Abstract
Aims
Collagen degradation in atherosclerotic plaques with thin fibrous caps renders them more prone to rupture. Fibroblast activation protein (FAP) plays a role in arthritis and tumour formation through its collagenase activity. However, the significance of FAP in thin-cap human fibroatheromata remains unknown.
Methods and results
We detected enhanced FAP expression in type IV–V human aortic atheromata (n = 12), compared with type II–III lesions (n = 9; P < 0.01) and healthy aortae (n = 8; P < 0.01) by immunostaining and western blot analyses. Fibroblast activation protein was also increased in thin-cap (<65 µm) vs. thick-cap (≥65 µm) human coronary fibroatheromata (n = 12; P < 0.01). Fibroblast activation protein was expressed by human aortic smooth muscle cells (HASMC) as shown by colocalization on immunofluorescent aortic plaque stainings (n = 10; P < 0.01) and by flow cytometry in cell culture. Although macrophages did not express FAP, macrophage burden in human aortic plaques correlated with FAP expression (n = 12; R2= 0.763; P < 0.05). Enzyme-linked immunosorbent assays showed a time- and dose-dependent up-regulation of FAP in response to human tumour necrosis factor α (TNFα) in HASMC (n = 6; P < 0.01). Moreover, supernatants from peripheral blood-derived macrophages induced FAP expression in cultured HASMC (n = 6; P < 0.01), an effect abolished by blocking TNFα (n = 6; P < 0.01). Fibroblast activation protein associated with collagen-poor regions in human coronary fibrous caps and digested type I collagen and gelatin in vitro (n = 6; P < 0.01). Zymography revealed that FAP-mediated collagenase activity was neutralized by an antibody directed against the FAP catalytic domain both in HASMC (n = 6; P < 0.01) and in fibrous caps of atherosclerotic plaques (n = 10; P < 0.01).
Conclusion
Fibroblast activation protein expression in HASMC is induced by macrophage-derived TNFα. Fibroblast activation protein associates with thin-cap human coronary fibroatheromata and contributes to type I collagen breakdown in fibrous caps.
Keywords: Atherosclerosis, Antibodies, Collagen, Inflammation, Smooth muscle cells
Introduction
Rupture of the fibrous cap in advanced atherosclerotic plaques is a critical trigger of acute coronary syndromes (ACS) that may lead to myocardial infarction and sudden cardiac death. One of the key events in promoting plaque instability is the degradation of the fibrous cap, which exposes the underlying thrombogenic plaque core to the bloodstream, thereby causing thrombosis and subsequent vessel occlusion.1–3 Fibrous cap rupture is facilitated by proteases which cleave type I collagen, the primary load-bearing molecule in fibrous caps, leading to fibrous cap thinning and destabilization.4–7 Therefore, activated proteases, which localize to thin fibrous caps, have attracted attention as potential diagnostic and therapeutic targets.
Candidate targets include matrix metalloproteinase (MMP)-2 and -9 and the cysteine protease cathepsin K each of which are enhanced in both stable and unstable lesions.8–11 Matrix metalloproteinase-2 and cathepsin K staining reveal diffuse localization throughout the plaque, whereas MMP-9 has been shown to colocalize with macrophages beneath the fibrous cap.12–14 Although these proteases have shown potential as markers of atherosclerotic plaques, their diffuse expression in all lesions -warrant careful assessment of their targeting potential towards clinically relevant unstable plaques. An ideal protease target would be specific to the rupture-prone fibrous cap; a site perhaps more easily accessible by intravenously injected targeting agents. Although MMPs and cysteine proteases have been well characterized as protease targets, the role of serine proteases in this context has not been investigated.
Fibroblast activation protein (FAP) is a membrane-bound, constitutively active serine protease expressed by activated fibroblasts in epithelial tumour stroma, arthritis, and wound healing, but remains virtually undetectable in healthy tissues.15–17 Fibroblast activation protein is an enzyme that exhibits dipeptidyl peptidase IV activity, prolyl endopeptidase activity, and specificity for type I collagen.17–19 However, the role of FAP in atherosclerosis is unknown. The aim of this study was to characterize FAP expression in human atherosclerosis and examine its association with features of plaque instability. Moreover, we sought to determine the mechanism of FAP induction, its downstream effects, and the capacity of a neutralizing FAP-specific antibody.
Methods
For the detailed Methods section, see Supplementary material online.
Characterization of atherosclerotic plaques
Biopsies of normal and plaque-bearing ascending aortae were obtained from patients undergoing surgical aortic valve replacement secondary to aortic stenosis [n = 20, age (years): 63 ± 14.5, body mass index: 27.8 ± 5.4, diabetes mellitus 3/20, C-reactive protein (mg/L): 2.1 ± 1.8, triglycerides (mmol/L): 2.2 ± 1.6, lactate dehydrogenase (IU/L): 218.9 ± 37.8]. Aortic plaques were sectioned and graded according to the American Heart Association (AHA) criteria20,21 using Movat pentachrome, Oil-Red-O, anti-CD68, and von Kossa staining (data not shown). Coronary arteries were obtained from patients who died after an acute myocardial infarction and embedded in paraffin for sectioning. Collagen in coronary artery plaques was characterized by Masson staining. Fibrous caps were identified as the collagen-rich tissue separating the lumen and the necrotic core.2 Plaques with a minimum fibrous cap thickness of <65 µm were classified as thin-cap, whereas plaques with a fibrous cap thickness ≥65 µm were classified as thick-cap atheromata.2
Immunofluorescence and immunohistochemistry
Cross-sections from human ascending aortae (10 µm thickness) and paraffin-embedded sections of coronary plaques (4 µm thickness) were mounted on glass slides. Tissue sections were labelled against FAP and cell-specific markers with purchased antibodies directed against CD68, von Willebrand factor (vWF), α-smooth muscle actin (αSMA), or type I collagen and visualization with either fluorescence-labelled secondary antibodies or biotin-labelled secondaries for immunostaining using an ABC staining kit for diaminobenzidine (Vector Labs, Burlingame, CA, USA).
Image analysis
For low-power imaging at spatial resolutions above 1 µm/pixel, a fluorescent microscope (DM60000B; Leica, Wetzlar, Germany) equipped with a fluorescent camera (DFC350 FX; Leica) was used. Colocalization analyses were performed at higher magnifications using a multichannel confocal microscope (TCS SP2; Leica) on a single optical plane.
Cells
Human aortic endothelial cells (HAEC) were isolated from biopsies of ascending aortae without macroscopic lesions obtained from patients undergoing operations for valve repair, human aortic smooth muscle cells (HASMC) were purchased (Promocell), and peripheral blood-derived monocytes were isolated from healthy subjects. Foam cells were generated by stimulating macrophages with 100 µg/mL of oxidized LDL (BT-910; BioConcept, Allschwil, Switzerland) for 48 h in serum-free macrophage medium (SFM; Gibco). Lipid uptake was assessed by Oil-red-O staining (O0624; Sigma-Aldrich).
Fibroblast activation protein induction assays
Quiescent HASMC were treated with starvation media supplemented with 3, 5, 10, 20, and 40% macrophage-conditioned SFM for 48 h. To determine the effects of tumour necrosis factor α (TNFα) on FAP expression, quiescent HASMC were treated with starvation media supplemented with 20% macrophage-conditioned SFM and a TNFα-neutralizing antibody (Ab6671; Abcam) or an IgG isotype control (Ab27478; Abcam) antibody. Recombinant human TNFα (300-01A; Peprotech) was used to induce FAP expression in quiescent HASMC in a dose- and time-dependent manner. Fibroblast activation protein levels were quantified by cell membrane enzyme-linked immunosorbent assay (see Supplementary material online, Figure S1).
Fibroblast activation protein-mediated type I collagen degradation assays
In situ zymography was performed on 5 µm cryosections of human aortic atherosclerotic plaques, which had been stained for FAP using a non-inhibitory antibody (F19). Sections were then incubated with an inhibitory antibody (A246) or isotype control (50 nM) overnight at 4°C. Subsequently, sections were mounted in warm 1% Agarose in phosphate-buffered saline (PBS) supplemented with 10% direct-quenched type I collagen from bovine skin (D12060; Invitrogen) and imaged after 2 h at 37°C using confocal microscopy. Image Quantification was performed as described in the Supplemental material online.
To evaluate the FAP-mediated type I collagen-specific cleavage, full-length native human type I collagen isolated from human placenta (purity > 90%) was used (288; Yo Proteins). Type I collagen (100 ng/mL) was treated with recombinant human FAP (rhuFAP; 200 nM) for 18 h at 37°C in PBS (pH = 7.2) and compared with an untreated collagen control. A246 (50 ng/mL) was added to the solution and compared with an isotype control antibody IgG (Ab27478; Abcam) to validate the neutralizing capacity of A246. Samples were separated by electrophoresis and visualized by silver staining (ProteoSilver Silver Stain Kit, Sigma).
Statistical analyses
Histological and cell culture results were compared using one-way ANOVA and associations calculated by Pearson's correlation coefficient. Student's t-test was used for comparisons of zymography. All statistical analyses were performed using MatLab (Version, R2007b). Data are presented as mean ± SD. Significance was accepted at the level of P < 0.05.
Results
Fibroblast activation protein is expressed by smooth muscle cells, but not macrophages in advanced human aortic plaques
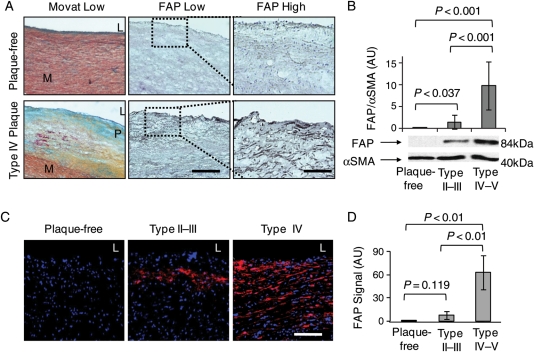
Immunofluorescent stainings for FAP in adjacent cryosections revealed enhanced expression of FAP in fibroatheromata vs. plaque-free aortae (Figure 1A). Positive staining for FAP was virtually absent in healthy ascending aortae, whereas a step-wise increase was observed in type II–III and type IV–V plaques by western blot analyses (Figure 1B; see Supplementary material online, Figure S2) and quantitative image analysis (Figure 1C and D).
Figure 1.
Fibroblast activation protein expression is enhanced in human atherosclerotic aortic plaques. (A) Movat and fibroblast activation protein stainings show cross-sections of representative plaque-free aortae and type IV aortic atherosclerotic plaques (L, lumen; M, media; P, atherosclerotic plaque; bar = 400 µm). Dotted boxes indicate regions of interest in adjacent sections at high magnification (bar = 50 µm). (B) Western blot analysis of fibroblast activation protein normalized to α-smooth muscle actin in plaque-free aortae (n = 8), type II–III plaques (n = 8), and type IV–V plaques (n = 7) shows a significant increase in fibroblast activation protein in advanced type IV–V plaques by immunoblot densitometry. (C) Immunofluorescent stainings in representative tissue sections of plaque-free aortae, type II–III plaque, and type IV plaque show fibroblast activation protein expression in red (DAPI in blue; bar = 50 µm). (D) The graph reveals a significant increase in fibroblast activation protein expression in type II–III aortic plaques (n = 9) and in type IV–V plaques (n = 12) compared with plaque-free aortae (n = 8).
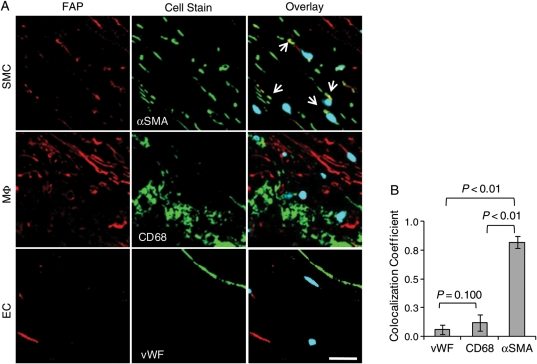
To characterize FAP-expressing cell types in human atherosclerotic plaques, we performed immunofluorescent co-stainings of FAP in macrophages (identified as CD68-positive cells), smooth muscle cells (αSMA-positive cells), and endothelial cells (vWF-positive cells) (Figure 2A). Confocal image analyses revealed that FAP expression by smooth muscle cells, but not by macrophages or endothelial cells (Figure 2B).
Figure 2.
Fibroblast activation protein expression in human aortic plaques colocalizes with smooth muscle cells, but not with macrophages or endothelial cells. (A) Overlays of confocal images of fibroblast activation protein (red) and DAPI (blue) with cell-specific stainings of α-smooth muscle actin, CD68, and von Willebrand factor (green) in representative sections illustrate fibroblast activation protein colocalization (arrows) with smooth muscle cells (bar = 20 µm). (B) The graph quantifies an increased colocalization of fibroblast activation protein with smooth muscle cells (α-smooth muscle actin), compared with endothelial cells (von Willebrand factor) and macrophages (CD68) in type IV–V atherosclerotic plaques (n = 10).
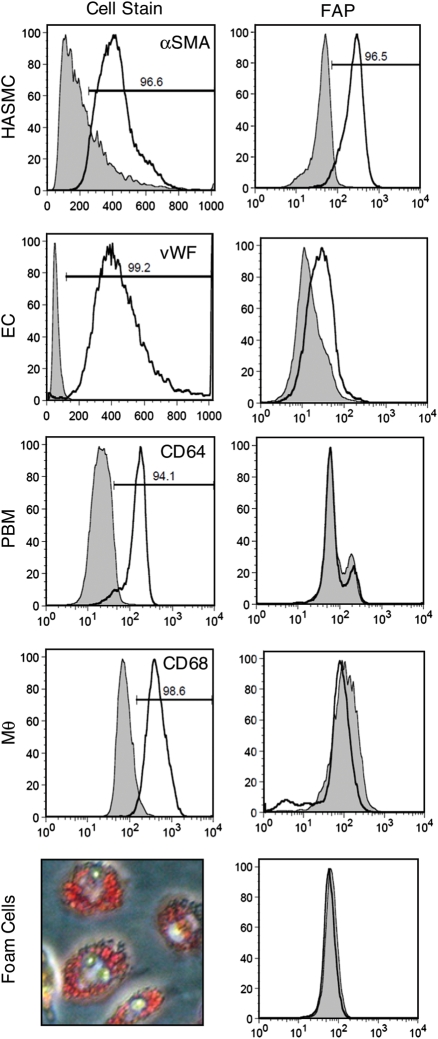
To validate FAP expression by vascular cells in vitro, we performed FACS analyses of FAP in HASMC (αSMA-positive cells), HAEC (vWF-positive cells), peripheral blood-derived monocytes (CD64-positive), macrophages (CD68-positive), and foam cells (Oil-Red-O-positive macrophages). FACS analyses revealed high constitutive FAP expression in HASMC and slight expression in HAEC, but no expression by peripheral blood-derived monocytes, macrophages, or foam cells (Figure 3).
Figure 3.
Fibroblast activation protein is constitutively expressed in cultured human aortic smooth muscle cells (HASMC) and human aortic endothelial cells (HAEC), but not in peripheral blood-derived monocytes (PBM), macrophages (MΦ), or foam cells. FACS analyses and Oil-Red-O staining of peripheral blood derived-macrophages laden with oxidized LDL characterize cells populations (left) and their respective fibroblast activation protein expression (right).
Fibroblast activation protein expression is enhanced in thin-cap vs. thick-cap human coronary atheromata
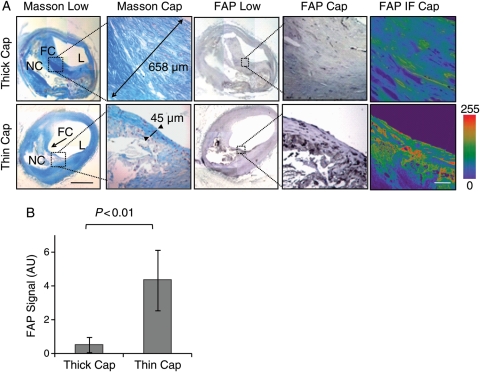
In order to determine the association of FAP with coronary fibrous cap thickness, we stained collagen applying the Masson method (stains collagen in blue) in rupture-prone human coronary arteries obtained from patients who died after myocardial infarction. On the basis of fibrous cap thickness, these specimens were characterized as thin-cap (<65 μm) or thick-cap (≥65 μm) fibroatheromata. Immunohistological and immunofluorescent stainings and subsequent confocal image analyses in adjacent sections revealed enhanced FAP expression in thin compared with thick fibrous caps (Figure 4A and B).
Figure 4.
Fibroblast activation protein expression is enhanced in thin-cap vs. thick-cap human coronary fibroatheromata. (A) Masson staining shows collagen-rich thick (658 µm) vs. thin (45 µm) fibrous caps (L, lumen; FC, fibrous cap; NC, necrotic core; bar = 1 mm). Fibroblast activation protein immunohistochemistry and immunofluorescence (intensity scale; bar = 50 µm) shows fibroblast activation protein expression in representative thin vs. thick caps. Dotted boxes indicate regions of interest in adjacent sections at high magnification. (B) The graph reveals a significant increase in fibroblast activation protein expression in thin vs. thick fibrous caps (n = 12 each).
Fibroblast activation protein expression associates with the macrophage burden in human aortic atherosclerotic plaques
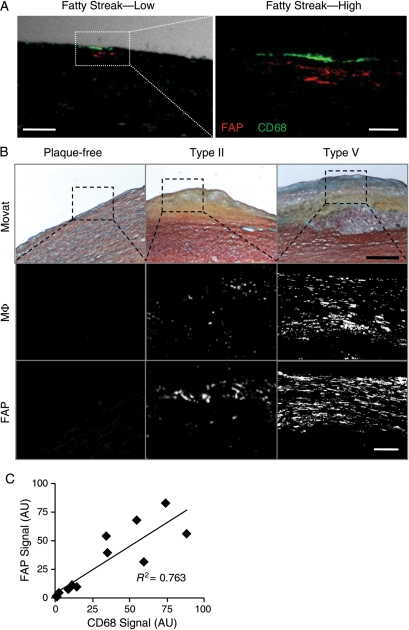
Immunofluorescence stainings revealed FAP expression in medial cells adjacent to macrophages in aortic fatty streaks (Figure 5A). To characterize the relationship between FAP and inflammation, we compared FAP and macrophage immunofluorescent signal intensity in human aortic plaques (Figure 5B). We observed a positive correlation between macrophage burden and FAP expression with plaque progression (R2= 0.763; n = 12; Figure 5C).
Figure 5.
Fibroblast activation protein expression correlates with macrophage burden in human aortic plaques. (A) Confocal immunofluorescent photomicrograph of an aortic fatty streak reveals fibroblast activation protein expression (red) adjacent to macrophages (CD68; green) at low (phase-contrast, white; bar = 100 μm) and high magnification (bar = 25 μm). (B) Movat staining (bar = 400 μm), fibroblast activation protein, or macrophage (CD68) immunofluorescent stainings in plaque-free aortae, type II, and type V atherosclerotic plaques show enhanced fibroblast activation protein expression with increasing macrophage burden (bar = 50 µm). (C) Comparisons of fibroblast activation protein and macrophage expression in serial adjacent sections from aortic plaques demonstrate a significant positive correlation (R2= 0.763; n = 12; P < 0.05); AU, arbitrary units.
Macrophage-derived tumour necrosis factor α induces fibroblast activation protein expression in cultured human aortic smooth muscle cells
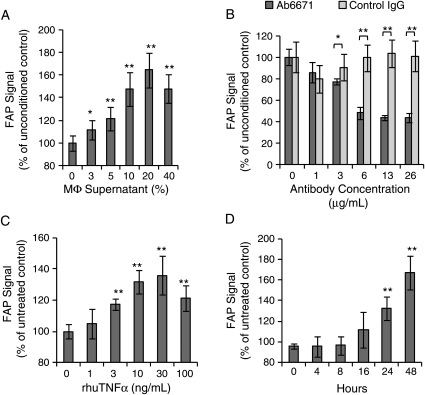
To elucidate a signalling mechanism between macrophages and FAP-expressing HASMC, we exposed HASMC to macrophage-conditioned media for 48 h to simulate conditions applicable to plaque inflammation. Cultured HASMC showed a dose-dependent increase in FAP expression in response to the macrophage-conditioned media with a maximal effect observed at 20% media concentration (Figure 6A) after 48 h. This effect was abolished when macrophage-conditioned media was supplemented with a TNFα-neutralizing antibody (Figure 6B). To confirm this paracrine effect of TNFα on FAP expression, experiments were repeated using recombinant human TNFα. Tumour necrosis factor α-mediated FAP expression was observed in a dose- and time-dependent manner, with a maximum response at 30 ng/mL after 48 h (Figure 6C and D).
Figure 6.
Macrophage-derived tumour necrosis factor α induces fibroblast activation protein expression in human aortic smooth muscle cells. (A) Macrophage-conditioned supernatant induces fibroblast activation protein in human aortic smooth muscle cells in a concentration-dependent manner following 48 h exposure (n = 6). (B) Using the same macrophage-conditioned medium, tumour necrosis factor α-blocking antibody (Ab6671) decreases fibroblast activation protein expression by 40% in human aortic smooth muscle cells compared with an isotype control antibody (n = 6). (C) Recombinant human tumour necrosis factor α induces fibroblast activation protein in human aortic smooth muscle cells in a dose-dependent manner after 48 h incubation (n = 6). (D) Recombinant human tumour necrosis factor α induces fibroblast activation protein in human aortic smooth muscle cells in a time-dependent manner (30 ng/mL). AU, arbitrary units (*P < 0.05, **P < 0.01).
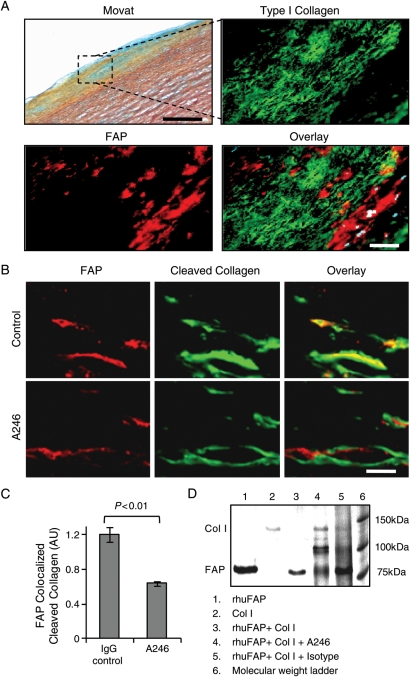
Fibroblast activation protein-mediated type I collagenase activity in aortic fibrous caps is inhibited by a fibroblast activation protein-neutralizing antibody
Immunofluorescence analyses revealed enhanced FAP expression in type I collagen-poor regions of aortic fibrous caps (Figure 7A). Aortic fibrous caps treated with an IgG control antibody showed a colocalization of FAP with cleaved DQ type I collagen, whereas Ab246-treated plaques demonstrated a significantly reduced colocalization of FAP with type I collagenase activity (Figure 7B). Confocal image analyses revealed that A246-treated fibrous caps exhibited decreased cleaved type I collagen at sites of FAP expression (Figure 7C). Type I collagenase activity of rhuFAP and the neutralizing capacity of A246 were demonstrated by incubation of native human type I collagen with rhuFAP in the presence of inhibiting antibody or isotype control (Figure 7D).
Figure 7.
Type I collagenase activity is inhibited in human aortic fibrous caps by the fibroblast activation protein-blocking antibody A246. (A) Movat staining of a fibrous cap in human aortic plaque. The region of interest (black box) is shown at higher magnification in an adjacent section stained for fibroblast activation protein (red), type I collagen (green), and overlay (DAPI = blue; bar = 150 µm). (B) Confocal images of in situ zymography show fibroblast activation protein (red) and cleaved DQ type I collagen (green) in fibrous caps shown by Movat (A) of aortic plaque treated with a control IgG antibody or neutralizing antibody A246 (bar = 10 μm). (C) The graph reveals a significant reduction of cleaved type I collagen colocalized with fibroblast activation protein expression by in situ zymography (n = 10/group). (D) Type I collagenase activity of recombinant human fibroblast activation protein and the neutralizing capacity of A246 was demonstrated by incubation of native human type I collagen with recombinant human fibroblast activation protein in the presence of inhibiting or isotype control antibodies (lanes: 1, rhuFAP; 2, collagen; 3, rhuFAP + collagen; 4, rhuFAP + collagen + A246; 5, rhuFAP + collagen + isotype control antibody; 6, molecular weight marker).
Discussion
The fibrous cap of an atherosclerotic plaque is essential for separating the bloodstream in the vessel lumen from its thrombogenic necrotic core. The mechanical strength of the fibrous cap is provided primarily by type I collagen, which is degraded by MMPs and cysteine proteases, both of which are associated with plaque instability and the occurrence of acute thrombotic events.22–26
This study links the constitutively active serine protease FAP to plaque progression and fibrous cap thinning and provides evidence that (i) FAP expression is enhanced in human aortic atheromata and in fibrous caps of thin-cap coronary plaques, (ii) FAP is expressed in HASMC and its expression correlates with macrophage burden, (iii) FAP expression is induced in HASMC by macrophage-derived TNFα via paracrine signalling, (iv) FAP cleaves collagen in fibrous caps of human atheromata, and (v) FAP-mediated type I collagenase activity is inhibited by an FAP-neutralizing antibody.
Numerous studies implicate matrix-degrading collagenases such as MMP-1, -2, and -9 as well as cysteine proteases such as cathepsins S and K in vascular remodelling and plaque rupture.13,23,27 Our findings provide evidence that FAP is the first known smooth muscle cell-derived serine protease involved in collagen degradation in human atherosclerosis. Fibroblast activation protein expression was particularly enhanced in fibrous caps of thin-cap human coronary plaques isolated from patients who died after myocardial infarction. Indeed, thin fibrous caps (<65 µm) have been associated with sudden cardiac death.2,28 The type I collagenase activity of FAP is demonstrated by both FAP-mediated collagenolysis in fibrous caps and the association of FAP with collagen-poor fibrous cap tissue in image analyses. These observations underline the potential clinical relevance of FAP as a diagnostic and/or therapeutic target in patients with plaques prone to rupture, i.e. patients at risk for ACS or stroke.
Inflammation constitutes a key feature of plaque vulnerability and inflammatory processes have been shown to induce collagenases in atherosclerotic plaques.4,29,30 Consistent with this paradigm, we demonstrate that FAP expression in HASMC is associated with macrophage burden in intermediate and advanced human atherosclerotic plaques. Fibroblast activation protein was not expressed by macrophages. However, macrophage-derived TNFα induced a dose- and time-dependent increase in FAP expression in cultured smooth muscle cells. These data indicate that a paracrine inflammatory pathway can induce FAP expression in smooth muscle cells. Thereby, our findings contribute to a growing body of evidence that supports the notion of inflammation-induced collagenase expression in atherosclerosis.6,11,22,31 Such findings could motivate future studies to investigate putative atheroprotective interventions involving either key anti-inflammatory mechanisms, such as the TNFα pathway, collagen-degrading enzymes themselves such as FAP, or both.
In addition to its expression in smooth muscle cells, we detected constitutive FAP expression in HAEC in vitro. Endothelial activation is a critical step in atherogenesis.32 Activated endothelial cells express fibrous cap-degrading collagenases and have also been shown to act in concert with fibrous cap-degrading smooth muscle cells.5,25,33 Indeed, the observed capacity of endothelial cells to express FAP in vitro supports the notion of a coordinated remodelling of the fibrous cap by both endothelial and smooth muscle cells. Furthermore, by recruiting blood-borne inflammatory cells to the plaque, activated endothelial cells may also enhance macrophage-derived cytokine release, activate smooth muscle cells, and thus induce FAP expression and/or activity.
Distinct from MMPs, FAP combines several unique properties: MMPs exhibit diffuse expression throughout both intermediate and advanced atherosclerotic plaques.14 In contrast, FAP expression is associated specifically with thin-cap atheromata. Moreover, MMP activity is modified by tissue inhibitors of metalloproteinases, whereas no natural inhibitors of the constitutively active FAP are identified. It is therefore plausible that FAP expression associates with its activity, rendering FAP a potential diagnostic target. Furthermore, we demonstrate that FAP is mainly expressed in activated smooth muscle cells in thin fibrous caps of advanced atherosclerotic plaques. Given its localization in the fibrous cap close to the bloodstream, FAP may be more accessible than MMPs by circulating targeting agents.34
Taken together, we found that FAP expression is induced by macrophage-derived TNFα in HASMC, associates with thin-cap human coronary plaques, and contributes to type I collagen breakdown in fibrous caps. Thus, FAP expression in thin-cap coronary plaques and endothelial cells renders this target attractive for further investigation in patients with ACS. Along this line, we plan to investigate whether soluble FAP may be a biomarker for ACS. Moreover, our present findings demonstrate that FAP-mediated collagenolysis is induced by inflammatory cues and may be blocked by neutralizing antibodies. At the experimental level, studies using genetic or pharmacological modulation of FAP will shed light onto the causal role of FAP in atherogenesis and its potential use as a target in atherosclerosis and other inflammatory diseases such as rheumatoid arthritis and tumour formation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the Swiss National Science Foundation 31-114094/1, 310030-130626/1 (C.M.M.) and 3100-068118 (T.F.L.), the Herzog-Egli Foundation (C.E.B. and C.M.M.) and the Hartmann Müller Foundation (C.E.B. and C.M.M.), both at the University of Zürich, the University Research Priority Program ‘Integrative Human Physiology’ at the University of Zurich (C.E.B., P.R., T.F.L., S.P.H., and C.M.M.). Further support was provided by unrestricted grants from the MERCATOR Foundation Switzerland. Funding to pay the Open Access publication charges for this article was provided by the Foundation for Cardiovascular Research.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Sonja Matter, Stephan Keller, and Sokrates Stein for their excellent support, the Zurich Center for Integrative Human Physiology (ZIHP), Andres Kaech at the Center for Microscopy and Image Analysis at the University of Zurich for access to imaging facilities, Thomas Wüest for his help in preparing targeting agents, and Irina Agarkova for providing expertise on immunofluorescent imaging.
References
- 1.Farb A, Burke AP, Tang AL, Liang Y, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. doi:10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 3.van der Wal A, Becker A, van der Loos C, Das P. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 4.Shah P, Falk E, Badimon J, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon J, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 5.Rajavashisth TB, Xu X-P, Jovinge S, Meisel S, Xu X-O, Chai N-N, Fishbein MC, Kaul S, Cercek B, Sharifi B, Shah PK. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques. Circulation. 1999;99:3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 6.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 7.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. doi:10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 8.Aikawa E, Nahrendorf M, Figueiredo J-L, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. doi:10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 9.Jaffer FA, Kim D-E, Quinti L, Tung C-H, Aikawa E, Pande AN, Kohler RH, Shi G-P, Libby P, Weissleder R. Optical visualization of cathepsin k activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. doi:10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 10.Schafers M, Riemann B, Kopka K, Breyholz H-J, Wagner S, Schafers KP, Law MP, Schober O, Levkau B. Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation. 2004;109:2554–2559. doi: 10.1161/01.CIR.0000129088.49276.83. doi:10.1161/01.CIR.0000129088.49276.83. [DOI] [PubMed] [Google Scholar]
- 11.Deguchi J-O, Aikawa M, Tung C-H, Aikawa E, Kim D-E, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. doi:10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 12.Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schönbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117–1126. doi: 10.1172/JCI10403. doi:10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins s and k in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. doi:10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong Y-Z, Yu X, Tang J-J, Ouyang X, Huang X-R, Fingerle-Rowson G, Bacher M, Scher LA, Bucala R, Lan HY. Macrophage migration inhibitory factor induces mmp-9 expression: implications for destabilization of human atherosclerotic plaques. Atherosclerosis. 2005;178:207–215. doi: 10.1016/j.atherosclerosis.2004.08.030. doi:10.1016/j.atherosclerosis.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S, Jendro M, Wadle A, Kleber S, Stenner F, Dinser R, Reich A, Faccin E, Godde S, Dinges H, Muller-Ladner U, Renner C. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8:R171. doi: 10.1186/ar2080. doi:10.1186/ar2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edosada CY, Quan C, Wiesmann C, Tran T, Sutherlin D, Reynolds M, Elliott JM, Raab H, Fairbrother W, Wolf BB. Selective inhibition of fibroblast activation protein protease based on dipeptide substrate specificity. J Biol Chem. 2006;281:7437–7444. doi: 10.1074/jbc.M511112200. doi:10.1074/jbc.M511112200. [DOI] [PubMed] [Google Scholar]
- 17.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–36512. doi: 10.1074/jbc.274.51.36505. doi:10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 18.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E, Rettig WJ, Gorrell MD. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–1778. doi: 10.1002/hep.510290631. doi:10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 19.Aertgeerts K, Levin I, Shi L, Snell GP, Jennings A, Prasad GS, Zhang Y, Kraus ML, Salakian S, Sridhar V, Wijnands R, Tennant MG. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein α. J Biol Chem. 2005;280:19441–19444. doi: 10.1074/jbc.C500092200. doi:10.1074/jbc.C500092200. [DOI] [PubMed] [Google Scholar]
- 20.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 21.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 22.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. doi:10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 23.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 24.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. doi:10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sluijter JPG, Pulskens WPC, Schoneveld AH, Velema E, Strijder CF, Moll F, de Vries J-P, Verheijen J, Hanemaaijer R, de Kleijn DPV, Pasterkamp G. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37:235–239. doi: 10.1161/01.STR.0000196986.50059.e0. doi:10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 26.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 27.Lutgens SPM, Cleutjens KBJM, Daemen MJAP, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007;21:3029–3041. doi: 10.1096/fj.06-7924com. doi:10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Farb A, Malcom GT, Liang Y-H, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. doi:10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 29.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kd gelatinase in human coronary atherosclerotic lesions: association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 30.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 31.Spagnoli LG, Bonanno E, Mauriello A, Palmieri G, Partenzi A, Sangiorgi G, Crea F. Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2002;40:1579–1588. doi: 10.1016/s0735-1097(02)02376-8. doi:10.1016/S0735-1097(02)02376-8. [DOI] [PubMed] [Google Scholar]
- 32.Szmitko PE, Wang C-H, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part i. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. doi:10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 33.Skinner MP, Raines EW, Ross R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type i collagen-coated membranes. Am J Pathol. 1994;1:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 34.Matter CM, Stuber M, Nahrendorf M. Imaging of the unstable plaque: how far have we got. Eur Heart J. 2009;30:2566–2574. doi: 10.1093/eurheartj/ehp419. doi:10.1093/eurheartj/ehp419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.