Abstract
Bax is a pro-death protein that plays a crucial role in developmental neuronal cell death. Bax−/− mice exhibit increased neuron number and the elimination of several neural sex differences. Here we examined the effects of Bax gene deletion on social behaviors (olfactory preference, social recognition, social approach, and aggression) and the neural processing of olfactory cues. Bax deletion eliminated the normal sex difference in olfactory preference behavior. In the social recognition test, both genotypes discriminated a novel conspecific, but wildtype males and Bax−/− animals of both sexes spent much more time than wildtype females investigating stimulus animals. Similarly, Bax−/− mice were more sociable than wildtype mice in a social approach test. Bax deletion had no effect on aggression in a resident/intruder paradigm where males, regardless of genotype, exhibited a shorter latency to attack. Thus, the prevention of neuronal cell death by Bax gene deletion results in greater sociability as well as the elimination of sex differences in some social behaviors. To examine olfactory processing of socially relevant cues, we counted c-Fos immunoreactive (Fos-ir) cells in several nodes of the accessory olfactory pathway after exposure to male-soiled or control bedding. In both genotypes, exposure to male-soiled bedding increased Fos-ir cells in the posterodorsal medial amygdala, principal nucleus of the bed nucleus of the stria terminalis, and medial preoptic nucleus (MPN), and the response in the MPN was greater in females than in males. However, a reduction in Fos-ir cells was seen in the anteroventral periventricular nucleus of Bax−/− mice.
Keywords: cell death, c-Fos, olfactory preference, sex difference
Developmental neuronal cell death is critically controlled by Bax, a member of the Bcl-2 protein family (Merry & Korsmeyer, 1997). Deletion of the Bax gene nearly eliminates neuronal cell death in postnatal mice (White et al., 1998). We previously reported that Bax deletion also eliminates neural sex differences that depend on differential cell death in males and females. For example, male mice normally have more neurons than females in the principal nucleus of the bed nucleus of the stria terminalis (BNSTp) and spinal nucleus of the bulbocavernosus (Forger et al., 2004; Holmes et al., 2009; Wee & Clemens, 1987). These sex differences correlate with greater cell death perinatally in females (Nordeen et al., 1985; Chung et al., 2000; Gotsiridze et al., 2007) and are eliminated in Bax−/− mice (Forger et al., 2004; Jacob et al., 2005). Conversely, females have more cells than males in the anteroventral periventricular nucleus (AVPV) and this sex difference also is eliminated in Bax−/− animals (Forger et al., 2004).
Bax deletion does not affect all neural sex differences, however. In the BNSTp, sex differences in NeuN-positive and androgen receptor expressing cells are absent in Bax knockout mice (Holmes et al., 2009), but this is not true for cells expressing vasopressin, a neuropeptide important in the control of social behaviors: Bax−/− mice of both sexes have increased vasopressin cell number in the BNST, but the sex difference (male > female) remains (De Vries et al., 2008). Similarly, in AVPV, Bax deletion eliminates the sex difference in total cell number but not sex differences in tyrosine hydroxylase- or kisspeptin-expressing cell number (Forger et al., 2004; Semaan et al., 2010).
Thus, Bax−/− animals present an interesting mosaic in which some sex differences are eliminated and others remain. This raises the question of whether sexually dimorphic behaviors are affected in Bax−/− mice. The behavior of Bax−/− mice is grossly normal (e.g., Knudson et al., 1995; Buss et al., 2006b). However, we found that Bax deletion eliminates the sex difference in female sexual receptivity (Jyotika et al., 2007). Many social behaviors are also sexually differentiated in mice and these behaviors are highly dependent on olfaction. For example, males prefer to chemoinvestigate sexually receptive females or bedding soiled by females, whereas females prefer to investigate males (e.g., Bodo & Rissman, 2007; Keller et al., 2006; Pankevich et al., 2006). The response to socially relevant olfactory cues (i.e., pheromones) begins with detection in the vomeronasal organ; information is then conveyed to the accessory olfactory bulb and, from there, to the medial amygdala and BNST (Baum, 2009), which in turn sends a strong projection to AVPV (Simerly, 2002).
Given the effects of Bax deletion on overall cell number in the BNSTp and AVPV, and on vasopressin cell number described above, we hypothesized that social behaviors might be altered in Bax−/− mice. Here we compared four social behaviors in Bax−/− and wildtype mice and used c-Fos immunohistochemistry to examine neural activity in response to olfactory cues within central projection sites of the vomeronasal circuitry.
Materials and Methods
Animals and housing
Wildtype (Bax+/+) and Bax knockout (Bax−/−) mice were generated in our colony by pairing mice heterozygous for the Bax gene deletion on a C57Bl/6J background. Genotyping was performed by PCR amplification of ear punch DNA using established primer sequences (White et al., 1998). Animals were single housed at approximately 8 weeks of age in a 12:12 light/dark cycle with ad libitum access to food and water in cages containing corn cob bedding. All procedures adhered to institutional and federal guidelines for the care and use of animals in research and were approved by the University Animal Care Committee at the University of Toronto. All behavior scoring and tissue analyses were performed by experimenters blind to the genotype of the animals. Experimenters were also blind to sex with the exception of tests where scoring was done live (e.g., social recognition and resident/intruder).
Social behavior tests
Between 10 and 14 weeks of age, a single cohort of animals (wildtype males: N = 12; wildtype females: N = 11; Bax−/− males: N = 12; Bax−/− females: N = 9) underwent a series of four behavioural tests. All animals were tested in the same order: olfactory preference, social recognition, social approach, and resident/intruder, as described below. All behavior testing was performed during the light phase of the animals’ light:dark cycle under dim light conditions and both subject and stimulus animals were transferred to test rooms at least one hour prior to testing. Testing apparatuses were cleaned with ethanol between each test.
Olfactory Preference
At 10 weeks of age, all animals were tested for olfactory preference (similar to that described in Bodo and Rissman, 2007). Testing consisted of a habituation trial followed by a test trial. Mice were individually transferred to a 10L glass aquarium with three circular ceramic containers (4 cm high × 8 cm diameter) located equidistant from each other and the aquarium walls. The aquarium and containers contained clean bedding and mice were allowed to freely explore the environment for a 10 min habituation period. Animals were then briefly returned to their home cage and the three containers were replaced with one containing female-soiled bedding (see below), one containing male-soiled bedding (see below), and one with clean bedding; the order of containers within the aquarium was randomized across animals. Animals were videotaped for 10 minutes and the time spent investigating each container was later scored from the recordings. A preference score was calculated for each animal by subtracting the total time spent investigating male-soiled bedding from the total time spent investigating female-soiled bedding.
Soiled bedding was collected from stimulus male and female mice heterozygous for the Bax gene. Four stimulus females were ovariectomized at least one month prior to test day. They were group housed and each received a single subcutaneous injection of estradiol benzoate (0.5μg in 0.05ml sesame oil) three days and two days prior to test day. The day before test day, stimulus females received a single subcutaneous injection of progesterone in sesame oil (0.05ml of 200μg/10ml) to induce estrus. Bedding was not changed during this period and was collected on the morning of test day. Male-soiled bedding was collected from the cages of four sexually experienced, gonadally-intact, single-housed males that had not been changed for four days. Bedding from all males was combined at the time of collection. Clean bedding was taken directly from vivarium stock.
Social Recognition
At 11 weeks of age all animals were tested for social recognition ability using a modification of the habituation/dishabituation paradigm described by Imwalle et al. (2002). The test consisted of 12 one-minute trials, each separated by five minutes. For the first 11 trials, the same stimulus animal was placed into the home cage of the experimental animal. On the twelfth trial, a novel stimulus animal was used. Stimulus animals were ovariectomized adult female mice heterozygous for the Bax gene that were placed into the subject’s home cage within a small 8.5 cm × 8.5 cm plastic aerated container. This allowed for chemoinvestigation of the stimulus animal without the interference of sexual or aggressive behavioral interactions. For all trials, the time spent investigating the stimulus animal was recorded live.
Social Approach
At 12.5 weeks of age, all animals were tested for sociability in a test similar to that described by Moy et al. (2004). Briefly, each mouse was placed in a 10L glass aquarium visually divided into three equal compartments using tape and containing two 7 cm × 24 cm aerated plastic cages, one at each end. The aquarium and cages contained clean bedding and mice were allowed to freely explore the environment for a 10 min habituation period. Following this, animals were briefly returned to their home cage and an unfamiliar stimulus animal (an ovariectomized adult female heterozygous for the Bax gene) was placed in one of the two plastic cages. Experimental animals were then returned to the aquarium, allowed to freely explore, and were videotaped for 10 minutes. Videotapes were scored by recording the time spent in each compartment: the two ends of the aquarium (one containing a stimulus mouse within a plastic cage and one containing an empty plastic cage) and the center of the aquarium. Sociability was calculated by subtracting the time spent in proximity to the empty cage from the time spent in proximity to the cage containing the stimulus animal.
Resident/Intruder
At 14 weeks of age, all animals were evaluated in a resident/intruder paradigm once a day for three consecutive days. Testing was performed in the home cage of the experimental animal. The intruders were 6–7 week old gonadally-intact, sexually-inexperienced male mice heterozygous for the Bax gene. Intruders were placed directly into the home cage of the experimental animal for 6 minutes or until the first attack occurred; latency for the resident to attack was scored live and averaged over the three tests.
Neural activation following exposure to olfactory cues
Two cohorts of Bax+/+ and Bax−/− mice were examined for neural activation following exposure to a socially relevant cue. One cohort was comprised of the mice run in the four behavior tests described above. The second cohort was age-matched and housed identically, but did not undergo previous behavior testing. Both cohorts were 15 weeks of age at testing and were equally distributed across the 12 experimental groups in this experiment (2 sexes × 2 genotypes × 3 olfactory stimuli). Because no significant effect of cohort was identified, data from both cohorts are combined in the data presented below; final N was between 8–12 animals for each sex × genotype × stimulus group.
Animals were transferred in their home cages to a testing room one day prior to testing. On the test day, a single ceramic container (4 cm high × 8 cm diameter) filled with either male-soiled bedding, vanilla-scented bedding, or clean bedding, was placed into the home cage of each experimental animal. This allowed us to compare the neural response to a socially-relevant odor (male-soiled bedding) with response to a novel odor that presumably has no social relevance. Male-soiled bedding was collected on test day, as described for Experiment 1 and vanilla-scented bedding was made on test day by mixing artificial vanilla extract with clean bedding. Ninety minutes after exposure, animals were weighed, deeply anesthetized, and transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were extracted, post-fixed for 2 hours and transferred to 30% sucrose in phosphate buffer for cryoprotection. Brains were then blocked (cerebellum and olfactory bulbs removed) and frozen-sectioned at 30 μm in the coronal plane into four series, which were stored at −20°C until processing.
c-Fos immunohistochemistry
Alternate sections (i.e., two of the four series) were processed for immunohistochemical detection of c-Fos, a marker of neural activity. Tissue was rinsed 3×5 minutes in PBS, and incubated for 90 minutes in TX100 [PBS + 4% normal goat serum (NGS) + 0.3% Triton X + 0.3% H2O2] at room temperature. Next, tissue was incubated in c-Fos primary antibody [1:500 rabbit anti-c-Fos polyclonal antibody (Santa Cruz Biotechnology, Inc., catalogue number sc-52)] in TX100 without H2O2 for approximately 20 hours at 4°C. Secondary antibody incubation [1:200 goat anti-rabbit (Vector Laboratories, Burlingame, CA) in TBS with 2% NGS and 0.3% Triton X for 90 min at room temperature] was followed by a 90 min ABC incubation (Vector Laboratories, Burlingame, CA). c-Fos immunoreactivity was visualized using a diaminobenzidine reaction and tissue was rinsed 3×5 min with PBS between all steps. Tissue was then mounted onto gel coated slides, counter-stained with methyl green, dehydrated and coverslipped using Permount (Fisher Scientific).
Brain analyses
All analyses were performed on slides coded to conceal the sex and genotype of the animals. The number of c-Fos immunoreactive (Fos-ir) cells was counted in the posterodorsal portion of the medial amygdala (MePD), the BNSTp, the medial preoptic nucleus (MPN), and the AVPV. Cell counts were obtained by placing a 430 μm × 325 μm sampling area on the region of interest and counting all immunoreactive cells within that area. The counter stain and other obvious anatomical features (e.g., ventricle borders) were used to ensure that counts only included cells within each region of interest; this was particularly important for the AVPV, which is smaller in cross sectional area than the counting frame. Cell counts were performed bilaterally in two sections for all brain areas. MePD counts were performed in tissue sections corresponding to bregma −1.70 mm to −1.81 mm [plates 45 and 46 in the Franklin & Paxinos (2008) mouse brain atlas]; BNSTp counts were performed in tissue sections corresponding to bregma −0.10 mm to −0.22 mm (plates 32 and 33); MPN and AVPV counts were performed in tissue sections corresponding to bregma 0.14 mm to 0.02 mm (plates 30 and 31).
Statistical analyses
Olfactory preference scores, time spent investigating the stimulus animal in the affiliation test and latency to attack in the resident/intruder paradigm were analyzed by two-way ANOVAs (sex-by-genotype). Time spent investigating stimulus animals for the social recognition test was analyzed using a sex-by-genotype-by-trial repeated measures ANOVA and the number of c-Fos immunoreactive cells in each brain area was analyzed by three-way ANOVAs (sex-by-genotype-by-olfactory stimulus). Planned comparisons using Fisher’s LSD were performed following significant main effects or interactions in the ANOVAs.
Results
Social behaviors
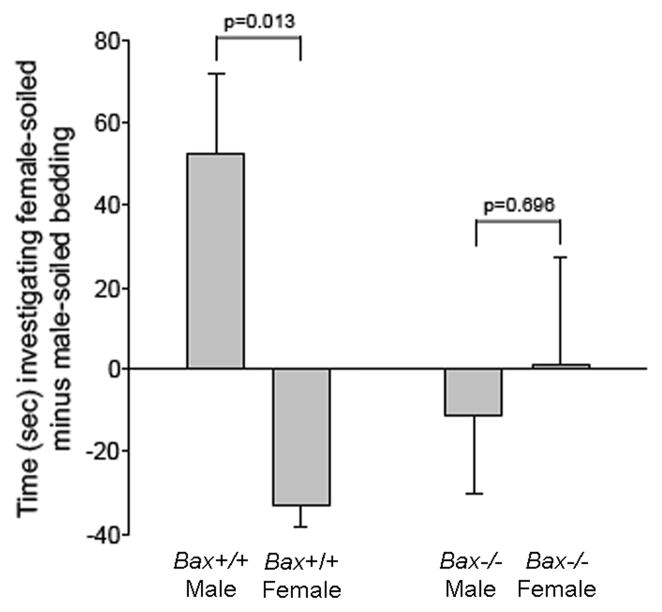
ANOVA revealed no significant main effect of sex or genotype, but a significant sex-by-genotype interaction on olfactory preference behavior (F1,19 = 4.97; p = 0.038) (Figure 1). As expected, wildtype mice showed a sex difference, with male subjects preferring to investigate female-soiled bedding and females showing a preference for male-soiled bedding (p = 0.013). In contrast, no preference was seen in Bax−/− animals of either sex and the sex difference in olfactory preference behavior was eliminated by Bax gene deletion.
Figure 1.
Mean (+SEM) time spent investigating female-soiled bedding minus time spent investigating male-soiled bedding. A significant sex-by-genotype interaction was detected: the sex difference present in wildtype animals was eliminated by Bax gene deletion.
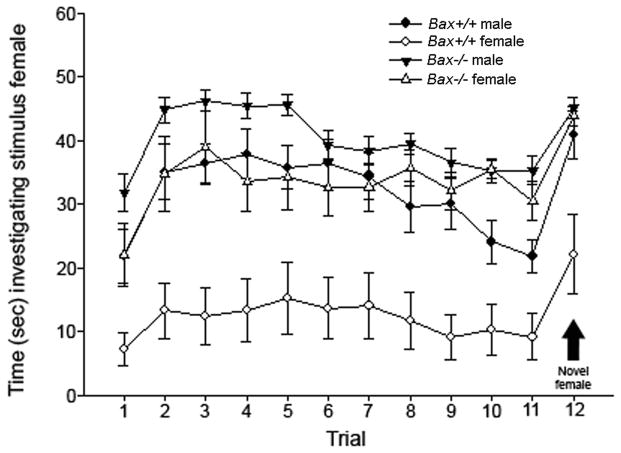
In the social recognition test, all four groups showed a significant increase in the time spent investigating the stimulus animal between Trial 11 (last habituation trial) and Trial 12 (introduction of a novel female; all p-values < 0.001). Thus, all animals were capable of short term social recognition memory. However, wildtype males and Bax−/− mice of both sexes spent much more time investigating the stimulus animal during the habituation trials than did wildtype females (Figure 2). This trend was evident beginning with the first trial and persisted over 11 repeated presentations of the stimulus animal: during habituation trials, wildtype females spent 129 ± 45 seconds investigating the stimulus animal, compared to 343 ± 33 seconds in wildtype males (p = 0.0009). Bax−/− females investigated the stimulus animal much more than wildtype females (p= 0.0008) and were similar to wildtype males on this measure (362 ± 32 seconds). Bax−/− males spent even more time investigating the stimulus animals (437 ± 13) than either wildtype males or Bax−/− females (p = 0.014 and p = 0.026, respectively). In the overall ANOVA, this resulted in both a main effect of sex (Figure 2; F1,40 = 19.63; p < 0.0001) as well as a sex-by-genotype interaction (F1,40 = 4.49; p = 0.04) on time spent investigating the stimulus animal.
Figure 2.
Mean (+/− SEM) time spent investigating the same stimulus animal over 11 habituation trials. On the twelfth trial, a novel stimulus animal was introduced. All groups showed increased chemoinvestigation towards the novel stimulus animal.
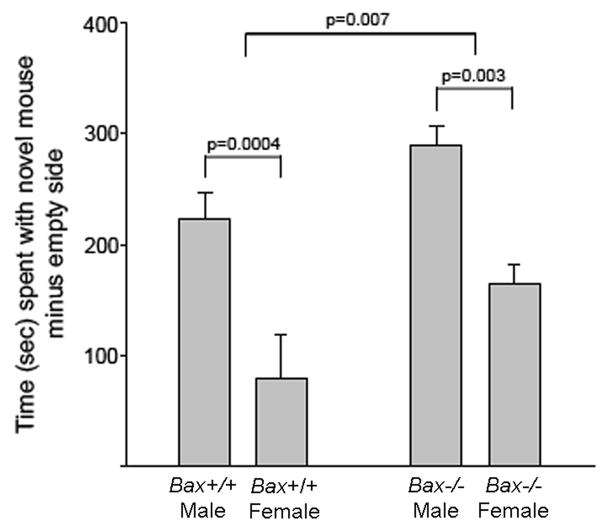
In the social approach test, we found significant main effects of both sex and Bax gene status. Males showed a greater preference for the compartment containing an unfamiliar stimulus animal than did females (F1,40 = 24.86; p < 0.001; Figure 3). In addition Bax−/− mice were more affiliative than wildtype mice (F1,40 = 7.97; p = 0.007), with no significant sex-by-genotype interaction (F1,40 = 0.11; p = 0.74).
Figure 3.
Mean (+SEM) sociability score (time spent in proximity to a cage containing a novel stimulus animal minus time spent in proximity to an empty cage). Overall, males spent significantly more time than females and Bax−/− mice spent more time than wildtype mice in contact with the stimulus animal.
Levels of aggression were relatively low across all groups in the resident / intruder test and no female of either genotype attacked. Males attacked with a mean latency of 299 ± 25 seconds (wildtype) and 316 ± 31 seconds (Bax−/−). In the ANOVA this resulted in a significant sex difference (F1,36 = 8.52; p = 0.006), no significant effect of genotype (F1,36 = 0.24; p = 0.62) and no sex-by-genotype interaction (F1,36 = 0.24; p = 0.62).
Neural activation
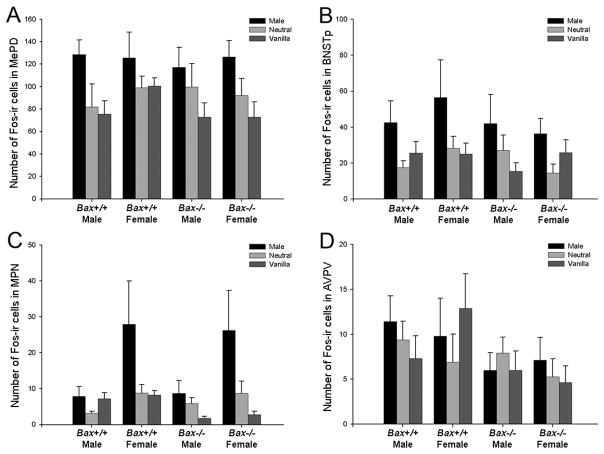
The density of Fos-ir cells was measured in the MePD, BNSTp, MPN, and AVPV after exposure to male-soiled, vanilla-scented or unscented (clean) bedding (Figure 4). In the MePD (F2,101 = 7.93; p = 0.001), BNSTp (F2,106 = 6.31; p = 0.003), and MPN (F2,100 = 7.492; p = 0.001), more Fos-ir cells were found in animals exposed to male-soiled bedding than those exposed to either vanilla-scented or unscented bedding (Figure 5A,B,C); the Fos response to vanilla-scented versus unscented bedding was not significantly different. This is consistent with previous work in rodents demonstrating activation in the MePD, BNSTp and MPN in response to pheromonal cues (e.g., Bakker et al., 1996; Fiber & Swann, 1996; Halem et al., 1999; Bodo and Rissman 2008). There was no significant effect of genotype and no stimulus-by-genotype interaction on c-Fos-immunoreactivity in the MePD, BNSTp, or MPN.
Figure 4.
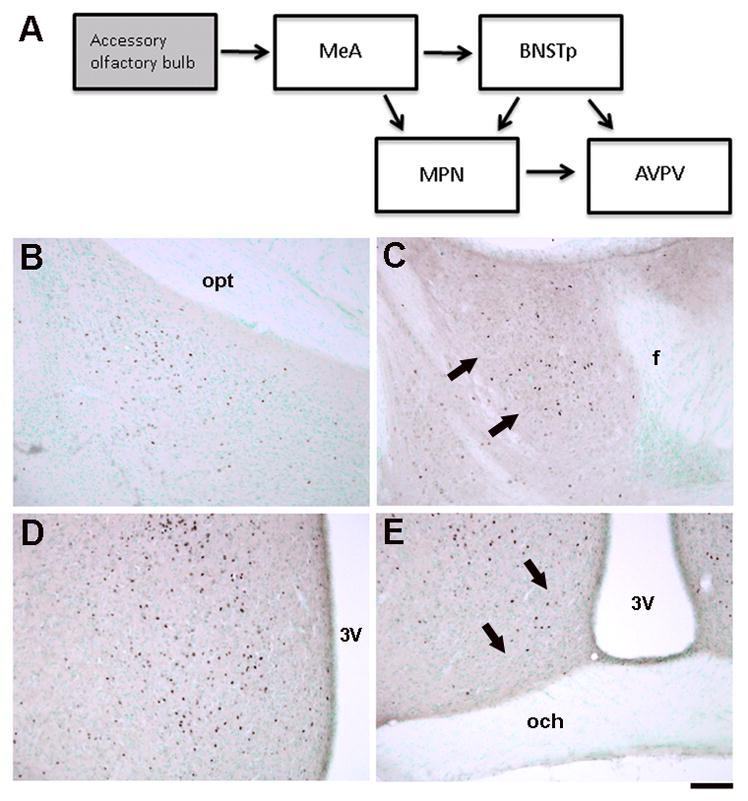
A) Schematic illustrating neural regions examined for c-Fos immunoreactivity after exposure to male-soiled bedding in the current study. The medial amygdala (MeA) receives direct projections from the accessory olfactory bulb. It projects to the BNSTp and, to a lesser extent, to the MPN. The BNSTp and MPN in turn project to the AVPV. Not all projections are shown and some, if not all, of the connections are bi-directional. B–D) Photomicrographs of c-Fos-immunoreactive cells in the MePD (A), BNSTp (B), MPN (C), and AVPV (D). 3V = 3rd ventricle; f = fornix; och = optic chiasm; opt = optic tract. Scale bar = 100 μm.
Figure 5.
Mean (+SEM) number of Fos-ir cells in the MePD (A), BNSTp (B), MPN (C), and AVPV (D). Male-soiled bedding increased Fos-ir in the MePD, BNSTp, and MPN; in the MPN this effect was seen only in female mice. Overall, Bax−/− mice had fewer Fos-ir cells in the AVPV than wildtype controls. Neutral = clean bedding.
No effect of sex or interaction of sex with other variables on Fos-ir cell number was detected in the MePD or BNSTp (Figure 5A,B). In contrast, a significant main effect of sex (F1,100 = 7.53; p = 0.007) and a sex-by-stimulus interaction (F2,100 = 3.50; p = 0.034) were seen in the MPN: the density of Fos-ir cells was higher in the MPN of females than in males, and this was true only in response to male-soiled bedding (Figure 5C).
In the AVPV, male-soiled bedding did not elicit a greater response than the other olfactory cues (and the density of Fos-ir cells was relatively low). However, Bax genotype influenced c-Fos-immunoreactivity (F1,100 = 5.01; p = 0.027), with fewer Fos-ir cells in AVPV of Bax−/− than wildtype animals (Figure 5D). There was no effect of sex and no sex-by-stimulus or sex-by-genotype interaction in this region.
Discussion
Deletion of the Bax gene results in the near complete suppression of developmental neuronal cell death; nonetheless, the appearance of Bax−/− mice as well as the structure and function of the nervous system are surprisingly normal (Knudson et al., 1995; Buss et al., 2006b). Most sensory and motor functions are unaffected (Buss et al., 2006a; Kim et al., 2007; Kim et al., 2009) and young adult Bax knockouts exhibit normal pre-pulse inhibition (cited as unpublished data in Kim et al., 2009), spatial learning ability, object recognition, fear conditioning and behavior in an open field (Perez et al., 2007; Kim et al., 2009). Sex differences were not investigated in the studies listed above, which either examined one sex or did not specify the sex of animals used. We previously observed that several neural sex differences are eliminated in Bax−/− mice within the neural circuitry that processes socially and sexually relevant cues (Forger et al., 2004; Holmes et al., 2009). Here we report that Bax gene deletion reduced or eliminated sex differences in olfactory preference and the time spent investigating conspecifics in a social recognition task. Sociability was also increased in Bax−/− animals in a social approach test. Thus, the prevention of developmental cell death alters social behaviors in mice.
One limitation of the current findings is that animals were gonadally intact and circulating gonadal hormones could have affected some behavioral measures. For example, the castration of male rats or mice reduces the time spent investigating conspecifics in tasks similar to those used here (Thor, 1980; Bluthé et al., 1993). Thus, the sex differences in time spent investigating stimulus animals in the social recognition and social approach tests may at least in part be due to activational effects of hormones. However, there is currently no evidence to suggest that gonadal hormone levels differ between Bax−/− and wildtype mice. Testosterone levels are unchanged in Bax−/− mice of both sexes (Forger et al., 2004), and seminal vesicle weights and anogenital distance (indirect measures of adult and developmental androgen exposure, respectively) are not affected in Bax−/− males (Forger et al., 2004). Similarly, although Bax−/− females have excess ovarian follicles, circulating estradiol levels do not differ (Perez et al., 2007; with the caveat that measures reported were for older adults). Thus, although we cannot rule out subtle hormonal changes in Bax−/− mice, circulating hormones do not seem likely to explain the effects of Bax deletion seen here. Indeed, social recognition memory and time spent investigating conspecifics during habituation trials does not vary across the estrous cycle in C57Bl/6 mice (Sanchez-Andrade & Kendrick, 2011), and the presence or absence of gonadal hormones does not influence c-Fos response to a socially relevant olfactory cue (Halem et al., 1999; see further discussion below), suggesting that these measures are relatively unaffected by variations in circulating steroids.
Consistent with previous findings (Bodo & Rissman, 2007; 2008), wildtype males in the current study preferred to investigate the bedding soiled by females, and wildtype females showed the opposite preference. Bax gene deletion eliminated this sex difference and neither male nor female knockouts showed a strong preference for one stimulus over the other. Olfactory preference is likely related to the motivation to remain in proximity of potential mates (Baum, 2009). Taken together with our previous observations that both feminine receptive behaviors and masculine mounting behaviors are suppressed in Bax−/− animals (Jyotika et al., 2007), we conclude that sexual motivation is reduced in Bax knockout mice of both sexes.
Social behaviors were not impaired, however, and if anything were enhanced in Bax−/−animals. All groups in the current study showed social recognition memory. Interestingly, however, Bax−/− mice of both sexes spent significantly more time than their wildtype counterparts investigating stimulus animals during habituation trials. We also observed a robust sex difference (male > female) on the time spent investigating stimulus animals, as is seen in rats across several different experimental paradigms (Bluthé & Danzer, 1990; Markham & Juraska, 2007; Kelly et al., 2009). Interestingly, there was also a sex-by-genotype interaction on this measure: Bax deletion affected females more than males and the magnitude of the sex difference was substantially reduced in Bax−/− mice. This parallels the previously reported effect of Bax deletion on several neural measures (Forger et al., 2004; Holmes et al., 2009).
In a simple social approach test, males in the current study were again more sociable than females, and Bax−/− mice showed greater sociability than wildtype mice. Although a sex difference in social affiliation has not received much attention in mice, previous studies suggest greater sociability in males of two strains (NIH Swiss and Swiss Webster; Hill et al., 2007; Moy et al., 2009). A similar sex difference is well established in rats, where it appears to be due to both neonatal and adult exposure to testosterone (Thor, 1980; Thor et al., 1982). Taken together, the results of the social recognition memory and sociability tests suggest that neuronal apoptosis normally reduces sociability in mice. Vasopressin produced by neurons of the BNST and MeA has been linked to the expression of several social behaviors (reviewed in Veenema & Neumann, 2008). Interestingly, Bax−/− mice of both sexes have more vasopressin neurons in the BNST than do wildtype mice (De Vries et al., 2008). Although vasopressin is often thought of as more important for sociality in males, recent data indicate that antagonism of vasopressin in the lateral septum (i.e., the target of BNST vasopressin neurons) modulates social investigation in rats of both sexes (Veenema et al., 2010).
The changes in social behaviors reported here apparently occurred in the absence of major differences in olfactory processing. Olfactory tasks that depend on the main olfactory bulb are normal in Bax−/− adults (Kim et al., 2007) and the current findings suggest that the processing of pheromonal cues by the accessory olfactory system is also largely normal in Bax knockouts. An increase in the number of Fos-ir cells was seen in the MePD, BNSTp and MPN in response to male-soiled bedding in both wildtype and Bax−/− mice and the magnitude of the effect was similar in the two genotypes. Thus, Bax knockouts detect and respond to socially relevant olfactory cues.
We also observed a sexually dimorphic response (females > male) to male-soiled bedding in the MPN of both genotypes, similar to findings reported by others in mice, rats, and ferrets (Bakker et al., 1996; Wersinger and Baum, 1997; Halem et al., 1999; Bodo and Rissman, 2007). Some previous studies have also reported sexually dimorphic Fos activation in the BNSTp in response to male odors (Halem et al., 1999; Bodo & Rissman, 2007). The absence of that effect here may be due to procedural differences [e.g., test animals were placed directly into soiled cages previously occupied by intact males in Halem et al., (1999) and Bodo & Rissman (2007)] or to hormonal differences (our animals were gonadally intact, whereas animals were gonadectomized and treated with estradiol in the previous studies). However, circulating steroids do not seem to play a large role in the expression of these sex differences in mice: the number of Fos-ir cells in the BNST and preoptic area after exposure to male odors did not differ between mice that were gonadectomized, or gonadectomized and treated with estradiol, and under both conditions females had a greater response (Halem et al., 1999). Exposure to androgens on the day of birth, on the other hand, eliminates the sex differences in neural response to male-soiled bedding in mice (Bodo & Rissman, 2008).
Bax deletion eliminated the sex difference in olfactory preference behavior, but not the sexually differentiated response to male-soiled bedding in the MPN. Similarly, estrogen receptor α knockout male mice show little interest in chemoinvestigating females, but nonetheless show a Fos response to female-soiled bedding similar to that of wildtype males (Wersinger & Rissman, 2000). This suggests that c-Fos immunoreactive cell number may not be a sensitive enough measure to detect behaviorally relevant differences in neural activity. Alternatively, neural activity in regions other than those examined here might underlie our behavioral findings. Bax deletion reduced Fos-ir cell number in the AVPV, which receives projections from both the MPN and BNST (Simerly and Swanson, 1988; Polston et al., 2004), and it would be interesting to determine how neural activation is affected in other downstream regions. In addition, although we focused here on the traditional vomeronasal pathway, there is growing evidence of cross talk between the main and accessory olfactory systems (Baum, 2009; Kang et al., 2011).
Although substantial data have now accumulated on cell number in various brain regions of Bax−/− animals, few studies have examined the projection patterns of cells rescued from death or the effect of excess neurons on the function of neural circuits. At least some of the supernumerary neurons in Bax−/− animals project to their expected targets (Jacob et al., 2005; Kim et al., 2009). A detailed examination of how these cells affect functional circuits has so far been examined only in the dentate gyrus of the hippocampus, where excess neurons accumulate throughout life in Bax−/− animals (Sun et al., 2004). These extra cells cause compensatory changes in afferent and efferent synapse number, alterations in electrophysiological properties of hippocampal circuits and, at least in older animals, a disruption in hippocampal-dependent behavior (Kim et al, 2009). We propose that the behavioural effects of Bax deletion seen in the current study might be due to similar changes in the circuitry underlying social and sexual behaviors.
Acknowledgments
This work was funded by a CIHR postdoctoral fellowship (FRN 76508) and NSERC Discovery Grant (MMH), a NSERC postdoctoral fellowship (LN), a NSERC Discovery Grant (DAM), and NIH grants RO1 MH068482 and K02 MH072825 (NGF).
Abbreviations
- AVPV
anteroventral periventricular nucleus
- BNSTp
principal nucleus of the bed nucleus of the stria terminalis
- Fos-ir
c-Fos immunoreactive
- MePD
posterodorsal portion of the medial amygdala
- MPN
medial preoptic nucleus
References
- Bakker J, Baum MJ, Slob AK. Neonatal inhibition of brain estrogen synthesis alters adult neural Fos responses to mating and pheromonal stimulation in the male rat. Neuroscience. 1996;74:251–260. doi: 10.1016/0306-4522(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 2009;55:579–588. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Euro J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Gould TW, Ma J, Vinsant S, Prevette D, Winseck A, Toops KA, Hammarback JA, Smith TL, Oppenheim RW. Neuromuscular development in the absence of programmed cell death: Phenotypic alteration of motoneurons and muscle. J Neurosci. 2006a;26:13413–13427. doi: 10.1523/JNEUROSCI.3528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006b;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- De Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Swann JM. Testosterone differentially influences sex-specific pheromone-stimulated Fos expression in limbic regions of Syrian hamsters. Horm Behav. 1996;30:455–473. doi: 10.1006/hbeh.1996.0050. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic co-ordinates. 3. Academic Press; New York: 2008. [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol. 2007;67:355–362. doi: 10.1002/dneu.20353. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomernasal neuroepithelium and forebrain fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- Hill JM, Cuasay K, Abebe DT. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp Neurol. 2007;206:101–113. doi: 10.1016/j.expneurol.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Holmes MM, McCutcheon J, Forger NG. Sex differences in NeuN- and androgen receptor-positive cells in the bed nucleus of the stria terminalis are due to Bax-dependent cell death. Neuroscience. 2009;158:1251–1256. doi: 10.1016/j.neuroscience.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behaviour and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 2011;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Leggett DC, Cronise K. Sexually dimorphic effects of alcohol exposure during development on the processing of social cues. Alcohol. 2009;44:555–560. doi: 10.1093/alcalc/agp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR, Kim Y, Eun B, Park OH, Kim H, Kim K, Park CH, Vinsant S, Oppenheim RW, Sun W. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J Neurosci. 2007;27:14392–14403. doi: 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WR, Park OH, Choi S, Choi SY, Park SK, Lee KJ, Rhyu IJ, Kim H, Lee YK, Kim HT, Oppenheim RW, Sun W. The maintenance of specific aspects of neuronal function and behavior is dependent on programmed cell death of adult-generated neurons in the dentate gyrus. Eur J Neurosci. 2009;29:1408–1421. doi: 10.1111/j.1460-9568.2009.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–888. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Madler JJ, Perex A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behaviour in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Young NB, Demyaneko GP, Maness PF. Impaired sociability and cognitive function in Nrcam-null mice. Behav Brain Res. 2009;205:123–131. doi: 10.1016/j.bbr.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120:925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez PI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, Tilly JL. Absence of proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci. 2007;104:5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrade G, Kendrick KM. Roles of α- and β-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Horm Behav. 2011;59:114–122. doi: 10.1016/j.yhbeh.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. Bax-dependent and Bax-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH. Testosterone and persistance of social investigation in laboratory rats. J Comp Physiol Psychol. 1980;94:970–976. doi: 10.1037/h0077831. [DOI] [PubMed] [Google Scholar]
- Thor DH, Wainwright KL, Holloway WR. Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol. 1982;15:1–8. doi: 10.1002/dev.420150102. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Program No. 387.13. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. Regulation of social recognition by vasopressin: Age- but not sex-specific effects. Online. [Google Scholar]
- Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Baum MJ. Sexually dimorphic processing of somatosensory and chemosensory inputs to forebrain luteinizing hormone-releasing hormone neurons in mated ferrets. Endocrinology. 1997;138:1121–1129. doi: 10.1210/endo.138.3.4969. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Oestrogen receptor alpha is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J Neuroendocrinol. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]