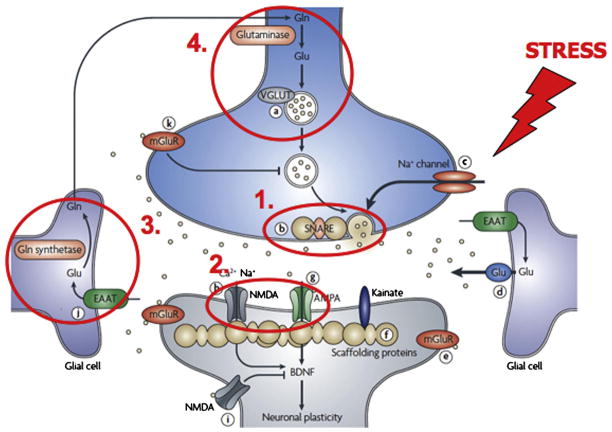
A remarkable feature of glutamate synapses is their ability to undergo structural, as well as functional change, in response to environmental stimuli (Sudhof and Malenka, 2008; Wong and Ghosh, 2002). Accordingly, synaptic terminals, spines and dendritic branches are constantly remodeled as a result of experience, learning and memory, emotion processing. As outlined above, environmental stress has a heavy impact on brain tissue morphology and is considered a risk factor for mood/anxiety disorders. However, the stress response is a complex physiological mechanism and, although much of this mechanism has been unveiled in recent years (deKloet et al., 2005; McEwen, 2010; Sorrels et al., 2009), the way whereby an incorrect stress response is linked to pathophysiology is still far from being completely understood. As often observed in pathophysiology, it can be envisaged that a continuum exists between physiological mechanisms of plasticity and more robust changes in structure and function, leading to pathological changes. It is well-known that higher than normal extracellular concentrations of glutamate cause excitotoxicity, neuronal degeneration and death, a phenomenon that is central to pathophysiology of neurodegenerative diseases (Hardingham and Bading, 2010). Considering that glutamate transmission is tightly regulated in the brain, if one or more of its regulatory aspects are endangered by stress this may in turn produce alterations in synaptic connections and circuits that, particularly in the presence of a genetic background of vulnerability, may play a relevant role in pathophysiology of mood/anxiety disorders. In this section we will briefly analyze the effects of stress on presynaptic release of glutamate, on postsynaptic ionotropic receptors for glutamate, and on glutamate clearance and metabolism at synapses (Fig. 3).
Figure 3.
The effects of stress and glucocorticoids on glutamate synapses and neurotransmission. Several sites/mechanisms of regulation of the glutamate synapse have been shown to be targets of stress and hormones released in the stress response (glucocorticoids): (1) presynaptic release of glutamate; (2) postsynaptic ionotropic receptors for glutamate (NMDA and AMPA receptors); (3) reuptake of glutamate by glial glutamate membrane transporters; (4) glutamate metabolism and recycling by the glutamate/glutamine cycle. Major consequences of stress/glucocorticoid exposure on these sites/mechanisms are: (1) increased glutamate release; (2) altered trafficking/expression/function of ionotropic glutamate receptors; (3) altered clearing of glutamate from the synapse; (4) reduced glutamate/glutamine cycling and glial cell density (see section 7). Adapted from Sanacora et al., 2008.
7.1. The effects of stress and glucocorticoids on presynaptic release of glutamate
Many studies have characterized the effect of acute environmental stress on presynaptic release of glutamate. Early in vivo microdialysis studies showed that exposure of rats to different stressors (tail-pinch, restraint, forced swim) or administration of corticosterone (the main stress hormone in rodents) rapidly and transiently induce a marked increase in the level of extracellular glutamate in different areas, including hippocampus, amygdala and prefrontal cortex (Bagley and Moghaddam, 1997; Lowy et al., 1993; Moghaddam, 1993; Venero and Borrell, 1999). Although the nature and origin of extracellular glutamate measured by microdialysis has been questioned (van der Zeyden et al., 2008), these results have been substantially confirmed by more recent works that used different methodologies measuring exocytotic release of glutamate (Cazakoff and Howland, 2010; Hascup et al., 2010; Karst et al., 2005; Musazzi et al., 2010; Reznikov et al., 2007; Satoh and Shimeki, 2010; Wang and Wang, 2009). Prefrontal cortex seems to be the area where stress induces the highest increase in glutamate release, although this effect is attenuated when stress is repeatedly applied over a short period of time (Bagley and Moghaddam, 1997; Moghaddam, 1993; Musazzi et al., 2010). The rapid effect of acute stress (or corticosterone) on glutamate release was shown to be mediated by membrane-located receptors of different types (mineralocorticoid receptor in hippocampus, glucocorticoid in prefrontal cortex) coupled to non-genomic (e.g., protein synthesis-independent) mechanisms. Related changes in molecular presynaptic mechanisms were shown in the two areas above (Olijslangers et al., 2008; Musazzi et al., 2010). For a detailed discussion of the effects of stress on glutamate release see Musazzi et al. (2011).
Much less is known on the effects of chronic stress on glutamate release, although this information is crucial in order to understand how environmental stress contributes to pathophysiology of mood/anxiety disorders (Fontella et al., 2004; Yamamoto and Reagan, 2006). Future studies should investigate how the response of glutamate synaptic terminals to acute stressors is modified by a previous period of chronic stress, possibly using different protocols for chronic stress and for the acute challenge (Armario et al., 2008).
7.2. The effects of stress and glucocorticoids on postsynaptic ionotropic receptors for glutamate
Converging evidence has revealed that mechanisms of synaptic plasticity in the hippocampus (e.g., LTP) are rapidly activated by stress. This initial period of activation is followed by a longer period in which the induction of new plasticity is inhibited (threshold for LTP is increased), probably to ensure the preservation of memory related to the stressful event and avoid interference of memories formed in the subsequent period of time (for a discussion see Diamond et al., 2007; Joels, 2008). There is now a wide consensus that the major mechanism of expression of LTP at hippocampal CA1 synapses (the most studied form of LTP) involves an increase in the number of α-amino-3-hydroxy-methyl-4-isoxazolepropionic acid receptors (AMPA-R) within the postsynaptic density, driven through activity-dependent changes in AMPA-R trafficking (Citri and Malenka, 2008). Recent studies have analyzed the effect of corticosterone on membrane trafficking at synapses of AMPA-R, crucial for the establishment of synaptic plasticity (Krugers et al., 2010). By using single particle tracking, it was shown that application of corticosterone to primary hippocampal neuronal cultures rapidly increased the lateral diffusion of GluA1/GluA2 AMPA-R subunits, and increased the synaptic number of GluA2-containing AMPA-R after induction of chemical LTP (Groc et al., 2008). This rapid effect was dependent on activation of membrane-located MR acting through non-genomic mechanism, as shown previously for corticosterone-induced enhancement of glutamate release in hippocampus (Karst et al., 2005). It is not known whether there is a causal link between the stress-induced enhancement of glutamate release and the increased trafficking of AMPA-R. This was shown to be followed by a slower phase of increased membrane recruitment of GluA2-containing AMPA-R, mediated by activation of GR acting through genomic mechanisms (Groc et al., 2008; Martin et al., 2009). This long-term stimulatory effect of corticosterone on GluA2-containing AMPA-R blocked any further attempt at inducing LTP, providing a possible explanation as to why the long-term corticosterone application hampers LTP.
In prefrontal cortex of juvenile rats, it was shown that both NMDA-R- and AMPA-R-mediated synaptic currents were markedly increased in pyramidal neurons by a variety of acute stress paradigms (Yuen et al., 2009, 2011). The effect was observed 1 hour after stress, was sustained for 24 hours after cessation of stress, and was mimicked by a short-term corticosterone application in vitro. This was paralleled by an increase in the surface expression of NMDA-R- and AMPA-R, and was shown to be a slow effect mediated by intracellular GR. The acute stress facilitated cognitive processes mediated by prefrontal cortex.
The impact of chronic stress on postsynaptic ionotropic glutamate receptors in prefrontal cortex has been less characterized. Decreased GluN2B and GluA2/3 subunit expression has been detected after chronic corticosterone exposure, providing a potential cellular mechanism underlying the corticosterone-induced impairment in fear extinction (Gourley et al., 2009). Recently, it was found that repeated restraint stress induced in prefrontal cortex neurons a significant reduction of AMPA-R- and NMDA-R-mediated synaptic currents, which was sustained for a few days after stress extinction, and of surface AMPA-R and NMDA-R subunits (Yuen et al., 2010). In particular the levels of GluA1 and GluN1 were markedly reduced, in contrast to the unchanged glutamate receptor protein expression by acute stress (Yuen et al., 2009). Thus, it was speculated that the loss of prefrontal cortex glutamate transmission in chronically stressed animals is due to a disruption of membrane trafficking or altered degradation/synthesis of glutamate receptors. For a discussion on the action of stress on postsynaptic glutamate receptors see Krugers et al., 2010; Yuen et al., 2011.
7.3. The effects of stress on synaptic clearance and metabolism of glutamate
Glial cells modulate glutamatergic neurotransmission at synapses by rapidly clearing glutamate from the extracellular space through EAAT1 and EAAT2, limiting the extent of synaptic transmission and preventing spillover to the extrasynaptic space. Glia also serve a central role in amino acid neurotransmitter metabolism by converting glutamate to glutamine for the glutamate/glutamine cycle, and providing the majority of the glutamine precursor needed for GABA synthesis. In light of the clinical findings showing marked reductions of glial density and numbers in mood disorders (see above), and glia’s critical role in glutamatergic signalling, it is important to consider glial contributions to the stress-related pathophysiology and potentially the pathogenesis of mood/anxiety disorders.
Stress has been shown to have structural and functional effects on glia. Chronic unpredictable stress (CUS) resulted in reduced proliferation of glial progenitor cells (Banasr et al., 2007), decreased numbers of GFAP-positive cells and reduced expression of GFAP in the prelimbic cortex (Banasr et al., 2007; Banasr and Duman, 2008). In conjuction with these findings, reduced levels of glutamate/glutamine cycling and GABA synthesis were seen in the same cortical regions following a 5-week period of CUS (Banasr et al., 2010). Interestingly, riluzole, a drug shown to modulate both glutamate release and uptake, was effective in attenuating or reversing the behavioural, structural and metabolic changes seen with CUS in this study. The chronic social defeat paradigm has also been shown to decrease gliogenesis in the medial prefrontal cortex of rats (Czeh et al., 2007). Suggesting that some of the regional stress-induced effects on glial cells are long-lasting, rats exposed to early-life stress were reported to have a reduced density of GFAP-immunoreactive astrocytes in the frontal cortex in adulthood (Leventopoulos et al., 2007). Other studies have reported a reduced density of GFAP-immunoreactive astrocytes in the hippocampus of rats and tree shrews following exposure to chronic social stress procedures (Fuchs, 2005; Liu et al., 2010).
Microdialysis findings of sustained increased extracellular glutamate levels in the rat hippocampus after acute stress following a period of chronic stress (Yamamoto and Reagan, 2006) suggest functional clearance is impaired by chronic stress exposure. A recent study showing both increased hippocampal glutamate release and decreased glial glutamate uptake following exposures to a variant of the CUS paradigm provides additional evidence for this claim (de Vasconcellos-Bittencourt et al., 2011). Yet another study has demonstrated reduced levels of glutamate uptake in the hippocampus, striatum, and prefrontal cortex 21 days after completing a learned helplessness paradigm (Almeida et al., 2010). This report is especially intriguing considering the findings of Zink et al. that showed helpless animals expressed significantly lower levels of EAAT2 (GLT1) in the hippocampus and cortex compared to littermates that did not exhibit helpless behavior (Zink et al., 2010). However, evidence of increased EAAT2 expression in the hippocampus shortly following chronic restraint stress exposure (Reagan et al., 2004) and activation of glucocorticoid receptors (Zschocke et al., 2005), suggest the relationship between stress, glial glutamate transporter expression, and glutamate uptake is complex.
8. Antidepressant agents and glutamate: preclinical evidence
The monoamine hypothesis was largely based on the serendipitous discovery of the first antidepressant agents. However, early findings that these drugs affect synaptic plasticity, modulate the function of NMDA-R, and that NMDA-R antagonists possess antidepressant activity, have started a variegated field of research that in time has contributed to disclose the role of glutamatergic system in mood disorder and in antidepressant action. In this section, we summarize the known effects of antidepressant agents on the glutamatergic system.
8.1. Antidepressant agents affect synaptic plasticity
Studies investigating the effect of antidepressant agents on LTP have generated a variety of results, depending on the brain area (Pittenger and Duman, 2008). However, the prevalent effect of antidepressant agents has been shown to be a reduction of hippocampal LTP, after acute (Kojima et al., 2003; Mnie-Filali et al., 2006; Shakesby et al., 2002; Tachibana et al., 2004) or chronic administration (Stewart et al., 2000; Ohashi et al., 2002; Ryan et al., 2009).
It has been speculated that antidepressant agents induce an LTP-like process, which saturates hippocampal synaptic plasticity and reduces capacity for further synaptic change (Moser et al., 1998; Huang et al., 2005; for a discussion see Pittenger and Duman, 2008; Popoli et al., 2002). It is interesting that acute administration of antidepressants (fluoxetine, imipramine, tianeptine) was shown to be able to rescue LTP after acute stress (Rocher et al., 2004; Shakesby et al., 2002; Von Frijtag et al., 2001). Recently, it has been shown that this effect of tianeptine (but not imipramine) may be linked to reversal of stress-induced down-regulation of MEK/ERK-MAPK signaling and activation of Ser831 phosphorylation in GluA1 AMPA-R subunit (Qi et al., 2009). However, it would be interesting to assess how chronic antidepressant treatments affect stress-induced impairment of LTP. In two studies it was shown that chronic antidepressant treatments were able to reverse the effects of conditioned fear stress and early-life stress, respectively, on LTP (Matsumoto et al., 2005; Ryan et al., 2009).
8.2. Antidepressant agents interfere with function of glutamate receptors
Several studies have shown that chronically administered antidepressants from different classes modulate the function of NMDA-R (Nowak et al., 1993; Paul et al., 1994; Skolnick et al., 1999), and reduce the synaptic expression of GluN1 subunit of NMDA-R (Boyer et al., 1998; Pittaluga et al., 2007). In addition, NMDA-R antagonists have been shown to possess antidepressant activity in animal models of depression (Skolnick et al., 1999).
In line with this rationale, ketamine, a nonselective NMDA-R antagonist mainly employed as a dissociative anesthetic, has been shown at subanesthetic doses to induce rapid (within 24 hours) antidepressant effects that are sustained for at least several days after a single infusion, in at least four blinded pilot clinical trials (Berman et al., 2000; Diazgranados et al., 2010; Valentine et al., 2011; Zarate et al., 2006). Additionally, CP 101–606, an antagonist of GluN2B subunit containing NMDA receptors was also shown to have antidepressant effects in previously treatment-resistant depressed patients (Preskorn et al., 2008). Ketamine has also been consistently shown to possess antidepressant-like properties in multiple different rodent models of depression and antidepressant action (see section 9) (Autry et al. 2011; Chaturvedi et al., 1999; Du et al., 2006; Koike et al., 2011; Li et al., 2010; Li et al., 2011; Maeng et al. 2008: Popik et al., 2008; Yilmaz et al., 2002). However, while some studies demonstrated sustained effects in rodent models similar to those seen in depressed patients (Autry et al. 2011; Du et al., 2006; Koike et al., 2011; Li et al., 2010; Li et al., 2011; Maeng et al. 2008; Yilmaz et al., 2002), others have failed to demonstrate the enduring effects of an acute ketamine dosing (Popik et al., 2008; Bechtholt-Gompf et al., 2011). Interestingly, other work has shown similar antidepressant-like effects with zinc, an inhibitor of NMDA-sensitive glutamate-gated channels, in preclinical and clinical studeis. (see Szewczyk et al., 2008, for a review).
Perhaps providing some insight into the mechanism of antidepressant action, recent studies suggest the antidepressant-like effects of ketamine and other NMDA antagonists may be dependent on enhancement of AMPA-R activation (Autry et al. 2011; Koike et al., 2011; Li et al. 2010; Maeng et al., 2008). Evidence that drugs such as AMPA potentiators possess antidepressant-like properties (see Alt et al., 2006) provides additional support to the hypothesis that a relative increase in AMPA-R activation may be critical to the antidepressant effects (Du et al., 2006). However, other studies question the universal need for AMPA activation in relation to NMDA antagonists’ antidepressant properties (Dybala et al., 2008; Popik et al., 2008). Lastly, a very recent study suggest the effects of ketamine are mediated through inhibition of spontaneous NMDAR-mEPSCs, leading to a rapid decrease in elongation factor 2 kinase activity permitting rapid increases in Brain Derived Neurotrophic factor (BDNF) translation (Autry et al., 2011).
Additional lines of evidence also support the view that increasing the function of AMPA-R may result in antidepressant action. First, it has been shown that AMPA-R activation increases the expression of BDNF (Lauterborn et al., 2000) and stimulates neurogenesis. Chronic treatments with different antidepressants have been shown to upregulate the membrane insertion of GluA1, GluA2/3 and synaptic expression of GluA1, and down-regulate the expression of GluA3 (Barbon et al., 2006; Du et al., 2004; Martinez-Turrillas et al., 2002). In contrast lithium was shown to reduce the synaptic expression of GluA1. As a consequence, AMPA-R potentiators (sometimes called AMPAkines) have been in development for some time as potential antidepressants.
A traditional limitation of strategies based on compounds targeting ionotropic glutamate receptors has been the presence of considerable side effects. An alternative has been offered by compounds affecting metabotropic glutamate receptors (mGlu), which are crucial for the regulation of glutamatergic neurotransmission as well as for other neurotransmitters involved in mood/anxiety disorders. Limited clinical proof of concept for mGlu receptor ligands in the treatment of affective disorders has been achieved. In particular, group II receptor agonists (mGlu2/3) and group I receptor antagonists (mGlu5) have shown activity in animal and/or human conditions of fear, anxiety, or stress (Wieronska and Pilc, 2009). Recently, much interest was raised on mGlu7 receptor, a presynaptic receptor at both glutamate and GABA terminals, highly expressed in hippocampus, amygdala and cortical regions (O’Connor et al., 2010). It would be interesting to assess whether mGlu7 has a role in the dampening effect of antidepressants on stress-induced glutamate release (see below).
8.3. Antidepressant agents interfere with the presynaptic release of glutamate
A number of studies have shown that chronic administration of antidepressant agents reduces the presynaptic release of glutamate. An early study showed that both chronic (3 weeks) and acute imipramine or phenelzine treatments significantly reduced depolarization-evoked glutamate overflow from slices of prefrontal cortex, while no effect was seen in striatal slices (Michael-Titus et al., 2000).
In a different work, by using the synaptosome superfusion technique, it was shown that chronic (2 weeks) treatments with different antidepressant agents (fluoxetine, desipramine, reboxetine) significantly reduced depolarization-evoked release of glutamate. GABA release was not modified by chronic treatments and release of neither amino acid was changed by acute treatments (Bonanno et al, 2005). This long-term adaptation in the mechanism of glutamate release was accounted for by changes in protein-protein interactions regulating the assembly of the presynaptic SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) protein complex (formed by synaptobrevin-2, syntaxin-1 and SNAP-25), that mediates fusion of synaptic vesicles with presynaptic membrane. In detail, in synaptic membranes of antidepressant-treated rats, phosphorylation of α-calcium/calmodulin-dependent protein kinase II (αCaMKII), a kinase enriched at synapses and involved in the mechanism of antidepressants (Barbiero et al., 2007; Celano et al., 2003), was markedly decreased. As a result, the binding of syntaxin-1 to αCaMKII was reduced and the binding to Munc-18 increased. This shift of interaction limits the participation of syntaxin-1 into the SNARE complex assembly and reduces depolarization-evoked release of glutamate (Agid et al., 2007; Bonanno et al., 2005).
A further study showed that chronic administration of imipramine reduced glutamatergic transmission in rat frontal cortex (Tokarski et al, 2008), with a reduction of the initial slope ratio of pharmacologically isolated NMDA-R to AMPA-R-mediated stimulation-evoked excitatory postsynaptic currents. Changes in postsynaptic glutamate receptors may represent a homeostatic mechanism compensating for alterations in neurotransmitter overflow. It was concluded that this depression of synaptic transmission induced by imipramine is likely due to an attenuation of glutamate release from presynaptic terminals.
In addition, application of fluoxetine in vitro for 10 min to Percoll-purified cerebrocortical synaptosomes dose-dependently (0.5–10 μM) inhibited 4-aminopyridine–evoked glutamate release, measured with a standard fluorimetric assay (Wang et al., 2003). This in vitro effect of fluoxetine was shown to be mediated by inhibition of P/Q-type calcium channels and not by a direct effect on the exocytotic machinery. Similar effects have been shown also with bupropion, a weak inhibitor of dopamine and noradrenaline reuptake (Lin et al., 2011), although it is difficult to correlate these effects in vitro of antidepressants with the outcome of chronic drug treatments.
8.4. Antidepressant agents prevent the stress-induced increase of glutamate release
The dampening effects of antidepressant agents on glutamate release discussed above prompted the hypothesis that these drugs may work by limiting the excess of glutamate overflow induced by stress (see section 7.1), which may also contribute to explain the anxiolytic action of antidepressants upon chronic treatment (Musazzi et al., 2011). If stress-induced enhancement of glutamate release and transmission is involved in the induction of functional changes and anatomical remodeling associated with depression, and antidepressants are able to reduce glutamate release, investigating whether these drugs are able to reduce or block stress-induced enhancement of glutamate release/transmission may reveal new targets for non-monoaminergic drugs. Recent studies analyzed this possibility.
First it was shown, by using microdialysis in vivo, that a single administration of the atypical antidepressant tianeptine, 30 min prior to acute restraint stress, completely abolished the increase of extracellular glutamate levels induced in basolateral amygdala by acute restraint stress (Reznikov et al., 2007). By contrast, acute administration of fluoxetine increased extracellular glutamate before the application of stress and amplified the glutamate levels when stress was applied. The stress-induced enhancement of glutamate levels was abolished by tetrodotoxin, strongly suggesting the procedure measures glutamate release dependent on neuronal activation. Accordingly, it was suggested that the acute fluoxetine-induced enhancement of glutamate levels may provide a potential mechanism for the anxiogenic properties of selective serotonin-reuptake inhibitors, often observed in the initial phases of treatment.
A second study, already mentioned above, investigated the effects of chronic treatment with different antidepressant agents on the enhancement of glutamate release induced in prefrontal/frontal cortex by acute footshock (FS)-stress, by using purified synaptosomes in superfusion and patch-clamp electrophysiology (Musazzi et al., 2010). The rats were treated for 2 weeks with four different antidepressants (fluoxetine, desipramine, venlafaxine, agomelatine), and then subjected to FS-stress, in order to assess the response to stress in drug-treated animals. All drugs nearly or completely prevented the stress-induced enhancement of depolarization-dependent glutamate release. Patch-clamp recordings in prefrontal cortex also showed that chronic desipramine treatment normalized the increase of sEPSCs amplitude, as well as the significant reduction of paired-pulse facilitation and its calcium-dependence measured in stressed rats. Interestingly, the different drugs used did not block the stress-induced rise of corticosterone level, showing the drug action is downstream of this mechanism. Also, it was shown that the increase of presynaptic SNARE complexes in presynaptic membranes and the increase in the readily releasable pool (RRP) of vesicles induced by acute stress were similar in drug- and vehicle-treated rats (Musazzi et al., 2010; Popoli et al., 2010). These results combined suggest that the action of drugs must be downstream of the increase in RRP, perhaps at the level of modulation of the SNARE complex function.
This work showed for the first time that chronically administered antidepressant agents are able to modify the response to acute stress (measured as glutamate release). This action likely represents a component of the therapeutic effect, perhaps with particular regard to the anxiolytic action of these drugs. It remains to be determined if this kind of action is typical of antidepressant agents. It is known that benzodiazepines have a similar dampening effect on glutamate release upon acute administration (Bagley and Moghaddam, 1997), and this, together with similar effects on noradrenaline, represents a clear n eurobiological substrate for their rapid anxiolytic action. However, pilot studies investigating the effect of chronic (21 days) treatment with lorazepam found that, while glutamate release from prefrontal cortex slices in superfusion was reduced, in hippocampus the same treatment resulted in a marked and significant increase of glutamate release (Bonavita et al., 2002; 2003). Further studies are in progress, to understand the long-term action of different psychiatric drugs on the release of glutamate.
9. Conclusions: future perspectives and novel targets
There is compelling accumulated evidence that long-term changes in an array of brain areas and circuits mediating complex cognitive-emotional behaviors represent the biological underpinnings of mood/anxiety disorders. As the vast majority of neurons and synapses in these areas and circuits use glutamate as neurotransmitter, today it would be limiting to maintain that glutamate is in some way ‘involved’ in these neuropsychiatric disorders; rather it should be recognized that the glutamatergic system is a primary mediator of psychiatric pathology and, potentially, also a final common pathway for the therapeutic action of antidepressant agents. A framework shift of this kind has already occurred for schizophrenia and addiction (Kalivas, 2009; Kantrowitz and Javitt, 2010). A limitation of this review article was that, for reason of space, we could not discuss at length a number of interesting drug targets in the glutamatergic system, including metabotropic and AMPA receptors.
In a way, this central role of glutamate was already implied in the neuroplasticity hypothesis of depression and mood disorders but, considering all the evidence that has been summarized here, openly stating this may represent a substantial progress in the working hypothesis that drives research for new drugs and therapies. What are the advantages offered by this research framework, alternative to the classic monoamine hypothesis?
First, despite the fact that ketamine and other NMDA antagonists seem to possess the long sought after properties of having both rapid and sustained antidepressant effects in previously treatment resistant patients, there are no drugs currently available for therapy of mood/anxiety disorders that act primarily on the glutamatergic system (with the possible exception of lamotrigine). This may suggest that our efforts should be shifted to the pursuit of new glutamatergic (or other alternative) avenues, rather than remaining on the well trodden path of monoaminergic drug development if we hope to identify truly novel drugs with unique and improved therapeutic profiles.
Second, we have registered in recent years a substantial improvement of our knowledge of neuroplastic changes associated with mood disorders, owing to the concomitant development of neuroimaging, cellular/molecular, physiological, behavioral technologies. Summarizing the evidence that was analyzed here from the point of view of a putative glutamate hypothesis, we know that pathophysiology is associated with dysfunction of the predominant glutamatergic system, measurable changes in glutamate release/transmission, malfunction in the mechanisms regulating clearance and metabolism of glutamate, and cytoarchitectural/morphological maladaptive changes in a number of brain areas mediating cognitive-emotional behaviors. Following further along this path is likely to give us a more detailed knowledge of the biological basis of mental disorders and, possibly, a new generation of drugs that more directly and specifically target the newly identified pathophysiological mechanisms and effectors identified.
Third, evidence obtained mostly from preclinical research with rodent models suggest that environmental stress, in particular through the action of corticosteroids, induces enhancement of excitatory transmission and possibly extra-synaptic glutamate spillover in a number of brain areas overlapping with those showing maladaptive changes in human studies. Viewing the relationship between stress and psychopathology through this lense may provide important new insights into the pathogenesis of mood and anxiety disorders. However, there are some gaps here between preclinical research with stress paradigms and clinical research. It is not clear how an enhancement of excitatory transmission may be causally related with the dendritic remodeling observed in rodent models, and whether this actually results in volume loss, as observed in neuroimaging studies in humans. Also, it is not clear yet if dendritic atrophy and remodeling is strictly a ‘pathological’ consequence of excessive glutamate transmission/spillover, or it is an adaptive protective strategy to reduce the excitatory input to postsynaptic neurons (Gorman and Docherty, 2010; McEwen, 2010). In hippocampus, obviously, the consequences of the inhibitory action of stress on neurogenesis and neurogenesis-related plasticity should be added to and integrated with the consequences of stress on structural remodeling (Duman, 2004). Fourth, research with drugs targeting glutamate release, clearance, or receptors, including riluzole, lamotrigine and ketamine, has shown that interfering with different sites of glutamate transmission regulation may result in behavioral changes similar to those elicited by classic monoaminergic drugs (Pittenger and Duman, 2008; Racagni and Popoli, 2008; Sanacora et al., 2008; Tokita et al., 2011). An intriguing finding was that a single infusion of a subanesthetic dose of ketamine was able to induce rapid and sustained antidepressant efficacy in depressed and treatment-resistant patients (Berman et al., 2000; Zarate et al., 2006), and rapidly reversed depressive-like behavior in the learned helplessness animal model with effects sustained for 2 weeks (Li et al., 2010). Recently, acute ketamine treatment was also shown to reverse behavioral and synaptic deficits induced by 21 days chronic unpredictable stress; similar effects were observed with a selective GluN2B antagonist (Li et al., 2011). Based on these results, it is tempting to speculate that drugs targeting select mechanisms in the glutamate system might bypass the traditional delay in the action of monoaminergic drugs, and show a much faster onset of action.
However, this strategy is not devoid of limitations and risks. Apart from cognitive and dissociative effects of ketamine, which limit its widespread application for treatment of depression, it is not clear yet whether ketamine targets mechanisms that are downstream of traditional antidepressant targets (whereby speeding up the therapeutic action), or its mechanism is entirely different from traditional drugs. Ketamine has been shown to affect rapidly a number of pathways (Autry et al., 2011; Li et al., 2010). Among these, ketamine activates the mammalian target of rapamycin (mTOR) pathway, leading to increased expression of some synaptic proteins and increased number and function of new synapses in the prefrontal cortex of rats, modifications reversing the synaptic deficits that result from exposure to stress (Li et al., 2010; 2011). Such a mechanism makes sense, if one envisages that repeated stress brings about a reduction in the number and activity of key glutamate synapses (e.g., medial prefrontal cortex), while activation of a key regulator of energy metabolism and cellular growth like mTOR may trigger the additional plasticity required to rescue synaptic contacts compromised by stress exposure. However, the possible risks involved in the stimulation of a pathway that is implicated in disease states where growth is deregulated and homeostasis is compromised, such as cancer, metabolic diseases and aging, cannot be underevaluated (Zoncu et al., 2011). It is also intriguing that, counterintuitively, rapamycin, the main mTOR inhibitor, was shown to exert antidepressant action in the forced swim test and tail suspension test, while sertraline showed antiproliferative activity due to the inhibition of the mTOR pathway (Lin et al., 2010; Cleary et al., 2008).
A reason for hope is that different sites of regulation of the glutamate synapse, such as NMDA receptors, AMPA receptors, glutamate transporters and metabotropic receptors are all targets of drugs in development: a process that makes the glutamate system a new frontier of psychopharmacology for depression and mood disorders. Therefore a paradigm shift in the role of glutamate in mood disorders and antidepressant action might bring new impulse to a field that, in spite of considerable advancement in the knowledge of pathophysiology, has not registered a comparable leap forward in available therapeutic treatments.
References
- Agid Y, Buzsáki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, Grace A, Lambert JJ, Manji H, Mayberg H, Popoli M, Prochiantz A, Richter-Levin G, Somogyi P, Spedding M, Svenningsson P, Weinberger D. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6:189–201. doi: 10.1038/nrd2217. [DOI] [PubMed] [Google Scholar]
- Almeida RF, Thomazi AP, Godinho GF, Saute JA, Wofchuk ST, Souza DO, Ganzella M. Effects of depressive-like behavior of rats on brain glutamate uptake. Neurochem Res. 2010;35:1164–1171. doi: 10.1007/s11064-010-0169-4. [DOI] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–88. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D’Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. European Neuropsychopharmacology. 1995;5:71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, Hellemann G, Vinters HV. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12:541–549. doi: 10.1111/j.1399-5618.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- Armario A, Escorihuela RM, Nadal R. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev. 2008;32:1121–1135. doi: 10.1016/j.neubiorev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Törk I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42:757–75. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiero VS, Giambelli R, Musazzi L, Tiraboschi E, Tardito D, Perez J, Drago F, Racagni G, Popoli M. Chronic antidepressants induce redistribution and differential activation of alphaCaM Kinase II between presynaptic compartments. Neuropsychopharmacol. 2007;32:2511–2519. doi: 10.1038/sj.npp.1301378. [DOI] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;25:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Jr, Cohen BM, Ongür D. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology (Berl) 2011;215:689–95. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- Begni B, Tremolizzo L, D’Orlando C, Bono MS, Garofolo R, Longoni M, Ferrarese C. Substrate-induced modulation of glutamate uptake in human platelets. Br J Pharmacol. 2005;145:792–799. doi: 10.1038/sj.bjp.0706242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.44. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Binesh N, Kumar A, Hwang S, Mintz J, Thomas MA. Neurochemistry of late-life major depression: a pilot two-dimensional MR spectroscopic study. J Magn Reson Imaging. 2004;20:1039–1045. doi: 10.1002/jmri.20214. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, Raiteri M, Racagni G, Popoli M. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavita CD, Bisagno V, Bonelli CG, Acosta GB, Rubio MC, Wikinski SI. Tolerance to the sedative effect of lorazepam correlates with a diminution in cortical release and affinity for glutamate. Neuropharmacology. 2002;42:619–625. doi: 10.1016/s0028-3908(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Bonavita C, Ferrero A, Cereseto M, Velardez M, Rubio M, Wikinski S. Adaptive changes in the rat hippocampal glutamatergic neurotransmission are observed during long-term treatment with lorazepam. Psychopharmacology (Berl) 2003;166:163–167. doi: 10.1007/s00213-002-1373-y. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–33. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Molecular Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–94. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- Castillo M, Kwock L, Courvoisie H, Hooper SR. Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. American Journal of Neuroradiology. 2000;21:832–838. [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Pan JW, Hetherington HP, Abi-Saab W, Zaveri HP, Vives KP, Krystal JH, Spencer SS, Spencer DD. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia. 2008;49:1358–1366. doi: 10.1111/j.1528-1167.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010;20:1327–1331. doi: 10.1002/hipo.20738. [DOI] [PubMed] [Google Scholar]
- Celano E, Tiraboschi E, Consogno E, D’Urso G, Mbakop MP, Gennarelli M, de Bartolomeis A, Racagni G, Popoli M. Selective regulation of presynaptic calcium/calmodulin-dependent protein kinase II by psychotropic drugs. Biol Psychiatry. 2003;53:442–449. doi: 10.1016/s0006-3223(02)01491-9. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Chaturvedi HK, Chandra D, Bapna JS. Interaction between N- methyl-D-aspartate receptor antagonists and imipramine in shock-induced depression. Indian J Exp, Biol. 1999;37:952–928. [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–73. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosi. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Research Bulletin. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Cousins JP, Harper G. Neurobiochemical changes from Taxol/Neupogen chemotherapy for metastatic breast carcinoma corresponds with suicidal depression. Cancer Letters. 1996;110:163–167. doi: 10.1016/s0304-3835(96)04486-2. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- Damasio AR. In: Descartes’ error: emotion, reason and the human brain. Putnam GP, editor. New York: 1994. [Google Scholar]
- Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, Venkatraman TN, Santoro E, Barnett S, McCracken J. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Vasconcellos-Bittencourt AP, Vendite DA, Nassif M, Crema LM, Frozza R, Thomazi AP, Nieto FB, Wofchuk S, Salbego C, da Rocha ER, Dalmaz C. Chronic stress and lithium treatments alter hippocampal glutamate uptake and release in the rat and potentiate necrotic cellular death after oxygen and glucose deprivation. Neurochem Res. 2011;36:793–800. doi: 10.1007/s11064-011-0404-7. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento CA, Nogueira CW, Borges VC, Rocha JB. Changes in [(3)H]-glutamate uptake into platelets from patients with bipolar I disorder. Psychiatry Res. 2006;141:343–347. doi: 10.1016/j.psychres.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Mapping the matrix: the ways of neocortex. Neuron. 2007;56:226–238. doi: 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Machado-Vieira R, Maeng S, Martinowich K, Manji HK, Zarate CA. Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov Today: Ther Strat. 2006;3:519–526. doi: 10.1016/j.ddstr.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–5. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Dybała M, Siwek A, Poleszak E, Pilc A, Nowak G. Lack of NMDA-AMPA interaction in antidepressant-like effect of CGP 37849, an antagonist of NMDA receptor, in the forced swim test. J Neural Transm. 2008;115:1519–20. doi: 10.1007/s00702-008-0128-2. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S, Gottesman II. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophrenia Research. 2004;69:317–323. doi: 10.1016/j.schres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Fontella FU, Vendite DA, Tabajara AS, Porciúncula LO, da Silva Torres IL, Jardim FM, Martini L, Souza DO, Netto CA, Dalmaz C. Repeated restraint stress alters hippocampal glutamate uptake and release in the rat. Neurochem Res. 2004;29:1703–1709. doi: 10.1023/b:nere.0000035805.46592.6c. [DOI] [PubMed] [Google Scholar]
- Francis PT, Poynton A, Lowe SL, Najlerahim A, Bridges PK, Bartlett JR, Procter AW, Bruton CJ, Bowen DM. Brain amino acid concentrations and Ca2+-dependent release in intractable depression assessed antemortem. Brain Research. 1989;494:315–324. doi: 10.1016/0006-8993(89)90600-8. [DOI] [PubMed] [Google Scholar]
- Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Frye MA, Watzl J, Banakar S, O’Neill J, Mintz J, Davanzo P, Fischer J, Chirichigno JW, Ventura J, Elman S, Tsuang J, Walot I, Thomas MA. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr. 2005;10:182–190. doi: 10.1017/s1092852900010038. [DOI] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Slowik A, McHugh P, Sobiecka B, Kozub J, Rich KE, Urbanik A, Szczudlik A. Single voxel proton magnetic resonance spectroscopy in post-stroke depression. Psychiatry Res. 2006;148:111–120. doi: 10.1016/j.pscychresns.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Docherty JP. A hypothesized role for dendritic remodeling in the etiology of mood and anxiety disorders. J Neuropsychiatry Clin Neurosi. 2010;22:256–264. doi: 10.1176/jnp.2010.22.3.256. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosi. 2008;11:868–70. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Yadav SK, Rangan M, Rathore RK, Thomas MA, Prasad KN, Pandey CM, Saraswat VA. Serum proinflammatory cytokines correlate with diffusion tensor imaging device metrics and (1)H-MR spectroscopy in patients with acute liver failure. Metab Brain Dis. 2010;25:355–61. doi: 10.1007/s11011-010-9206-x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biological Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, Gerhardt GA. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yang CH, Hsu KS. Do stress and long-term potentiation share the same molecular mechanisms? Mol Neurobiol. 2005;32:223–235. doi: 10.1385/MN:32:3:223. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosterois in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Archiv fur Psychiatrie und Nervenkrankheiten. 1982;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsumoto M, Togashi H, Tachibana K, Kemmotsu O, Yoshioka M. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anesthetized rats: an effect mediated via 5-HT1A receptors. Brain Res. 2003;959:165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, Shi X, Renshaw PF. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675–81. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Kucukibrahimoglu E, Saygin MZ, Caliskan M, Kaplan OK, Unsal C, Goren MZ. The change in plasma GABA, glutamine and glutamate levels in fluoxetine-or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol. 2009;65:571–577. doi: 10.1007/s00228-009-0650-7. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MJ, McLoughlin GA, Griffin JL, Tsang TM, Huang JT, Yuan P, Manji H, Holmes E, Bahn S. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2009;14:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosi. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–126. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Robert F, Sukarieh R, Michnick S, Pelletier J. The antidepressant sertraline inhibits translation initiation by curtailing mammalian target of rapamycin signaling. Cancer Res. 2010;70:3199–208. doi: 10.1158/0008-5472.CAN-09-4072. [DOI] [PubMed] [Google Scholar]
- Lin TY, Yang TT, Lu CW, Wang SJ. Inhibition of glutamate release by bupropion in rat cerebral cortex nerve terminals. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:598–606. doi: 10.1016/j.pnpbp.2010.12.029. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosi. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. J Affect Disord. 2010;128:309–13. doi: 10.1016/j.jad.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lowy M, Gault L, Yamamoto B. Adrenolectomy attenuates stress induced elevation in extracellular glutamate concentration in hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Psychosomatic disease and the ‘visceral brain’: recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4- propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatrica Scandinavica. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, Joëls M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Frechilla D, Del Río J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Tachibana K, Togashi H, Tahara K, Kojima T, Yamaguchi T, Yoshioka M. Chronic treatment with milnacipran reverses the impairment of synaptic plasticity induced by conditioned fear stress. Psychopharmacology(Berl) 2005;179:606–612. doi: 10.1007/s00213-004-2094-1. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003a;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychological Medicine. 2003b;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W, Pfleiderer B. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl) 2003c;168:344–346. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- Michael-Titus AT, Bains S, Jeetle J, Whelpton R. Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex--a possible mechanism of neuroprotection in major depression? Neuroscience. 2000;100:681–684. doi: 10.1016/s0306-4522(00)00390-0. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosi. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biological Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza Y, Tang J, Russell A, Banerjee SP, Bhandari R, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J Am Acad Child Adolesc Psychiatry. 2004;43:341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnie-Filali O, El Mansari M, Espana A, Sànchez C, Haddjeri N. Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neurosci Lett. 2006;395:23–27. doi: 10.1016/j.neulet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Fayed N, Artal J, Olmos S. Correlation of findings in advanced MRI techniques with global severity scales in patients with Parkinson disease. Acad Radiol. 2011;18:235–41. doi: 10.1016/j.acra.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learn-ing after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Racagni G, Popoli M. Environmental stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.05.002. (in press) [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse. 2001;42:151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J Pharmacol Exp Ther. 1993;265:1380–1386. [PubMed] [Google Scholar]
- O’Connor RM, Finger BC, Flor PJ, Cryan JF. Metabotropic glutamate receptor 7: at the interface of cognition and emotion. Eur J Pharmacol. 2010;639:123–131. doi: 10.1016/j.ejphar.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, Mori K, Togashi H, Ueno K, Kaku A, Yoshioka M. Changes in synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joëls M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosi. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: a critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56:539–555. doi: 10.1016/0306-4522(93)90355-j. [DOI] [PubMed] [Google Scholar]
- Othmer E, Othmer JP, Othmer SC. Brain functions and psychiatric disorders. A clinical view. Psychiatr Clin North Am. 1998;21:517–566. doi: 10.1016/s0193-953x(05)70022-8. [DOI] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pittaluga A, Raiteri L, Longordo F, Luccini E, Barbiero VS, Racagni G, Popoli M, Raiteri M. Antidepressant treatments and function of glutamate ionotropic receptors mediating amine release in hippocampus. Neuropharmacol. 2007;53:27–36. doi: 10.1016/j.neuropharm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacol. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Popik P, Kos T, Sowa-Kućma M, Nowak G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacol. 2008;198:421–30. doi: 10.1007/s00213-008-1158-z. [DOI] [PubMed] [Google Scholar]
- Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002;4:166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- Popoli M, Musazzi L, Perego C, Mallei A, Villa A, Milanese M, Treccani G, Tardito D, Corbelli A, Racagni G, Bonanno G. Acute behavioural stress affects the readily releasable pool of vesicles in prefrontal/frontal cortex. Soc Neurosci. 2010:Abstr 667.7. [Google Scholar]
- Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. 2008;162:113–121. doi: 10.1016/j.pscychresns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Pralong E, Magistretti P, Stoop R. Cellular perspectives on the glutamate-monoamine interactions in limbic lobe structures and their relevance for some psychiatric disorders. Prog Neurobiol. 2002;67:173–202. doi: 10.1016/s0301-0082(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, McEwen B, Jay TM, Svenningsson P. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacol. 2009;56:37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M. Cellular and molecular mechanisms in thelong-term action of antidepressants. Dial Clin Neurosci. 2008;10:385–400. doi: 10.31887/DCNS.2008.10.4/gracagni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–92. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosi. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Macmaster FP, Mirza Y, Smith JM, Easter PC, Banerjee SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Villafuerte RA, Moore GJ, Renshaw P. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Ryan B, Musazzi L, Mallei A, Tardito D, Gruber SH, El Khoury A, Anwyl R, Racagni G, Mathé AA, Rowan MJ, Popoli M. Remodelling by early-life stress of NMDA receptor-dependent synaptic plasticity in a gene-environment rat model of depression. Int J Neuropsychopharmacol. 2009;12:553–9. doi: 10.1017/S1461145708009607. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of GABA and glutamate in major depression. Archives of General Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh E, Shimeki S. Acute restraint stress enhances calcium mobilization and glutamate exocytosis in cerebrocortical synaptosomes from mice. Neurochem Res. 2010;35:693–701. doi: 10.1007/s11064-009-0120-8. [DOI] [PubMed] [Google Scholar]
- Schildraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotoner-gic and antidepressant agents. J Neurosi. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gómez AB, Rojas D, Juárez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Antidepressants for the new millennium. Eur J Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–76. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Poleszak E, Sowa-Kućma M, Siwek M, Dudek D, Ryszewska-Pokraśniewicz B, Radziwoń-Zaleska M, Opoka W, Czekaj J, Pilc A, Nowak G. Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep. 2008;60:588–9. [PubMed] [Google Scholar]
- Tachibana K, Matsumoto M, Togashi H, Kojima T, Morimoto Y, Kemmotsu O, Yoshioka M. Milnacipran, a serotonin and noradrenaline reuptake inhibitor, suppresses long-term potentiation in the rat hippocampal CA1 field via 5-HT1A receptors and alpha 1-adreno- ceptors. Neurosci Lett. 2004;357:91–94. doi: 10.1016/j.neulet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Mannie ZN, Norbury R, Near J, Cowen PJ. Elevated cortical glutamate in young people at increased familial risk of depression. Int J Neuropsychopharmacol. 2011;14:255–259. doi: 10.1017/S1461145710001094. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Bobula B, Wabno J, Hess G. Repeated administration of imipramine attenuates glutamatergic transmission in rat frontal cortex. Neuroscience. 2008;153:789–795. doi: 10.1016/j.neuroscience.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Tokita K, Yamaji T, Hashimoto K. Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.04.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Toro CT, Hallak JE, Dunham JS, Deakin JF. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett. 2006;404:276–281. doi: 10.1016/j.neulet.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Beretta S, Ferrarese C. Peripheral markers of glutamatergic dysfunction in neurological diseases: focus on ex vivo tools. Crit Rev Neurobiol. 2004;16:141–146. doi: 10.1615/critrevneurobiol.v16.i12.150. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G, Malatynska E. Central GABAergic systems and depressive illness. Neurochemical Research. 2003;28:965–976. doi: 10.1023/a:1023287729363. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–7. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–147. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosi. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Kamal A, Reijmers LG, Schrama LH, van den Bos R, Spruijt BM. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett. 2001;309:153–156. doi: 10.1016/s0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosi. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang X, Li YH, Li MH, Lu J, Zhao JG, Sun XJ, Zhang B, Ye JL. Glutamate level detection by magnetic resonance spectroscopy in patients with post-stroke depression. Eur Arch Psychiatry Clin Neurosci. 2011 doi: 10.1007/s00406-011-0209-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang SJ, Su CF, Kuo YH. Fluoxetine depresses glutamate exocytosis in the rat cerebrocortical nerve terminals (synaptosomes) via inhibition of P/Q-type Ca2+ channels. Synapse. 2003;48:170–177. doi: 10.1002/syn.10200. [DOI] [PubMed] [Google Scholar]
- Wang CC, Wang SJ. Modulation of presynaptic glucocorticoid receptors on glutamate release from rat hippocampal nerve terminals. Synapse. 2009;63:745–751. doi: 10.1002/syn.20654. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem Int. 2009;55:85–97. doi: 10.1016/j.neuint.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto B, Reagan LP. The glutamatergic system in neuronal plasticity and vulnerability in mood disorders. Neuropsych Dis Treat. 2006;2(Suppl 2):7–14. [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E, Wei J, Yan Z. Repeated stress suppresses glutamate receptor expression and function in prefrontal cortex and impairs object recognition memory. Soc Neurosci. 2010:Abstr. 389.21. [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate- related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–94. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–73. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Zoia C, Cogliati T, Tagliabue E, Cavaletti G, Sala G, Galimberti G, Rivolta I, Rossi V, Frattola L, Ferrarese C. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer’s disease. Neurobiol Aging. 2004;25:149–57. doi: 10.1016/s0197-4580(03)00085-x. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2010;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J, Bayatti N, Clement AM, Witan H, Figiel M, Engele J, Behl C. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J Biol Chem. 2005;280:34924–32. doi: 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]