Abstract
Aims
Little evidence is available regarding restrictions from driving following implantable cardioverter defibrillator (ICD) implantation or following first appropriate or inappropriate shock. The purpose of the current analysis was to provide evidence for driving restrictions based on real-world incidences of shocks (appropriate and inappropriate).
Methods and results
A total of 2786 primary and secondary prevention ICD patients were included. The occurrence of shocks was noted during a median follow-up of 996 days (inter-quartile range, 428–1833 days). With the risk of harm (RH) formula, using the incidence of sudden cardiac incapacitation, the annual RH to others posed by a driver with an ICD was calculated. Based on Canadian data, the annual RH to others of 5 in 100 000 (0.005%) was used as a cut-off value. In both primary and secondary prevention ICD patients with private driving habits, no restrictions to drive directly following implantation, or an inappropriate shock are warranted. However, following an appropriate shock, these patients are at an increased risk to cause harm to other road users and therefore should be restricted to drive for a period of 2 and 4 months, respectively. In addition, all ICD patients with professional driving habits have a substantial elevated risk to cause harm to other road users during the complete follow-up after both implantation and shock and should therefore be restricted to drive permanently.
Conclusion
The current analysis provides a clinically applicable tool for guideline committees to establish evidence-based driving restrictions.
Keywords: Device therapy, Driving legislation, Driving restriction, Implantable cardioverter defibrillator, Ventricular arrhythmias
Introduction
It has been recognized that patients treated with an implantable cardioverter defibrillator (ICD) have an ongoing risk of sudden incapacitation that might cause harm to others when driving a car. Although numerous recommendations exist, thus far evidence is scarce to justify them. As a result, a large variation exists between different countries concerning the legislation of driving restriction after both primary prevention and secondary prevention ICD implantation.1–3 Since driving restrictions are often being perceived as difficult for patients and their families, clear evidence on the necessity of these restrictions is vital. Furthermore, these restrictions should take into account the indication for ICD implantation (primary or secondary prevention). In the end, however, it must be recognized that the goal of a zero per cent risk is unobtainable and that society has to accept a certain level of risk by allowing patients at risk to resume driving.4–6
With the constant increase in ICD implants worldwide, clear guidelines regarding driving restrictions in both primary and secondary ICD patients are warranted. In this analysis, we determined the risk for ICD therapy following ICD implantation or following previous device therapy (appropriate and inappropriate shock) in relation with driving restriction for private and professional drivers in a large number of primary and secondary ICD patients.
Methods
Patients
The study population consisted of patients from the south-western part of the Netherlands (comprising 1 500 000 people) who received an ICD for primary prevention or secondary prevention in the Leiden University Medical Center, the Netherlands. Since 1996, all implant procedures were registered in the departmental Cardiology Information System (EPD-Vision®, Leiden University Medical Center). Characteristics at baseline, data of the implant procedure, and all follow-up visits were recorded prospectively. The data collected for the current registry ranged from January 1996 up to September 2009.
Eligibility for ICD implantation in this population was based on international guidelines for primary and secondary prevention. Due to evolving guidelines, indications will have changed over time.7,8
Device implantation and programming
All defibrillator system implantations were performed transvenously, without thoracotomy. Testing of sensing and pacing thresholds and defibrillation threshold testing was performed during the implant procedure. Implanted systems were manufactured by Biotronik (Berlin, Germany), Boston Scientific [Natick, MA, USA, formerly CPI, Guidant (St Paul, MN, USA)], Medtronic (Minneapolis, MN, USA), and St Jude Medical/Ventritex (St Paul, MN, USA).
Defibrillators were programmed as follows: a ventricular arrhythmia monitor zone was programmed in all patients (150–188 b.p.m.). No therapy was programmed in this zone until arrhythmias were detected during follow-up. Ventricular arrhythmias faster than 188 b.p.m. were initially attempted to be terminated with two bursts of antitachycardia pacing (ATP) and, after continuation of the arrhythmia, device shocks were the indicated therapy. Ventricular arrhythmias faster than 210 b.p.m. were directly attempted to be terminated by device shocks. Furthermore, atrial arrhythmia detection was set to >170 b.p.m. with supraventricular arrhythmia discriminators enabled. Settings were adapted, only when clinically indicated (e.g. haemodynamic well-tolerated ventricular tachycardia (VT) at high rate; VT in the monitor zone).
According to Dutch legislation, updated in June 2004, private driving was prohibited for the first 2 months after implantation for both primary prevention and secondary prevention ICD patients. Furthermore, private drivers are restricted from driving for a period of 2 months following an appropriate shock, and professional drivers are permanently restricted from driving following ICD implantation.9
Patient follow-up
Patient check-up was scheduled every 3–6 months, which included device interrogation. In case of unplanned hospitalization or symptomatic episodes of arrhythmia, additional device interrogations were performed. During device interrogation, episodes were assessed for appropriate and inappropriate ICD therapy (ATP or shocks) and verified by an electrophysiologist. Shocks were classified as appropriate when they occurred in response to VT or ventricular fibrillation (VF) and as inappropriate when triggered by sinus tachycardia or supraventricular tachycardia, T-wave oversensing, or electrode dysfunction. After delivery of an appropriate shock, efforts were made by a trained electrophysiologist to reduce the recurrence rate of arrhythmic events. When clinically indicated, ICD settings and/or anti-arrhythmic medication were adjusted.
Since periodical follow-up was performed every 3–6 months, patients without data for the most recent 6 months prior to the end of the study were considered as lost to follow-up. However, these patients were included in the analysis as far as data were acquired.
Endpoints
The first shock (appropriate or inappropriate) was considered the primary endpoint. For the second shock analysis, only those patients who received a first shock were considered at risk for a second shock, and only subsequent shocks occurring >24 h after first shock were considered second shocks. Noteworthy, ATP therapy was discarded from the analysis since the number of patients experiencing syncope—and therefore incapacitation—during ATP therapy is low.10,11
Risk assessment
Currently, prospective controlled studies in which ICD patients have been randomized to permit driving are not available. In 1992, a ‘risk of harm' formula was developed to quantify the level of risk to drivers with ICDs by the Canadian Cardiovascular Society Consensus Conference.12,13 This formula, with the following equation: RH = TD × V × SCI × Ac, calculates the yearly risk of harm (RH) to other road users posed by a driver with heart disease and is directly proportional to:
proportion of time spent on driving or distance driven in a given time period (TD),
type of vehicle driven (V),
yearly risk of sudden cardiac incapacitation (SCI),
the probability that such an event will result in a fatal or injury producing accident (Ac).
Based on the literature, it is known that on average a private driver spends ∼4% (TD = 0.04) and a professional driver spends ∼25% (TD = 0.25) of his time driving.14,15 In addition, it was shown that more injurious accidents were caused by heavy truck or passenger-carrying vehicles when compared with private automobiles. In the Ontario Road Safety Annual Report, truckers were involved in ∼2% of all road accidents but in ∼7.2% of all lethal accidents. Based on this data, V = 1 for a professional driver and V = 0.28 for a private driver in the RH formula.14,15 Furthermore, <2% of reported incidents of driver sudden death or loss of consciousness has resulted in injury or death to other road users or bystanders (Ac = 0.02).16–18 In this analysis, the yearly risk of SCI was based on the cumulative incidence of ICD shocks (appropriate or inappropriate), which were calculated for different follow-up periods as described previously. However, the actual influence of an ICD shock on the capacity to drive is unknown. According to the literature, 31% of the patients experience syncope or near syncope during an appropriate shock.19 Since this proportion of patients receiving an appropriate shock will then be incapacitated to drive, it was assumed that the SCI is equal to the cumulative incidence of appropriate ICD shocks times 0.31. So far, no reports exist that describe the proportion of patients experiencing syncope or near syncope during an inappropriate shock. Based on the causes of inappropriate shocks (atrial fibrillation, sinus tachycardia, T-wave oversensing, and lead failure), it is less likely that inappropriate shocks coincide with more haemodynamic consequences than appropriate shocks do. With the assumption that 31% of the patients with appropriate shocks experience syncope, it was supposed that at most the same proportion of patients receiving an inappropriate shock will experience syncope. Therefore, similar to appropriate shocks, the SCI is equal to the cumulative incidence of inappropriate ICD shocks times 0.31.
Considering the fact that driving restrictions for ICD patients are implemented as a protection for both ICD patients, as well as other road users, the RH formula is an easy tool to calculate the potential harm brought to other road users on a yearly basis when ICD patients are not restricted to drive.
Unfortunately, data regarding an acceptable level of risk for private and professional drivers with an ICD in society are scarce. However, in Canada an annual risk of death or injury to others of 5 in 100 000 (0.005%) appeared to be in general acceptable.3 Therefore, this generally accepted level of risk will be used as a cut-off value in the current study.
Private and professional drivers
Criteria to distinguish a private driver from a professional driver were defined on the basis of the Canadian Cardiovascular Society Consensus Conference.12,13 According to these criteria, a private driver was defined as follows: (i) driving <36 000 km per year; (ii) spending <720 h per year driving; (iii) driving a vehicle weighting <11 000 kg, and (iv) does not earn a living by driving. Any licenced driver who does not fulfil one of these criteria was considered to be a professional driver.
Statistical analysis
Continuous data are expressed as mean with standard deviation (SD) or median and first and third quartile when appropriate; dichotomous data are presented as numbers and percentages. Cumulative incidences for first and second appropriate shock were determined by the Kaplan–Meier method to take different follow-up times per patient into account. Cumulative incidences were determined for several periods of time after implantation and presented with a 95% confidence interval (CI) as the estimate ±1.96 times the standard error.
Standard errors were derived from the binomial distribution, and the CI constructed with the normal approximation. The RH formula was used to calculate the yearly RH to other road users posed by an ICD-treated driver. With this formula, various outcomes were calculated on the basis of distinct ICD indication (i.e. primary and secondary prevention), type of driver (i.e. private and professional driver), and type of vehicle driven (i.e. heavy truck and passenger-carrying vehicle or a private automobile). All statistical analyses were performed with the SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA).
Results
Patients
Since 1996, data of 2786 consecutive patients receiving an ICD for primary (n= 1718, 62%) or secondary (n= 1068, 38%) prevention were prospectively collected. One hundred and ninety-eight of these patients [n= 126 (64%) primary prevention; n= 72 (36%) secondary prevention] received an ICD for diagnosed congenital heart disease or monogenetic heart disease. A total of 196 (7.0%) patients were lost to follow-up; however, they are included in the analysis as far as data were acquired. Median follow-up time was 996 days (inter-quartile range, 428–1833 days). The majority of patients [79% men, mean age 61 years (SD 13 years)] had ischaemic heart disease. Baseline patient characteristics are summarized in Table 1.
Table 1.
Baseline patient characteristics
| Total (n = 2786) | Primary prevention (n = 1718) | Secondary prevention (n = 1068) | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 61 ± 13 | 62 ± 13 | 61 ± 14 |
| Male (%) | 2192 (79) | 1336 (78) | 856 (80) |
| Left ventricular ejection fraction (%) | 33 ± 15 | 31 ± 14 | 39 ± 16 |
| QRS, mean (SD), ms | 125 ± 34 | 129 ± 35 | 119 ± 32 |
| Renal clearance, mean (SD), mL/min | 81 ± 37 | 81 ± 36 | 82 ± 39 |
| Ischaemic heart disease (%) | 1800 (65) | 1077 (63) | 723 (68) |
| History of atrial fibrillation/flutter (%) | 683 (25) | 447 (26) | 236 (22) |
| Medication | |||
| ACE-inhibitors/AT II antagonist (%) | 2107 (76) | 1407 (82) | 700 (66) |
| Aspirin (%) | 1107 (40) | 649 (38) | 458 (43) |
| Beta-blocker (%) | 1513 (54) | 1074 (63) | 439 (41) |
| Diuretics (%) | 1738 (62) | 1221 (71) | 517 (48) |
| Statins (%) | 1610 (58) | 1075 (63) | 535 (50) |
| Anti-arrhythmic medicationa | |||
| Amiodarone (%) | 497 (18) | 221 (13) | 276 (26) |
| Sotalol (%) | 386 (14) | 184 (11) | 202 (19) |
ACE, angiotensin-converting enzyme; AT, angiotensin; SD, standard deviation.
aPatients could be taking >1 anti-arrhythmic drug.
Device therapy in primary prevention patients
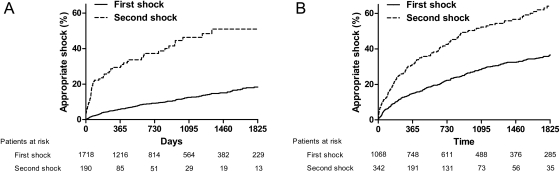
In the group of primary prevention patients, median follow-up was 784 days (inter-quartile range, 363–1495 days). During this follow-up, a total of 190 (10%) patients received an appropriate shock. Median time to first appropriate shock was 417 days (inter-quartile range, 134–960 days). From those 190 patients who received a first appropriate shock, 65 patients (34%) received a second appropriate shock. Median time between first and second appropriate shock was 66 days (inter-quartile range, 29–379 days). Cumulative incidences for first and second appropriate shock are displayed in Figure 1.
Figure 1.
Kaplan–Meier curve for first and second appropriate shock in primary (A) and secondary (B) prevention implantable cardioverter defibrillator patients. Only patients who received a first appropriate shock were included in the analysis for the second appropriate shock. The time to the occurrence of a second appropriate shock was counted (in days) from the first appropriate shock.
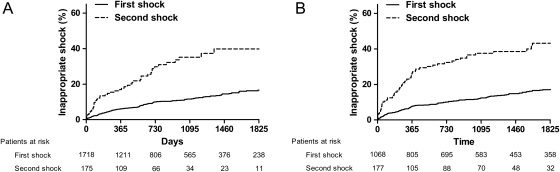
Inappropriate shocks occurred in 175 (10%) patients with a median time of 320 days (inter-quartile range, 124–711days). From the 175 patients with a first inappropriate shock, 47 patients (27%) received a second inappropriate shock. Median time between first and second inappropriate shock was 224 days (inter-quartile range, 77–580 days). Cumulative incidences for first and second inappropriate shock are displayed in Figure 2.
Figure 2.
Kaplan–Meier curve for first and second inappropriate shock in primary (A) and secondary (B) prevention implantable cardioverter defibrillator patients. Only patients who received a first inappropriate shock were included in the analysis for the second inappropriate shock. The time to the occurrence of a second inappropriate shock was counted (in days) from the first inappropriate shock.
Device therapy in secondary prevention patients
In the group of secondary prevention patients, median follow-up time was 1442 days (inter-quartile range, 618–2469 days). During this follow-up, a total of 342 (32%) patients received an appropriate shock. Median time to first appropriate shock was 509 days (inter-quartile range, 141–1137 days). From those 342 patients with a first appropriate shock, 166 (49%) patients received a second appropriate shock. Median time between the first and second appropriate shock was 400 days (inter-quartile range, 107–1072 days). Cumulative incidences for first and second appropriate shock are displayed in Figure 1.
Inappropriate shocks occurred in 177 (17%) patients with a median time of 639 days (inter-quartile range, 190–1676 days). From the 177 patients with a first inappropriate shock, 60 patients (34%) received a second inappropriate shock. Median time between first and second inappropriate shock was 243 (inter-quartile range, 47–435 days). Cumulative incidences for first and second inappropriate shock are displayed in Figure 2.
Risk assessment in primary prevention implantable cardioverter defibrillator patients
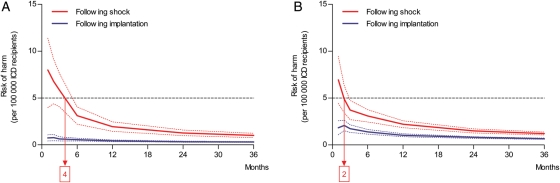
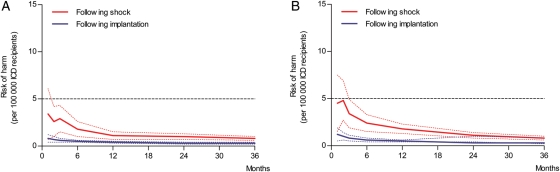
In the RH formula (RH = TD × V × Ac × SCI), the annual RH per specific time point is calculated with the pre-specified variables TD, V, and Ac and with the SCI. Sudden cardiac incapacitation equals the cumulative incidence of ICD shocks multiplied by the proportion of patients experiencing syncope (31%). For instance, for primary prevention ICD patients, the cumulative incidence for an appropriate shock at 1 month following implantation is 0.9%. Since the formula uses yearly incidences, the monthly incidence is converted to a yearly incidence of 10.8% (0.9% × 12) and hereafter multiplied by the proportion of patients experiencing syncope or near syncope during an ICD (i.e. 31%) shock. Therefore, SCI in this example equals 0.03 (0.009 × 12 × 0.31). Accordingly, the RH to other road users per 100 000 ICD patients for primary prevention ICD patients with private driving habits 1 month after implantation is calculated as follows: 0.04 × 0.28 × 0.02 × 0.009 × 12 × 0.31 = 0.75. After 1 year, the cumulative incidence for appropriate shocks in these patients is 6.0% following implantation. Consequently, the RH to other road users for these patients declines to 0.43 (RH = 0.04 × 0.28 × 0.02 × 0.062 × 0.31) per 100 000 ICD patients per year (Figures 1 and 3). Directly after implantation, the RH to other road users in primary and secondary prevention ICD patients with private driving habits remains below the acceptable cut-off value of 5 per 100 000 ICD patients. Also, after experiencing a first inappropriate shock, the RH to other road users remains below the accepted cut-off value (Figure 4).
Figure 3.
The annual risk of harm to other road users (y-axis) in primary (A) and secondary (B) prevention implantable cardioverter defibrillator patients based on the cumulative incidence of appropriate shocks is illustrated. Risk of harm (solid lines) is calculated in the months (x-axis) following implantation or appropriate shock. The horizontal dotted line represents the cut-off value for the accepted level of risk of harm (5 per 100 000). Blue and red dotted lines represent the range of the risk of harm, based on the confidence interval of the cumulative incidence for appropriate shocks. In primary prevention implantable cardioverter defibrillator patients (A), driving is acceptable directly following implantation (blue line) and should be restricted for 4 months following appropriate shock (red line). In secondary prevention implantable cardioverter defibrillator patients (B), driving is acceptable directly following implantation (blue line) and should be restricted for 2 months following appropriate shock (red line).
Figure 4.
The annual risk of harm to other road users (y-axis) in primary (A) and secondary (B) prevention implantable cardioverter defibrillator patients based on the cumulative incidence of inappropriate shocks is illustrated. Risk of harm (solid lines) is calculated in the months (x-axis) following implantation or inappropriate shock. The horizontal dotted line represents the cut-off value for the accepted level of risk of harm (5 per 100 000). Blue and red dotted lines represent the range of the risk of harm, based on the confidence interval of the cumulative incidence for inappropriate shocks. In primary prevention implantable cardioverter defibrillator patients (A), driving is acceptable directly following implantation (blue line) as well as directly following inappropriate shock (red line). Similar results were found in secondary prevention implantable cardioverter defibrillator patients (B), where driving is again acceptable directly following implantation (blue line) as well as directly following inappropriate shock (red line).
Following an appropriate shock, the annual RH declines from 8.0 (RH = 0.04 × 0.28 × 0.02 × 0.096 × 12 × 0.31) after 1 month to 2.1 (RH = 0.04 × 0.28 × 0.02 × 0.302 × 0.31) per 100 000 ICD patients after 1 year (Figures 1 and 3). In Figure 3, it is shown that the RH declines below the accepted cut-off value after 4 months following an appropriate shock in primary prevention ICD patients with private driving habits. However, following an inappropriate shock, the RH in these patients is again directly below the accepted cut-off value (Figure 4).
Due to the heavy type of vehicle driven and the hours spent driving, the annual RH following both implantation and appropriate shock was found to be 22.3 times higher in primary prevention ICD patients with professional driving habits when compared with private drivers. Consequently, the RH to other road users following implantation or shock remains above the acceptable cut-off value during the complete follow-up.
Risk assessment in secondary prevention implantable cardioverter defibrillator patients
In secondary prevention ICD patients with private driving habits, the annual RH based on an appropriate shock was found to be 1.8 (RH = 0.04 × 0.28 × 0.02 × 0.022 × 12 × 0.31) per 100 000 ICD patients 1 month following implantation (Figures 1 and 3). Similar to primary prevention ICD patients with private driving habits, the RH to other road users of these patients remained below the cut-off value of 5 per 100 000 ICD patients during follow-up. Also if the RH to other road users after implantation was based on the cumulative incidence of inappropriate shocks, outcomes were directly following implantation below the accepted cut-off value (Figure 4).
However, after an appropriate shock, the RH to other road users declined from 6.9 (RH = 0.04 × 0.28 × 0.02 × 0.083 × 12 × 0.31) to 2.2 (RH = 0.04 × 0.28 × 0.02 × 0.315 × 0.31) casualties on an annual basis per 100 000 ICD patients 1 month and 12 months following appropriate shock, respectively. This risk following appropriate shock declined below the accepted cut-off value after 2 months in the group of secondary prevention ICD patients with private driving habits (Figures 1 and 3). Following an inappropriate shock, the RH in these patients is again directly below the accepted cut-off value (Figure 4).
Professional driving in secondary prevention ICD patients was above the cut-off value following both implantation and shock during the complete follow-up.
Discussion
In this evidence-based assessment of driving restrictions using the RH formula, the findings can be summarized as follows: (i) following device implantation, primary and secondary prevention ICD patients with private driving habits have an acceptable RH and therefore can be directly permitted to drive; (ii) after an inappropriate shock, the level of risk remains below the accepted cut-off value and therefore no restrictions should be applied in all ICD patients with private driving habits; (iii) in the case of an appropriate shock, primary and secondary prevention ICD patients with private driving habits should be restricted to drive for 4 and 2 months, respectively; (iv) ICD patients with professional driving habits do not reach an acceptable level of risk during follow-up and therefore should be permanently restricted to drive.
Risk of driving in primary prevention implantable cardioverter defibrillator patients
With increasing rates of primary prevention ICD implantations worldwide, clear guidelines regarding driving restrictions are essential. Although the risk for sudden incapacitation while driving is considered lower in this group of ICD patients than in secondary prevention ICD patients, no distinction is made in driving restrictions following ICD treatment. These differences in event rates are based on mortality data, rates of sudden cardiac death, and rate of ICD discharges reported from primary prevention trials.20–27 With the lack of randomized controlled trials concerning ICD patients and the risk of driving, recommendations of the European Heart Rhythm Association (EHRA) and American Heart Association (AHA) on driving restrictions in the group of primary prevention ICD patients are based on the data from these trials.1,3
The current study shows a cumulative incidence of 6.0% appropriate shocks after 1 year. Furthermore, ICD discharges were highest in the first period following implantation and showed a slight decline in the years thereafter (Figure 1). These data are not comparable with the MADIT I trial, which described a shock rate of 30.0% on an annual basis during 2 years follow-up or with the MADIT II trial, which described a shock rate of 11.7% on an annual basis during 3 years follow-up. However, the appropriateness of the defibrillator discharges could not be assessed reliably in the MADIT I trial.26,28 Furthermore, the utilized devices of the MADIT II trial were unable to deliver ATP therapy, which might explain the shock rate discrepancy between the MADIT II trial and the current study. In the SCD-HeFT trial, the annual rate of appropriate ICD discharge during 5 years of follow-up was 7.5% per year.20 In the DEFINITE trial, a shock rate of 7.4% occurred on an annual basis; however, only 44.9% of discharges were appropriate.25 Data of the SCD-HeFT and DEFINITE trials are comparable with the data from the current study.
In the current analysis, 10% of the primary prevention ICD patients received an inappropriate shock that is more or less comparable with the 11.5% of the MADIT II trial.29
Currently, the EHRA and AHA recommend primary prevention ICD patients with private driving habits not to drive for 1 month and 1 week, respectively. It should be noted that this is not because of an increased risk of SCI, but to improve recovery from implantation of the defibrillator.1–3 The current study demonstrates that the RH for private drivers remains well below the acceptable cut-off level after implantation and therefore is in agreement with these recommendations (Figures 3 and 4). In addition, for professional drivers, the outcomes of the RH formula in the current analysis are unfavourable during the entire period of ICD implantation. As a result, based on the outcomes of this study, these drivers should be permanently restricted from driving, which is in line with the current recommendations of the EHRA and AHA.1–3
Risk of driving in secondary prevention implantable cardioverter defibrillator patients
Secondary prevention ICD patients have already experienced a life-threatening arrhythmia (e.g. VT or VF). The probability that patients will experience a recurrent arrhythmia is therefore an important factor determining the RH, both with respect to themselves as well as others in car accidents. With regard to inappropriate shocks, only 17% of the secondary prevention ICD patients in the current analysis received such a shock. This proportion is more or less comparable with the 15% found in secondary prevention ICD patients included in the PainFREE Rx II trial.30 However, the 5-year cumulative incidence of appropriate shock ranged between 55 and 70% in various trials, compared with a 36% cumulative incidence of appropriate shock in the current analysis.19,31–34 This difference is at least, in part, explained by the ATP therapy, which was less frequently applied in the older secondary prevention studies which could prevent degeneration of VT in VF resulting in a lower cumulative incidence of appropriate shock therapy in the present study. Almost similar to Lubinski et al.,35 the probability of arrhythmic episodes resulting in appropriate shocks in the current analysis was 2.2% in the first month, 2.9% in the second month, and remained below 2% per month in the months thereafter. However, it was assumed that the risk for road accidents is just a fraction of the monthly probability of appropriate shocks, as described previously. Therefore, in patients with defibrillators implanted for secondary prevention, the risk of symptoms that may lead to incapacity while driving is low. Consequently in the current analysis, the RH to other road users, based on both the cumulative incidence of appropriate and inappropriate shocks, remains below the acceptable risk. Therefore, no driving restrictions for secondary prevention ICD patients with private driving habits following implantation should be implemented. However, this outcome is in contrast with the current recommendations for secondary ICD patients with private driving habits, where the EHRA and AHA recommend a 3 and 6 months driving restriction, respectively.1–3
With respect to professional drivers, outcomes of the RH formula are unfavourable during the entire period. Therefore, similar to primary prevention patients, secondary ICD patients should be restricted from professional driving.
Risk of driving following appropriate or inappropriate shock
A particularly difficult issue for patients and physicians is the consideration of driving restrictions in an ICD patient who has received an appropriate ICD shock. Following appropriate ICD therapy, recommendations of the EHRA and AHA prescribe a 3 and 6-month period of driving restriction in ICD patients, respectively.1,3,36 When patients experience an appropriate shock for a spontaneous ventricular arrhythmia during follow-up, the risk of driving is determined by the probability of a subsequent arrhythmic event and by the likelihood of symptoms of impaired consciousness. However, symptoms of impaired consciousness during the first appropriate ICD therapy are not unambiguously predictive for future syncope during subsequent shocks.31,37 In a study of 125 ICD patients by Freedberg et al.,19 the median freedom from ICD therapy for the second shock was only 22 days, with a 1-year cumulative incidence of a second appropriate shock being 79%. These were all secondary prevention ICD patients and the cumulative incidence for a second appropriate shock shows large dissimilarity when compared with the 1-year cumulative incidence of 32% observed in the secondary prevention group in the present study. However, since these are all older devices without the option of ATP, shock rates in the study by Freedberg et al. are probably comparable with cumulative incidence of all ICD therapy in the current analysis.
Finally, substituting these cumulative incidences for appropriate shock in the RH formula results in a significant increase in the RH to other road users when ICD patients are allowed to drive in the period following this shock. This RH to others is above the cut-off value of 5 per 100 000 on an annual basis for a period of 4 months and 2 months following appropriate shock in primary and secondary ICD patients, respectively (Figure 3). These outcomes are more or less in line with the recommendations of the EHRA and AHA.1–3
Since, to our knowledge, the incidence of syncope following an inappropriate shock is unknown, calculating the corresponding RH is problematic. Therefore, it was assumed that the incidence of syncope or near syncope during an inappropriate shock is equal to the incidence of syncope or near syncope during an appropriate shock. Even with this apparent defensive approach in which the potential RH could be overestimated, the actual RH following an inappropriate shock remained below the acceptable cut-off value for both primary and secondary ICD patients. Therefore, in line with the current recommendations of the EHRA and AHA, no driving restrictions following an inappropriate shock should be applied in these patients.1–3 However, it is needless to say that all efforts should be made to prevent subsequent inappropriate shock before those patients should be permitted to drive again.
Private and professional drivers
It is, however, important to recognize the difference between the Canadian and European classification of private and commercial drivers. In Canada, a private driver is defined as one who drives <36 000 km per year or spends <720 h driving per year, drives a vehicle weighing <11 000 kg, and does not earn a living by driving. A commercial driver is defined as any licenced driver who does not fulfil the definition of a private driver. In Europe, two groups of drivers are defined: Group 1 comprises drivers of motor cycles, cars, and other small vehicles with or without a trailer. Group 2 includes drivers of vehicles over 3.5 metric tonnes or passenger-carrying vehicles exceeding eight seats excluding.3
As the RH estimations are based on the Canadian data, it may be necessary to re-evaluate the strict European rules. For example, a private driver with a motor-home exceeding the 3.5 metric tonne limit automatically is a Group 2 driver and restricted from driving after ICD implant, which seems to be an unnecessary restriction.
Clinical implications
Recently, EHRA and AHA provided consensus documents on driving restriction for ICD patients. Since no data from routine clinical practice were available at that time, restrictions were based on data from randomized clinical trials, which to a certain extent—differ from routine clinical practice. This study is the first to provide accurate data on the incidences of appropriate and inappropriate shocks during follow-up in routine clinical practice and based on this, established driving restrictions. However, it is of course up to the guideline committees and national regulatory authorities to determine final driving restrictions for ICD patients. It should be emphasized that for the current study, an acceptable RH of 5 per 100 000 ICD patients was used based on Canadian consensus. Increasing or decreasing this cut-off value may hold significant consequences for the recommendations. Moreover, in the current formula, Ac was considered 2% (i.e. 2% of reported incidents of driver sudden death or loss of consciousness has resulted in injury or death to other road users or bystanders). These data are derived from the Ontario Road Safety Annual Report, since exact data usable for the formula are scarce. It should be noted that differences in these data will exist between different countries or areas affected by population density, driving habits, and type of vehicle driven. This could affect the RH to other road users. However, if available, data from other countries can be implemented in the formula.2 Finally, guidelines committees and national regulatory authorities must taken into account the serious impact of driving restrictions on patient's life and the fact that ICD patients will ignore (too rigorous) driving restrictions.38–40
Limitations
This was a prospective observational study assessing the incidence of SCI in ICD patients. Since patients received ICDs in a single center over a long period of time, evolving guidelines could have created a heterogeneous population. Moreover, median follow-up time was 2.1 years in primary prevention and 4.0 years in secondary prevention ICD patients, which resulted in relatively broad CIs of the cumulative incidences at long-term follow-up. In addition, ATP was discarded from the analysis since, according to the literature, minority of patients receiving ATP experience syncope.10,11 As a result, the calculated RH to others might be underestimated. Moreover, ICD programming was not homogeneous since ICD settings were adapted when clinically indicated. Finally, only the first and second shock (appropriate or inappropriate) of the ICD patients were taken into account. Although patients sometimes received more than two shocks, the number of patients receiving three or more shocks was small and had limited follow-up making assessment of the SCI unreliable.
Conclusion
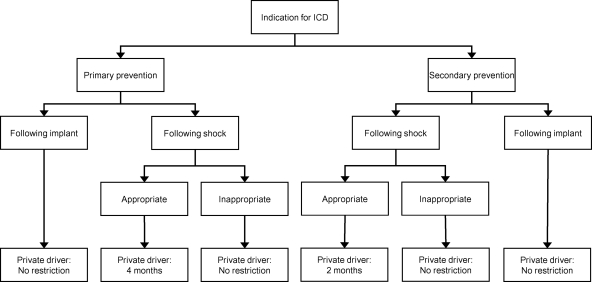
The current study provides reports on the cumulative incidences of SCI in ICD patients following ICD implantation and following first appropriate or inappropriate shock. The RH to others was assessed using this SCI multiplied by the estimated risk of syncope, which resulted in specific outcomes for the RH to other road users per different scenario (Figure 5). This study may serve as a basis and founding of driving recommendations that can be used by national regulatory authorities.
Figure 5.
Flowchart demonstrating the recommended driving restrictions for implantable cardioverter defibrillator patients with private driving habits. Based on the current analysis, implantable cardioverter defibrillator patients with professional driving habits should be restricted to drive in all circumstances and therefore are not in the figure.
Contributors
J.T., C.J.W.B., and J.B.R. conceived and designed the study and have written the manuscript. J.T., C. J.W.B., E.T.V., and M.K.B. collected all relevant data. L.E., J.J.B., and M.J.S. supervised this project. S.C.C. helped by analysing the data and suggested specific statistical tests.
Funding
Department of Cardiology, Leiden University Medical Center, the Netherlands. Funding to pay the Open Access publication charges for this article was provided by the Department of Cardiology, Leiden University Medical Center.
Conflict of interest: Prof. J.J.B. received research grants from GE Healthcare, BMS Medical Imaging, Edwards Lifesciences, Boston Scientific, Medtronic, Biotronik, and St Jude. Prof. M.J.S. received research grants from Biotronik, Medtronic, and Boston Scientific. Prof. M.J.S. and Dr J.T. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Epstein AE, Baessler CA, Curtis AB, Estes NA, III, Gersh BJ, Grubb B, Mitchell LB. Addendum to ‘'Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations: a medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology’: public safety issues in patients with implantable defibrillators: a scientific statement from the American Heart Association and the Heart Rhythm Society. Circulation. 2007;115:1170–1176. doi: 10.1161/CIRCULATIONAHA.106.180203. doi:10.1161/CIRCULATIONAHA.106.180203. [DOI] [PubMed] [Google Scholar]
- 2.Katritsis DG, Webb-Peploe MM. Occupational and regulatory aspects of heart disease. In: Camm AJ, Lüscher TF, Serruys PW, editors. The ESC Textbook of Cardiovascular Medicine. 2nd ed. 2009. pp. 1359–1369. [Google Scholar]
- 3.Vijgen J, Botto G, Camm J, Hoijer CJ, Jung W, Le Heuzey JY, Lubinski A, Norekval TM, Santomauro M, Schalij M, Schmid JP, Vardas P. Consensus statement of the European Heart Rhythm Association: updated recommendations for driving by patients with implantable cardioverter defibrillators. Europace. 2009;11:1097–1107. doi: 10.1093/europace/eup112. doi:10.1093/europace/eup112. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M, Camm AJ. Implantable cardioverter defibrillators and fitness to drive. Lancet. 1994;343:358. doi: 10.1016/s0140-6736(94)91196-7. doi:10.1016/S0140-6736(94)91196-7. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MH, Camm AJ. Legal and ethical aspects of driving and working in patients with an implantable cardioverter defibrillator. Am Heart J. 1994;127:1185–1193. doi: 10.1016/0002-8703(94)90108-2. doi:10.1016/0002-8703(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 6.Petch MC. Implantable cardioverter defibrillators and fitness to drive. Lancet. 1994;343:674. doi: 10.1016/s0140-6736(94)92669-7. doi:10.1016/S0140-6736(94)92669-7. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. doi:10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 8.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. doi:10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 9.2010. http://www.cbr.nl/brochure/2000%20Rijgeschiktheid%20bij%20ICD.pdf. Ref Type: Internet Communication.
- 10.Leitch JW, Gillis AM, Wyse DG, Yee R, Klein GJ, Guiraudon G, Sheldon RS, Duff HJ, Kieser TM, Mitchell LB. Reduction in defibrillator shocks with an implantable device combining antitachycardia pacing and shock therapy. J Am Coll Cardiol. 1991;18:145–151. doi: 10.1016/s0735-1097(10)80232-3. doi:10.1016/S0735-1097(10)80232-3. [DOI] [PubMed] [Google Scholar]
- 11.Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, Canby RC, Khalighi K, Machado C, Rubenstein DS, Volosin KJ. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. doi:10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 12.Assessment of the cardiac patient for fitness to drive. Can J Cardiol. 1992;8:406–419. [PubMed] [Google Scholar]
- 13.Assessment of the cardiac patient for fitness to drive: 1996 update. Can J Cardiol. 1996;12:1164–1182. [PubMed] [Google Scholar]
- 14.Ontario Ministry of Transportation. Ontario Road Safety Annual Report. Toronto:: Ontario Ministry of Transportation; 1987. [Google Scholar]
- 15.Ottawa:: Statistics Canada; 1987. Fuel consumption survey annual report: October 1981 to September 1982 and October 1982 to September 1983 (Catalogue 53–226) [Google Scholar]
- 16.Hossack DW. Death at the wheel. A consideration of cardiovascular disease as a contributory factor to road accidents. Med J Aust. 1974;1:164–166. doi: 10.5694/j.1326-5377.1974.tb50782.x. [DOI] [PubMed] [Google Scholar]
- 17.Ostrom M, Eriksson A. Natural death while driving. J Forensic Sci. 1987;32:988–998. [PubMed] [Google Scholar]
- 18.Parsons M. Fits and other causes of loss of consciousness while driving. Q J Med. 1986;58:295–303. [PubMed] [Google Scholar]
- 19.Freedberg NA, Hill JN, Fogel RI, Prystowsky EN. Recurrence of symptomatic ventricular arrhythmias in patients with implantable cardioverter defibrillator after the first device therapy: implications for antiarrhythmic therapy and driving restrictions. CARE Group. J Am Coll Cardiol. 2001;37:1910–1915. doi: 10.1016/s0735-1097(01)01226-8. doi:10.1016/S0735-1097(01)01226-8. [DOI] [PubMed] [Google Scholar]
- 20.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. doi:10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 21.Bigger JT., Jr Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. N Engl J Med. 1997;337:1569–1575. doi: 10.1056/NEJM199711273372201. doi:10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 22.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De MT, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 23.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. doi:10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 24.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. doi:10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 25.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. doi:10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 26.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. doi:10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. doi:10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 28.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. doi:10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 29.Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. doi:10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney MO, Wathen MS, Volosin K, Abdalla I, DeGroot PJ, Otterness MF, Stark AJ. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111:2898–2905. doi: 10.1161/CIRCULATIONAHA.104.526673. doi:10.1161/CIRCULATIONAHA.104.526673. [DOI] [PubMed] [Google Scholar]
- 31.Bansch D, Brunn J, Castrucci M, Weber M, Gietzen F, Borggrefe M, Breithardt G, Block M. Syncope in patients with an implantable cardioverter-defibrillator: incidence, prediction and implications for driving restrictions. J Am Coll Cardiol. 1998;31:608–615. doi: 10.1016/s0735-1097(97)00543-3. doi:10.1016/S0735-1097(97)00543-3. [DOI] [PubMed] [Google Scholar]
- 32.Curtis JJ, Walls JT, Boley TM, Stephenson HE, Schmaltz RA, Nawarawong W, Flaker GC. Time to first pulse after automatic implantable cardioverter defibrillator implantation. Ann Thorac Surg. 1992;53:984–987. doi: 10.1016/0003-4975(92)90371-a. doi:10.1016/0003-4975(92)90371-A. [DOI] [PubMed] [Google Scholar]
- 33.Grimm W, Flores BF, Marchlinski FE. Symptoms and electrocardiographically documented rhythm preceding spontaneous shocks in patients with implantable cardioverter-defibrillator. Am J Cardiol. 1993;71:1415–1418. doi: 10.1016/0002-9149(93)90602-9. doi:10.1016/0002-9149(93)90602-9. [DOI] [PubMed] [Google Scholar]
- 34.Levine JH, Mellits ED, Baumgardner RA, Veltri EP, Mower M, Grunwald L, Guarnieri T, Aarons D, Griffith LS. Predictors of first discharge and subsequent survival in patients with automatic implantable cardioverter-defibrillators. Circulation. 1991;84:558–566. doi: 10.1161/01.cir.84.2.558. [DOI] [PubMed] [Google Scholar]
- 35.Lubinski A, Bissinger A, Truszcz-Gluza M, Filipecki A, Kargul W, Zajac T, Aarons D. Potentially syncopal arrhythmias in ICD secondary prevention patients. Europace. 2008;10:233. Ref Type: Abstract. [Google Scholar]
- 36.Epstein AE, Miles WM, Benditt DG, Camm AJ, Darling EJ, Friedman PL, Garson A, Jr, Harvey JC, Kidwell GA, Klein GJ, Levine PA, Marchlinski FE, Prystowsky EN, Wilkoff BL. Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations: A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation. 1996;94:1147–1166. doi: 10.1161/01.cir.94.5.1147. [DOI] [PubMed] [Google Scholar]
- 37.Kou WH, Calkins H, Lewis RR, Bolling SF, Kirsch MM, Langberg JJ, de BM, Sousa J, el-Atassi R, Morady F. Incidence of loss of consciousness during automatic implantable cardioverter-defibrillator shocks. Ann Intern Med. 1991;115:942–945. doi: 10.7326/0003-4819-115-12-942. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama T, Powell JL, Mitchell LB, Ehlert FA, Baessler C. Resumption of driving after life-threatening ventricular tachyarrhythmia. N Engl J Med. 2001;345:391–397. doi: 10.1056/NEJM200108093450601. doi:10.1056/NEJM200108093450601. [DOI] [PubMed] [Google Scholar]
- 39.Albert CM, Rosenthal L, Calkins H, Steinberg JS, Ruskin JN, Wang P, Muller JE, Mittleman MA. Driving and implantable cardioverter-defibrillator shocks for ventricular arrhythmias: results from the TOVA study. J Am Coll Cardiol. 2007;50:2233–2240. doi: 10.1016/j.jacc.2007.06.059. doi:10.1016/j.jacc.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 40.Maas R, Ventura R, Kretzschmar C, Aydin A, Schuchert A. Syncope, driving recommendations, and clinical reality: survey of patients. BMJ. 2003;326:21. doi: 10.1136/bmj.326.7379.21. doi:10.1136/bmj.326.7379.21. [DOI] [PMC free article] [PubMed] [Google Scholar]