Abstract
Background:
The pterional approach is the most common for AComm aneurysms, but we present a unilateral approach to a midline region for addressing the AComm complex. The pure subfrontal approach eliminates the lateral anatomic dissection requirements without sacrificing exposure. The subfrontal approach is not favored in the US compared to Asia and Europe. We describe our experience with the subfrontal approach for AComm aneurysms treated at a single institution.
Methods:
We identified 28 patients treated for AComm aneurysms through the subfrontal approach. Patient records and imaging studies were reviewed. Demographics and case data, as well as clinical outcome at 6 weeks and 1 year were collected.
Results:
Mean patient age was 48 (range 21–75) years and 64% suffered subarachnoid hemorrhage (SAH). All aneurysms were successfully clipped. Gyrus rectus was resected in 57% of cases, more commonly in ruptured cases. Intraoperative rupture occurred in 11% of cases. The average operative time was 171 minutes. There were two patient deaths. Ninety-two percent of patients had a Glasgow Outcome Scale (GOS) of 5 at 6 weeks. All unruptured patients had a GOS of 5. At 12 months, 96% of all patients had a GOS of 5.
Conclusions:
The subfrontal approach provides an efficient avenue to the AComm region, which reduces opening and closing friction but still yields a comprehensive operative window for access to the anterior communicating region.
Keywords: Aneurysm clipping, anterior communicating artery, craniotomy, subfrontal
INTRODUCTION
Anterior communicating artery (AComm) aneurysms are the most common and some of the most complex aneurysms, accounting for approximately 30–37% of intracranial aneurysms.[6] A variety of operative approaches to the anterior communicating complex for intracranial aneurysms have been described,[1–11,14–25,27–48] but the most commonly used is the pterional approach best described by Yasargil.[44] Neurosurgeons are comfortable with this approach because it is familiar and applicable to a wide variety of lesions, and provides relatively easy and rapid access to the basal cisterns. With aggressive sphenoid and posterolateral orbital bone removal, the pterional corridor provides exposure to the proximal A1 segment of the anterior cerebral artery (A1) when there is asymmetry in the supply to the AComm complex. It also provides access to other common aneurysm locations when multiple aneurysms are present ipsilateral to the chosen side of approach, and provides an anterolateral trajectory to the AComm region that allows for easier visualization of perforating vessels supplying the septal region and chiasm that originate off the back of the AComm artery.
There are, however, some disadvantages to the pterional approach. This is a unilateral approach to a midline structure with complex bilateral anatomical relationships. In some instances, retraction on the frontal lobe cannot be achieved safely or adequately without widely opening the sylvian fissure, increasing the temporal lobe, insula, draining veins and middle cerebral artery (MCA) dissection trauma. Extensive and time-consuming bone removal is required to minimize brain retraction and can be cosmetically disfiguring because of temporalis muscle atrophy or damage to the frontalis branch of the facial nerve, especially when paired with orbital osteotomy as frequently described.[1,11,20,36] Superiorly projecting AComm aneurysms, and sometimes the AComm artery itself, are often poorly visualized in the interhemispheric fissure when approached anterolaterally unless the gyrus rectus is widely resected, or the orbital rim is removed, both of which lengthen the procedure and increase cosmetic and neurologic risks further.
Like most neurosurgeons in the United States, we were trained to approach AComm aneurysms through the pterional approach, but we have found that during AComm aneurysm surgery through the pterional approach, we generally performed much or all of the procedure through the anterior and medial portions of the craniotomy. This led the most senior surgeon (PKM) to consider a modified unilateral anterior subfrontal craniotomy for approaching AComm aneurysms. The subfrontal approach, either unilateral or bilateral, to AComm aneurysms has been described[6,18,25,41] but has not been widely adopted in the United States for treatment of AComm aneurysms. Additionally, Hernesniemi has described extensively the European experience with the lateral supraorbital approach – a similar although minimally invasive approach to AComm aneurysms.[12,13]
We adopted and modified a technique that limits the pterional bone removal and temporal lobe exposure, similar to what has been described by others.[2,5–7,11,18,20,21,23–25,28,32,36,37,44,48] It is important to emphasize that the purpose was not to create a minimally invasive exposure with limited access, but rather to tailor the pterional exposure and blend it with the subfrontal approach to this region to create a rapid, efficient and ample exposure for most AComm aneurysms. We present recent results in the patients treated through this approach because we are convinced that this exposure is similar to but more direct than the pterional approach, and offers an excellent option for rapid and adequate exposure and treatment of most AComm aneurysms.
MATERIALS AND METHODS
Approval to conduct this study was obtained after review of the research protocol by both the University of Rochester Research Subjects Review Board and the Institutional Review Board of Rochester General Hospital. We performed a retrospective review of the medical records of a consecutive series of patients with AComm aneurysms who were treated either at the University of Rochester Medical Center or Rochester General Hospital. Patients with other lesions and pathologies treated with this approach were excluded. We identified 28 patients treated for AComm aneurysms through the subfrontal approach, out of a total of 568 patients treated for aneurysms between May 2000 and August 2007.
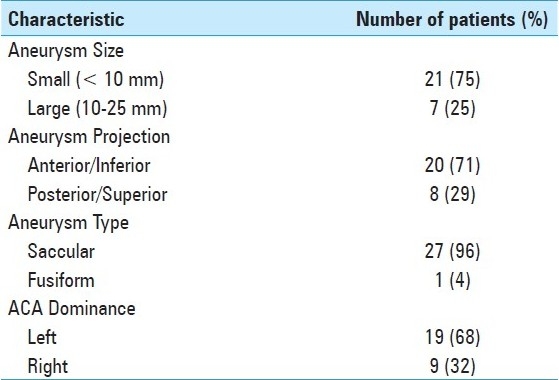
The admission characteristics of the patients are summarized in Table 1. All patients underwent diagnostic cerebral angiography, although one patient with an unruptured aneurysm had surgery based on computed tomography angiography/magnetic resonance angiography (CTa/MRa) alone. Data regarding aneurysm morphology including mode of presentation, location/size of the aneurysm, and anterior cerebral artery (ACA) dominance were collected and are summarized in Table 2.
Table 1.
Clinical characteristics of 28 patients with Acomm aneurysms treated through the subfrontal approach*
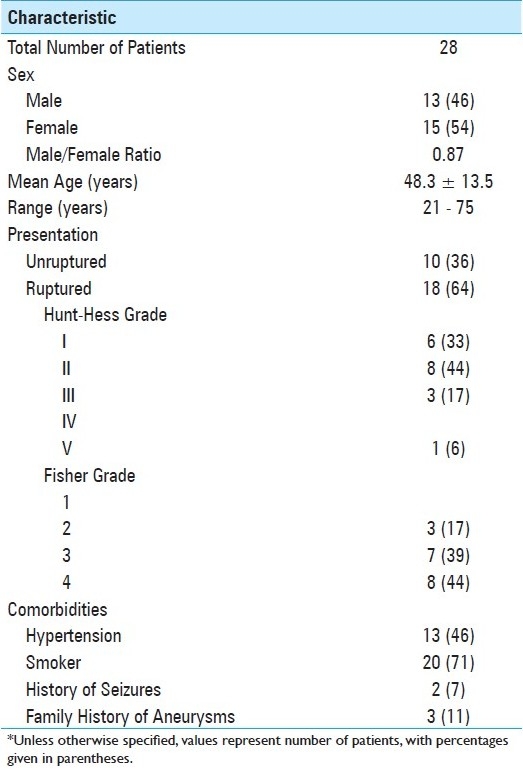
Table 2.
Summary of morphological characteristics
Surgical case characteristics and details regarding postoperative course were reviewed and are summarized in Tables 3 and 4, respectively. The clinical outcome of the patients was assessed at the time of discharge, at 6 weeks follow-up and 1 year follow-up by Glasgow Outcome Scale (GOS), defined as follows: GOS 5 – good recovery (resumption of normal life despite deficits), GOS 4 – moderate disability (disabled but independent), GOS 3 – severe disability (conscious but disabled), GOS 2 – persistent vegetative state, and GOS 1 – death.
Table 3.
Summary of surgical case characteristics*
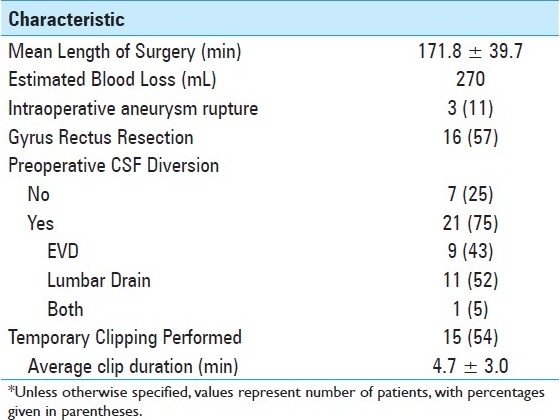
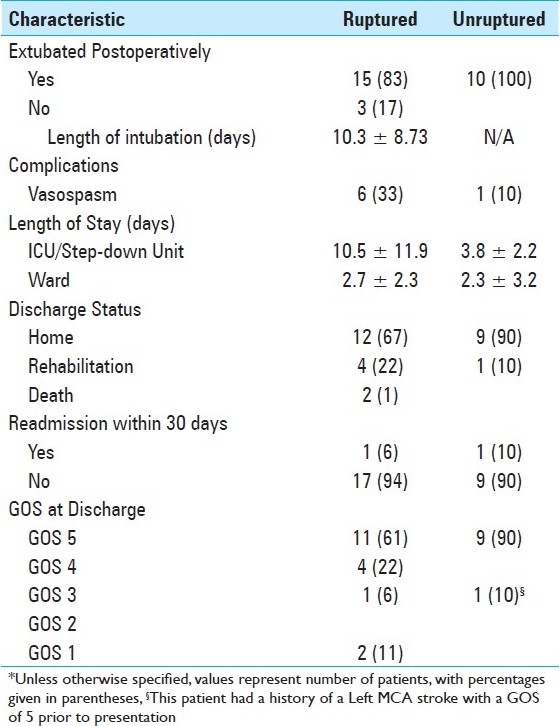
Table 4.
Summary of post-operative course*
Surgical technique
Positioning and preparation
After induction of endotracheal general anesthesia, a lumbar drain [for unruptured or Hunt Hess grade 1–2 subarachnoid hemorrhage (SAH) patients] is recommended to provide brain relaxation; SAH patients presenting with Hunt Hess grade 3–5 routinely get a ventriculostomy as part of their initial management on admission. We remove up to or more than 100 ml of cerebrospinal fluid (CSF) during the approach phase of the procedure to allow for adequate brain relaxation. Mannitol is also administered at the time of skin incision (0.5–1 g/kg body weight, up to 100 g total). Patients are positioned supine. The head is placed in three-point fixation, the head pointing either straight up or turned 10°–20° from midline opposite the side of the planned craniotomy, with adjustments of the head rotation made during surgery as needed by tilting the operating table. The neck is extended approximately 20° to facilitate frontal lobe retraction, and then reverse Trendelenberg positioning is used as needed to reduce venous congestion and also provide better CSF relaxation. Each patient has a Foley catheter, arterial catheter, and multiple peripheral intravenous lines, with more invasive monitoring utilized in higher grade subarachnoid patients.
Skin incision and craniotomy
The skull can be exposed by either a supraorbital skin incision just superior to the eyebrow through a pre-existing skin crease or a modified pterional skin incision [Figure 1a]. We prefer the modified pterional incision in most cases, unless the patient is a bald male, in which case the supraorbital incision provides equal cosmetic results. The modified pterional incision starts 0.5–1 cm in front of the tragus and slightly above the zygomatic arch, and curves supero-anteriorly behind the hairline to just past midline, allowing for exposure of the frontal bone up to midline. The superficial temporal artery is identified early and controlled with bipolar cautery and divided as necessary; the incision is carried through the galea but care is taken not to interrupt the underlying pericranium, in case it is needed to cover a defect in the frontal air sinus. The scalp flap is gently elevated and turned anterior and inferior by dissecting between the temporal fascia and the aponeurosis of the temporal muscle. In order to avoid damage to the frontal branch of the facial nerve, dissection is stopped at the level of the supraorbital and temporalis fat pad (if the orbital rim needs to be removed, an interfascial dissection of the temporal fat pad can be performed, with the fat pad mobilized continuous with the scalp flap and dissection of the periorbita performed at this time to expose the lateral orbital rim and wall). Once the scalp flap is mobilized, it is retracted anteriorly and inferiorly using scalp hooks. The pericranium is then incised as a flap based on the orbital rim, and gently elevated to preserve its vascular pedicle over the orbital rim. Unless the orbital rim is to be removed, the dissection of the pericranium proceeds inferiorly until the orbital rim and supraorbital nerve are encountered. The pericranial flap is then swaddled in a moist sponge, retracted anteroinferiorly over the scalp flap, and preserved for later use if needed. The fascia of the temporal muscle is incised by monopolar cautery at the temporal line for 1–2 cm, and the temporal muscle is elevated in the region of the orbital pillar and the anterior aspect of the linea temporalis using a sharp curved dissector, with care taken to preserve the neurovascular layer on the undersurface of the muscle. No soft tissue dissection is needed lateral to the key point at the frontozygomatic rim [Figure 1a, b].
Figure 1.
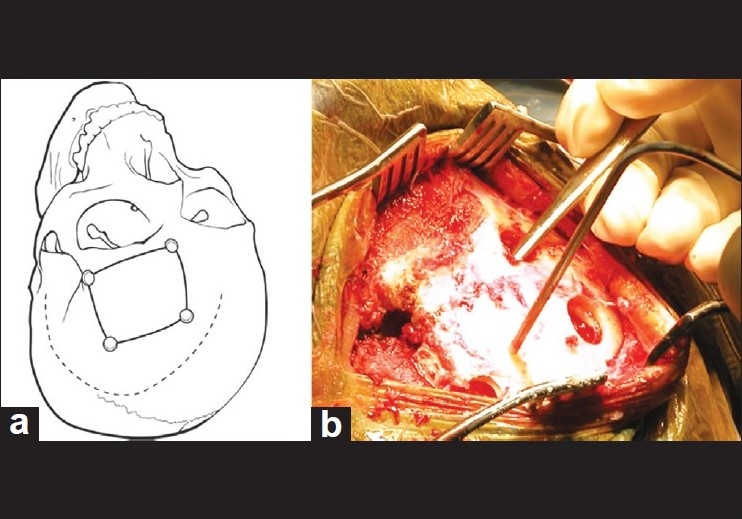
Placement of incision and burr holes for the subfrontal approach. (a) Incision can be a modified pterional incision as shown in the drawing or a supraciliary incision. (b) Through a supraciliary incision, burr holes are placed identically as shown in the drawing
Following placement of a burr hole at the key point, a burr hole is placed just lateral to the midline and just superior to the orbital rim. The orbital rim itself is not removed routinely, although it can be if needed for lesions located high in the interhemispheric fissure. Frontal sinus entry, if present, is addressed by removing the mucosa and then pericranial coverage at closure. The superior burr holes are placed approximately 4–6 cm superior to the burr holes previously described, with a larger craniotomy used in cases when it is anticipated that the brain may be tight [younger patients or higher grade SAH patients in whom the previously placed ventriculostomy indicates elevated intracranial pressure (ICP)]. After the bone flap is removed [Figure 2], the dura is elevated off the inner cortical layer of the orbital rim, and this rim is drilled flush with the outer cortical layer, so that it provides a better viewing angle along the orbital roof. In cases where there are extreme protuberances of bone along the orbital roof, the dura may be elevated and these protuberances are drilled flat [Figure 3]. Similarly, if it is anticipated that an interhemispheric approach may also be needed, the craniotomy can be extended medially by separating the dura and the anteriormost portion of the superior sagittal sinus from the bone under direct visualization and then extension of the bone flap across midline, although we have not needed this additional exposure in any cases thus far.
Figure 2.
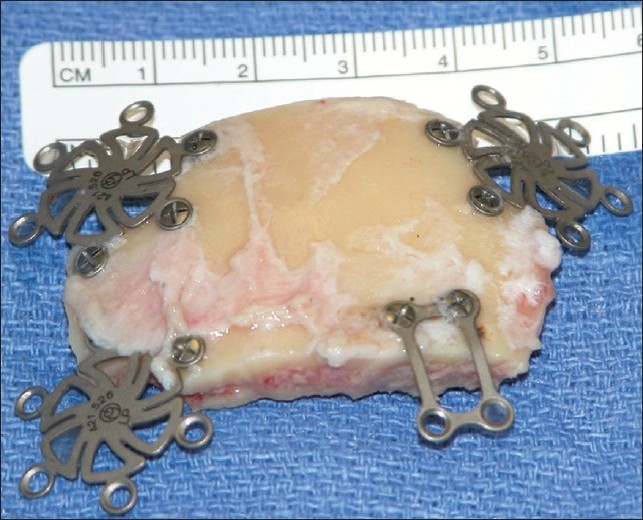
Standard unilateral subfrontal bone flap. While the bone flapbeing is smaller than a standard pterional bone flap, it is not a “mini” craniotomy. Reconstruction is easily achieved with standard titanium miniplates and screws
Figure 3.
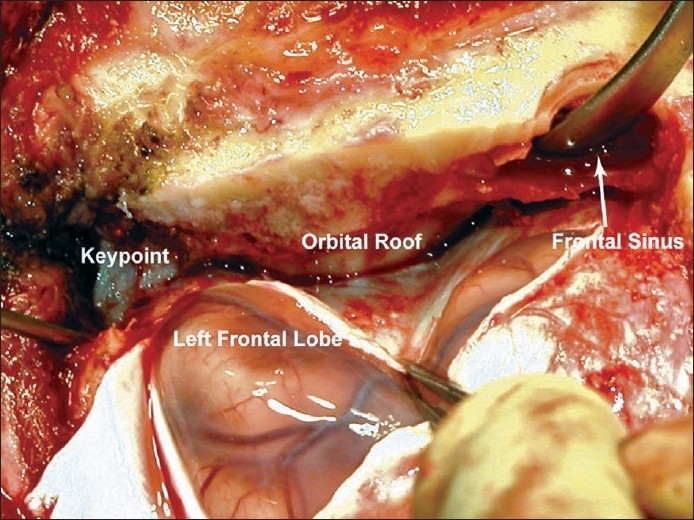
Interior bone removal for the subfrontal approach. The inner table of the cranial opening can be drilled flat, along with any bony protuberances on the superior orbital roof, providing a smooth corridor for approach. Entry into the frontal sinus can be excluded during closure using a pericranial flap (not shown)
Approach
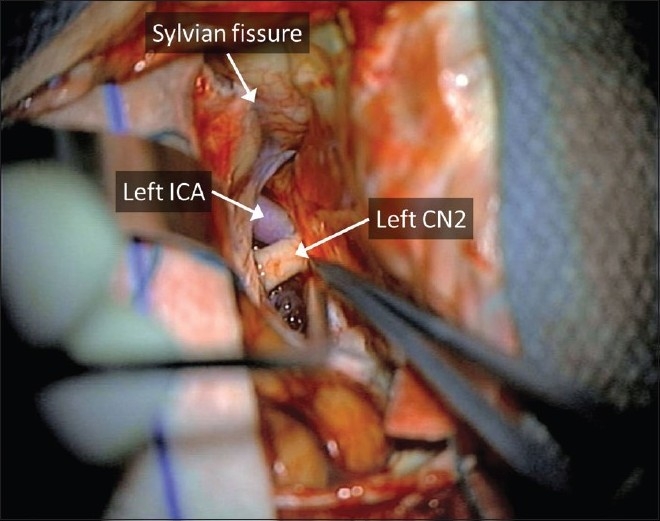
The dura is opened as a flap based on the orbital roof and retracted over the orbital rim, allowing for temporary occlusion of any entry into the frontal air sinus and also a smooth surface for the introduction of microinstruments. Greenberg retractors are used to provide gentle elevation of the orbitofrontal cortex. The olfactory nerve is identified, the olfactory cistern is opened and the frontal lobe is progressively retracted until the carotid cistern is visualized along with the optic nerve and proximal carotid artery [Figure 4]; if the patient has a short supraclinoid carotid segment and a long A1 segment, this usually leads right to the proximal A1 and makes exposure of the internal carotid artery (ICA) bifurcation (and the delicate perforators that originate there) unnecessary. Once the proximal A1 segment is seen, it is inspected for a perforator-free zone that could serve as a point for temporary clipping, if needed. From this point on, dissection proceeds along identical to the pterional approach described by Yasargil, with the retractors progressively deepened to allow for a smooth application of pressure along the orbital cortex. A retractor is placed lateral to the olfactory nerve to allow exposure of the gyrus rectus.
Figure 4.
Operative corridor of the subfrontal approach. With CSF removal and gentle elevation of the frontal lobe, easy access is achieved to the optic nerve and internal carotid artery, from which standard dissection toward an AComm aneurysm can proceed
The ICA, if needed, can be readily dissected distally with this exposure. The initial dissection is always weighted toward lateral frontal lobe retraction to avoid unnecessary early elevation and potential avulsion of the medially located aneurysm, especially if the aneurysm points anteroinferiorly toward the tuberculum sella. The A1 is followed toward the interhemispheric fissure to the AComA complex. In cases where the AComm or aneurysm is wedged high in the interhemispheric fissure, it may still be necessary to resect a small portion of gyrus rectus. The aneurysmal dissection and clip placement are standard in technique [Figure 5], although once the aneurysm is clipped it must be mobilized and inspected carefully, especially on its posterior surface, to make sure that no perforators coming off the back of the aneurysm or AComm complex are caught in the clip reconstruction. In cases where the aneurysm points superiorly, we frequently will perform a "temporary" clipping of the aneurysm with the clip purposefully placed higher on the neck, leaving a small remnant but effectively eliminating the risk of aneurysm occlusion while more aggressive mobilization of the aneurysm allows for better visualization of posterior located perforating vessels. Once these have been defined, a permanent clip application can be performed [Figure 5].
Figure 5.
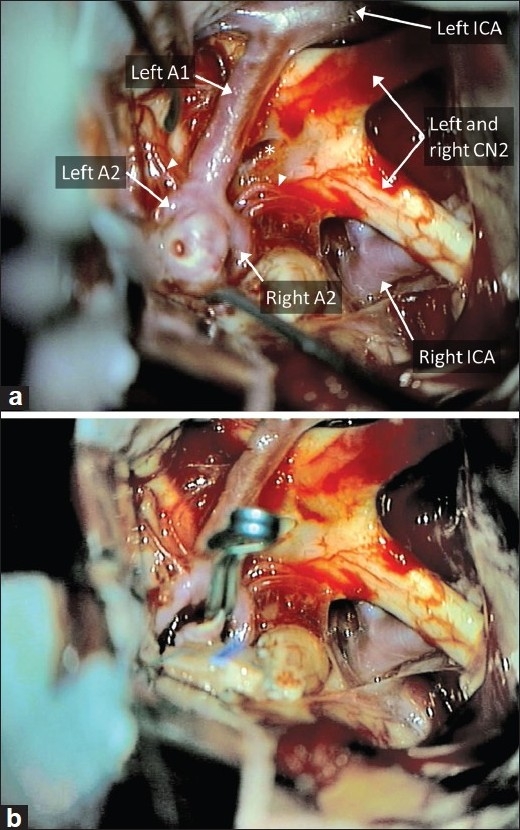
Microscopic visualization of the AComm complex through the subfrontal approach. (a) The entire AComm region and aneurysm, extending over to the contralateral side, can be easily visualized through the subfrontal approach, in this case with no gyrus rectus resection. Note that this patient did not have a right A1 segment. Note lamina terminalis (star) and perforators (arrowheads). A temporary clip is applied to the left A1 in preparation for final dissection around the aneurysm dome. (b) The view after aneurysm clipping
Closure
Closure is rapid due to the limited dissection of the opening. The dura is closed in watertight fashion with interrupted 4-0 nylon sutures. The frontal sinus is dealt with by mucosal stripping, cranialization of the part of the sinus present on the free bone flap with removal of the inner table and drilling of the sinus to eliminate any nests of mucosal cells, no plugging of the frontal sinus aperture, and a pericranial flap tacked down to the dura to exclude the sinus from the intracranial space. The bone plate is fixed in position with titanium burr hole covers, the galea is closed with interrupted 2-0 Vicryl sutures (Ethicon, Somerville, NJ, USA) and the scalp with staples behind the hairline (pterional incision) or subcuticular Monocryl sutures (Ethicon) for exposed areas not covered by hair, or in cases where a supraciliary (forehead) incision is used.
RESULTS
The surgical case characteristics are summarized in Table 3. All aneurysmal lesions were successfully clipped. We frequently use CSF diversion to facilitate exposure and currently generally favor the option of lumbar drainage over ventricular catheter placement in most cases. When CSF diversion was used in unruptured cases (four cases), a lumbar drain was placed to facilitate brain relaxation with the drain being removed at the end of the operation. Gyrus rectus was resected to facilitate exposure in 50% of unruptured aneurysm cases and 61% of ruptured cases. The average length of surgery was approximately 171 minutes. Intraoperative rupture occurred in 11% of cases, all of which were previously ruptured aneurysms. Temporary clips were used in 54% of cases, generally to reduce wall tension at final dissection and clipping. The average temporary clip time was 4.67 minutes; with only ipsilateral A1 clip placement in all cases of temporary occlusion, except for three cases of bilateral A1 clip placement.
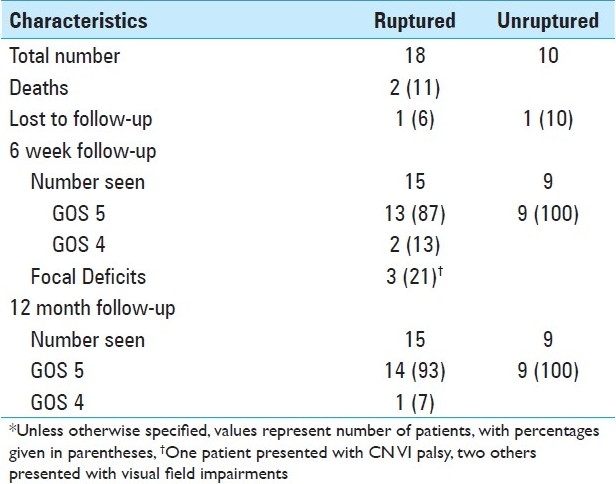
Neurologic outcome is as summarized in Tables 4 and 5. Most patients did extremely well without complications postoperatively. It should be noted, though, that very few patients were high grade in this series. The most common complication postoperatively was vasospasm (25%), and no patient developed a CSF leak or bacterial meningitis. The majority of patients had an excellent outcome at the time of discharge, with 90% of unruptured and 61% ruptured patients having a GOS score of 5. After discharge, one patient was readmitted for hydrocephalus and another patient for headache. In addition, we had two patient deaths in this series secondary to complications of vasospasm.
Table 5.
Follow-up Data*
DISCUSSION
A wide variety of approaches to the AComm region have been described,[7,9,15,18,20,23,25,33–35,37,38,41,45–47] and our description of the subfrontal approach only differs from other reports in subtle ways, but these subtleties have made big differences in our practice. All of the approaches provide appropriate exposure and achieve satisfactory results. Each individual neurosurgeon's experience, level of comfort, assessment of aneurysm anatomy and how this plays to the strengths and weaknesses of a particular approach and the frequency of complications for different approaches, all weigh into the final decision as to the approach.
Our described modified subfrontal approach provides a number of advantages that we further describe below, including: 1) a bilateral view of a bilateral structure, 2) minimized temporal lobe exposure and sylvian fissure dissection, 3) easier access to the interhemispheric fissure and better visualization of superiorly projecting aneurysms, and 4) quick access to both A1 vessels when there is no dominance.
Merits of the unilateral subfrontal approach
The pterional exposure can provide rapid access to the basal cisterns and easy identification of the ipsilateral carotid artery and access to the A1 artery, which can be important if the ipsilateral A1 artery is the dominant supply to the AComm region and the aneurysm (often this dictates the side of approach). A downside of the pterional approach is that it is an anterolateral corridor to the midline AComm region; while this may be advantageous for delineation of the posterior septal perforators coming off the back of the AComm, it puts the contralateral structures of the AComm region on the far side of the aneurysm neck, making dissection of the necessary anatomy complicated by having to work around and past the aneurysm neck. Some authors describe liberal use of temporary clips,[18,26,30,37] but this puts downstream brain at risk and clutters the operative corridor with clips that make definitive aneurysm dissection and clip reconstruction more difficult. In addition, from the anterolateral trajectory of the pterional approach, superiorly projecting AComm aneurysms and often the AComm itself are hidden in the interhemispheric fissure; visualization of the relevant anatomy then requires tangential dissection of the interhemispheric fissure or resection of the gyrus rectus that, in our experience, is more extensive than what is required in our approach.
The subfrontal approach we have adopted solves many of the issues of the anterolateral corridor by focusing on the anterior exposure available from the pterional approach and extending it nearly to the midline, while limiting the lateral exposure that is not always necessary for aneurysm exposure and treatment. By focusing on the anterior trajectory, the unilateral subfrontal approach provides an en face and panoramic view of the AComm region while limiting the risk of bifrontal injury attendant in a true interhemispheric or bilateral subfrontal approach. Often this obviates the need for gyrus rectus resection, although when a better view of the aneurysm is desired, we have resected only a small portion of gyrus rectus, but even in these cases our impression is that this amount of gyrus rectus resection is reduced compared to the standard pterional approach. We also feel this makes the final dissection in cases of ruptured lesions safer, often facilitates early A1 segment exposure and, consequently, proximal control. In those cases where the internal carotid segment is relatively short, the A1 segment often is readily exposed without needing to dissect the bifurcation, and in these cases no gyrus resection may be necessary. Nonetheless, the topic of gyrus rectus resection continues to generate controversy, and we have no way of comparing the extent of resection in our patient cohort to that of other reported series, but as many others have noted, unilateral resection does not seem to cause significant neurologic consequences.[1,14,22,25,35,36,40] However, as far as resection of “normal brain” is concerned (regardless of approach), it is logical to believe less is more. Thus, if some gyrus rectus resection is possible, some surgeons may not prefer this surgical approach, despite any potential advantages.
Our craniotomy also eliminates the need for extensive resection of the sphenoid wing that is often necessary for the pterional craniotomy. This is straightforward to accomplish, but is time consuming and creates a cranial defect that must be repaired, especially if the bone is thinned out to the point where the orbital walls are removed as is frequently advocated.[7,10,11,20,21,36] An additional benefit of the subfrontal approach is that the temporal lobe and sylvian fissure are left undisturbed. When a pterional approach is used to expose the AComm region, it is almost always necessary to split the sylvian fissure; without this dissection, retraction on the frontal lobe “drags” the temporal lobe into the operative corridor. Splitting the sylvian fissure carries with it the risk of injury to the superficial and deep sylvian veins, the MCA, and the perisylvian cortex. With modern microsurgical technique, this risk is low but not negligible. We feel that the exposure through the subfrontal corridor narrows the “at risk” territory to the orbitofrontal cortex that would be at risk with either approach.
Another question that arises in any approach to AComm aneurysms is the side of the approach, which is usually dictated by three factors: side of A1 dominance, direction of aneurysm projection and location of frontal lobe hemorrhage if present. AComm aneurysms frequently arise because of asymmetry in the A1 supply to the AComm region, with aneurysms typically occurring ipsilateral to the dominant A1 and the fundus projecting toward the contralateral side in up to 80% of cases in many series.[33,34,41] This has led many surgeons, including the authors of this manuscript, to advocate that the craniotomy should be performed ipsilateral to the side of the dominant A1, so that relative proximal control is available.[3,14,33,41]
The relative disadvantages of this technique include possible exposure of the frontal air sinus necessitating repair and presumably increasing the risk for potential CSF leak, infection and mucocele formation. Other possible disadvantages include: risk of bifrontal injury (although this can be minimized by using a unilateral low frontal craniotomy), an anterior view of the AComm complex that leaves delicate septal perforators at risk on the backside of an aneurysm and early exposure of the aneurysm fundus for anteroinferiorly projecting AComm aneurysms that are adherent to the optic chiasm or tuberculum sella. Thus, anteroinferiorly projecting aneurysms may benefit from the visualization/operative corridor offered by the pterional craniotomy, as compared to subfrontal approaches.
It is important to emphasize that this is not designed to be a “minimally invasive” approach to the AComm region, as described by others,[2,5,9,11,20,21,27,28,32,36,48] and it was never our intent to limit bone removal at the expense of the surgeon's ability to maneuver. Aneurysm surgery is difficult and risky as it is, and the additional hurdle of doing it through a minimal exposure seems unwarranted if an equally acceptable cosmetic result can be achieved with a modest alteration in the bone flap. The subfrontal bone flap, while small, is still not a “keyhole” approach per se. Well-established senior neurosurgeons, most notably Hernesniemi et al,[12,13] with extensive aneurysm surgery experience, have reported success with a similar, although less invasive, approach – the lateral supraorbital approach. This highlights an important point of consideration. Without a doubt, there is increased pressure to advance microsurgical cererbovascular technique and strive for the least invasive approach possible, especially at a time when so many of these aneurysms are treated endovascularly. Ultimately; however, the surgeon's experience dictates the needed size of exposure. Thus, we wanted to share our practice and describe what we feel is an adequate and familiar operative corridor for most surgeons, while still being less invasive than the standard approaches.
In our experience, patients understand (perhaps better than the surgeons who do not face the risks of surgery) that they are confronting a major problem with risks no matter which way they turn. Brain aneurysm surgery is well known in popular culture as the most technically challenging and potentially risky procedure in modern medicine, and it is the rare patient indeed who asks that we make the incision or bone opening smaller if it means increasing even slightly the risk of death, stroke, paralysis or other neurological complications. There must be a balance between the concerns of safe, efficacious surgery and cosmetic result, and we feel that our approach strikes that balance nicely, but each surgeon must weigh these considerations based on their experience and patient preference.
CONCLUSION
We feel that this pure frontal craniotomy/subfrontal approach provides an efficient avenue to the AComm region. Rather than a minimally invasive approach for a very dynamic and sometimes anatomically variable region, we feel this provides a less invasive option, which reduces opening and closing friction but still yields a very thorough operative window for access to the anterior communicating region.
ACKNOWLEDGMENT
The authors wish to acknowledge J. Bone and thank him for his hard work and intellectual contributions.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/124/85056
Contributor Information
Anthony L. Petraglia, Email: Anthony_Petraglia@urmc.rochester.edu.
Vasisht Srinivasan, Email: Vasisht_Srinivasan@urmc.rochester.edu.
Michael J. Moravan, Email: Michael_Moravan@urmc.rochester.edu.
Michelle Coriddi, Email: Michelle_Coriddi@urmc.rochester.edu.
Babak S. Jahromi, Email: Babak_Jahromi@urmc.rochester.edu.
G Edward Vates, Email: Edward_Vates@urmc.rochester.edu.
Paul K. Maurer, Email: Paul_Maurer @urmc.rochester.edu.
REFERENCES
- 1.Andaluz N, Van Loveren HR, Keller JT, Zuccarello M. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2003;52(5):1140–8. discussion 1148-9. [PubMed] [Google Scholar]
- 2.Cheng WY, Lee HT, Sun MH, Shen CC. A pterion keyhole approach for the treatment of anterior circulation aneurysms. Minim Invasive Neurosurg. 2006;49:257–62. doi: 10.1055/s-2006-954575. [DOI] [PubMed] [Google Scholar]
- 3.Czochra M, Kaminski S, Kozniewska H, Muszynski A, Brzozowski S. [Results of surgical treatment of aneurysms of the anterior communicating artery] Neurol Neurochir Pol. 1980;14:203–8. [PubMed] [Google Scholar]
- 4.Czochra M, Kozniewska H, Muszynski A, Trojanowski T. [Surgical treatment of aneurysms of the anterior communicating artery using Yasargil's approach] Neurol Neurochir Pol. 1979;13:71–4. [PubMed] [Google Scholar]
- 5.Diraz A, Kobayashi S, Toriyama T, Ohsawa M, Hokama M, Kitazama K. Surgical approaches to the anterior communicating artery aneurysm and their results. Neurol Res. 1993;15:273–80. doi: 10.1080/01616412.1993.11740148. [DOI] [PubMed] [Google Scholar]
- 6.El-Noamany H, Nakagawa F, Hongo K, Kakizawa Y, Kobayashi S. Low anterior interhemispheric approach--a narrow corridor to aneurysms of the anterior communicating artery. Acta Neurochir (Wien) 2001;143:885–91. doi: 10.1007/s007010170018. [DOI] [PubMed] [Google Scholar]
- 7.Fujitsu K, Kuwabara T. Orbitocraniobasal approach for anterior communicating artery aneurysms. Neurosurgery. 1986;18:367–9. doi: 10.1227/00006123-198603000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara H, Yasui N, Nathal-Vera E, Suzuki A. Anosmia after anterior communicating artery aneurysm surgery: Comparison between the anterior interhemispheric and basal interhemispheric approaches. Neurosurgery. 1996;38:325–8. doi: 10.1097/00006123-199602000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima T, Miyazaki S, Takusagawa Y, Reichman M. Unilateral interhemispheric keyhole approach for anterior cerebral artery aneurysms. Acta Neurochir Suppl (Wien) 1991;53:42–7. doi: 10.1007/978-3-7091-9183-5_8. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez LF, Zabramski JM. Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery. 2004;54:1031–2. doi: 10.1227/01.neu.0000117124.32806.37. author reply 1032. [DOI] [PubMed] [Google Scholar]
- 11.Grand W, Landi MK, Dare AO. Transorbital keyhole approach to anterior communicating artery aneurysms. Neurosurgery. 2001;49:483–4. doi: 10.1097/00006123-200108000-00058. [DOI] [PubMed] [Google Scholar]
- 12.Hernesniemi J, Dashti R, Lehecka M, Niemela M, Rinne J, Lehto H, et al. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol. 2008;70:8–28. doi: 10.1016/j.surneu.2008.01.056. discussion 29. [DOI] [PubMed] [Google Scholar]
- 13.Hernesniemi J, Ishii K, Niemela M, Smrcka M, Kivipelto L, Fujiki M, et al. Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl (Wien) 2005;94:17–21. doi: 10.1007/3-211-27911-3_4. [DOI] [PubMed] [Google Scholar]
- 14.Horikoshi T, Nukui H, Mitsuka S, Kaneko M. Partial resection of the gyrus rectus in pterional approach to anterior communicating artery aneurysms. Neurol Med Chir (Tokyo) 1992;32:136–9. doi: 10.2176/nmc.32.136. [DOI] [PubMed] [Google Scholar]
- 15.Huber PJ, Stearns AB. The bi-frontal approach for aneurysm of anterior communicating artery: Case report using hypotensive anesthesia. Harper Hosp Bull. 1954;12:50–4. [PubMed] [Google Scholar]
- 16.Hyodo A, Mizukami M, Tazawa T, Togashi O, Eguchi T. [Some considerations on surgical approaches to the anterior communicating artery aneurysms--radiological study of 122 cases] No Shinkei Geka. 1984;12:469–75. [PubMed] [Google Scholar]
- 17.Ito S, Fujimoto S, Saito K, Tada H, Tanaka T. [Postoperative olfactory dysfunction in interhemispheric approach for ruptured anterior communicating artery aneurysms] No Shinkei Geka. 1996;24:625–8. [PubMed] [Google Scholar]
- 18.Ito Z. The microsurgical anterior interhemispheric approach suitably applied to ruptured aneurysms of the anterior communicating artery in the acute stage. Acta Neurochir (Wien) 1982;63:85–99. doi: 10.1007/BF01728859. [DOI] [PubMed] [Google Scholar]
- 19.Jain KK. Surgery of anterior communicating aneurysms: Experience with various techniques and evolution of a modified approach. Surg Neurol. 1974;2:31–3. [PubMed] [Google Scholar]
- 20.Jeon BC, Chen SY, Zheng YR, Cho YW, Kwon KY. Superior orbital rim approach for anterior communicating artery aneurysms: A surgical series of 27 patients. J Korean Med Sci. 2003;18:566–72. doi: 10.3346/jkms.2003.18.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher MO, Kamel MH, O’Sullivan MG. Cranio-orbital approach for complex aneurysmal surgery. Br J Neurosurg. 2005;19:413–5. doi: 10.1080/02688690500390110. [DOI] [PubMed] [Google Scholar]
- 22.Kempe LG, Vander Ark GD. Anterior communicating artery aneurysms. Gyrus rectus approach. Neurochirurgia (Stuttg) 1971;14:63–70. doi: 10.1055/s-0028-1090556. [DOI] [PubMed] [Google Scholar]
- 23.Keogh AJ, Sharma RR, Vanner GK. The anterior interhemispheric trephine approach to anterior midline aneurysms: Results of treatment in 72 consecutive patients. Br J Neurosurg. 1993;7:5–12. doi: 10.3109/02688699308995050. [DOI] [PubMed] [Google Scholar]
- 24.Khandelwal P, Kato Y, Sano H, Yoneda M, Kanno T. Treatment of ruptured intracranial aneurysms: Our approach. Minim Invasive Neurosurg. 2005;48:325–9. doi: 10.1055/s-2005-915633. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi K, Watanabe K. Modified bifrontal interhemispheric approach to aneurysms of the anterior communicating artery with the use of a trephine craniotomy. A review of personal experience with 25 cases. Acta Neurochir (Wien) 1993;125:127–31. doi: 10.1007/BF01401839. [DOI] [PubMed] [Google Scholar]
- 26.Kodama N, Ebina T, Suzuki J. [Surgery of anterior communicating artery aneurysm--from the experiences of 346 cases (author's transl)] No To Shinkei. 1978;30:895–909. [PubMed] [Google Scholar]
- 27.Krayenbuhl HA, Yasargil MG, Flamm ES, Tew JM., Jr Microsurgical treatment of intracranial saccular aneurysms. J Neurosurg. 1972;37:678–86. doi: 10.3171/jns.1972.37.6.0678. [DOI] [PubMed] [Google Scholar]
- 28.Lan Q, Chen J, Qian ZY, Zhang QB, Huang Q. [Microsurgical treatment of complex intracranial aneurysms via keyhole approaches] Zhonghua Yi Xue Za Zhi. 2007;87:872–6. [PubMed] [Google Scholar]
- 29.Meskhiia N, Rurua VG, Sekaniia VI, Dzandzava DG, Kukhalashvili N. [Surgical approach to an aneurysm of the anterior cerebral-anterior communicating artery] Zh Vopr Neirokhir Im N N Burdenko. 1984:56–8. [PubMed] [Google Scholar]
- 30.Okamoto S, Itoh A. [Craniotomy side for neck clipping of the anterior communicating aneurysm via the pterional approach] No Shinkei Geka. 2002;30:285–91. [PubMed] [Google Scholar]
- 31.Proust F, Debono B, Hannequin D, Gerardin E, Clavier E, Langlois O, et al. Treatment of anterior communicating artery aneurysms: Complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99:3–14. doi: 10.3171/jns.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 32.Revuelta-Gutierrez R, Arriada-Mendicoa N, De Juambelz-Cisneros P, Fernandez-Alvarado B, Flores-Orozco J, Gil-Ortiz C, et al. [Minimally invasive surgery in intracranial aneurysms] Rev Neurol. 2001;32:1–5. [PubMed] [Google Scholar]
- 33.Sekhar LN, Kalia KK, Yonas H, Wright DC, Ching H. Cranial base approaches to intracranial aneurysms in the subarachnoid space. Neurosurgery. 1994;35:472–81. doi: 10.1227/00006123-199409000-00016. discussion 481-3. [DOI] [PubMed] [Google Scholar]
- 34.Speakman TJ, Barlass JL. The bifrontal approach to aneurysms of the anterior communicating artery. Can J Surg. 1963;6:237–43. [PubMed] [Google Scholar]
- 35.Srour A, el Tantawi AM, Khouja N, Zouaoui A, Lassau JP, Philippon J, et al. Neurosurgical anatomy of the anterior interhemispheric approach for aneurysms of the anterior communicating artery (26.6.92) Surg Radiol Anat. 1994;16:117–9. doi: 10.1007/BF01627935. [DOI] [PubMed] [Google Scholar]
- 36.Steiger HJ, Schmid-Elsaesser R, Stummer W, Uhl E. Transorbital keyhole approach to anterior communicating artery aneurysms. Neurosurgery. 2001;48:347–51. doi: 10.1097/00006123-200102000-00021. discussion 351-2. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki J, Mizoi K, Yoshimoto T. Bifrontal interhemispheric approach to aneurysms of the anterior communicating artery. J Neurosurg. 1986;64:183–90. doi: 10.3171/jns.1986.64.2.0183. [DOI] [PubMed] [Google Scholar]
- 38.Tamatani S, Toyama M, Kawaguchi T, Yamamoto K, Hara N. [Evaluation of the surgical results of the interhemispheric approach in comparison with the pterional approach for anterior communicating artery aneurysms] No Shinkei Geka. 1992;20:657–61. [PubMed] [Google Scholar]
- 39.Vander Ark GD, Kempe LC. Classification of anterior communicating aneurysms as a basis for surgical approach. J Neurosurg. 1970;32:300–3. doi: 10.3171/jns.1970.32.3.0300. [DOI] [PubMed] [Google Scholar]
- 40.Vander Ark GD, Kempe LG, Smith DR. Anterior communicating aneurysms: The gyrus rectus approach. Clin Neurosurg. 1974;21:120–33. doi: 10.1093/neurosurgery/21.cn_suppl_1.120. [DOI] [PubMed] [Google Scholar]
- 41.Wakai S. Subfrontal-basal interhemispheric approach for anterior communicating artery aneurysms.Technical note. Acta Neurochir (Wien) 1991;108:78–80. doi: 10.1007/BF01407671. [DOI] [PubMed] [Google Scholar]
- 42.Yasargil MG, Antic J, Laciga R, Jain KK, Hodosh RM, Smith RD. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol. 1976;6:83–91. [PubMed] [Google Scholar]
- 43.Yasargil MG, Boehm WB, Ho RE. Microsurgical treatment of cerebral aneurysms at the bifurcation of the internal carotid artery. Acta Neurochir (Wien) 1978;41:61–72. doi: 10.1007/BF01809137. [DOI] [PubMed] [Google Scholar]
- 44.Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3:7–14. [PubMed] [Google Scholar]
- 45.Yasui N, Nathal E, Fujiwara H, Suzuki A. The basal interhemispheric approach for acute anterior communicating aneurysms. Acta Neurochir (Wien) 1992;118:91–7. doi: 10.1007/BF01401292. [DOI] [PubMed] [Google Scholar]
- 46.Yasui N, Suzuki A, Sayama I, Kawamura S. [A basal interhemispheric operative approach for anterior communicating artery aneurysms] Neurol Med Chir (Tokyo) 1987;27:756–61. doi: 10.2176/nmc.27.756. [DOI] [PubMed] [Google Scholar]
- 47.Yeh H, Tew JM., Jr Anterior interhemispheric approach to aneurysms of the anterior communicating artery. Surg Neurol. 1985;23:98–100. doi: 10.1016/0090-3019(85)90327-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Ao XS, Huang X, Hu KQ, Liu HD, Zhang QS, et al. [Application of keyhole approach in operation of intracranial aneurysms] Zhonghua Yi Xue Za Zhi. 2005;85:2250–3. [PubMed] [Google Scholar]


