Abstract
Background:
Based on numerous reports citing high sensitivity and specificity of non-invasive imaging [e.g. computed tomography angiography (CTA) or magnetic resonance angiography (MRA)] in the detection of intracranial aneurysms, it has become increasingly difficult to justify the role of conventional angiography [digital subtraction angiography (DSA)] for diagnostic purposes. The current literature, however, largely fails to demonstrate the practical application of these technologies within the context of a “real-world” neurosurgical practice. We sought to determine the proportion of patients for whom the additional information gleaned from 3D rotational DSA (3DRA) led to a change in treatment.
Methods:
We analyzed the medical records of the last 361 consecutive patients referred to a neurosurgeon at our institution for evaluation of “possible intracranial aneurysm” or subarachnoid hemorrhage (SAH). Only those who underwent non-invasive vascular imaging within 3 months prior to DSA were included in the study. For asymptomatic patients without a history of SAH, aneurysms less than 5 mm were followed conservatively. Treatment was advocated for patients with unruptured, non-cavernous aneurysms measuring 5 mm or larger and for any non-cavernous aneurysm in the setting of acute SAH.
Results:
For those who underwent CTA or MRA, the treatment plan was changed in 17/90 (18.9%) and 22/73 (30.1%), respectively, based on subsequent information gleaned from DSA. Several reasons exist for the change in the treatment plan, including size and location discrepancies (e.g. cavernous versus supraclinoid), or detection of a benign vascular variant rather than a true aneurysm.
Conclusions:
In a “real-world” analysis of intracranial aneurysms, DSA continues to play an important role in determining the optimal management strategy.
Keywords: Aneurysm, angiography, computed tomography angiography, magnetic resonance angiography
INTRODUCTION
The non-trivial risk of conventional catheter angiography [digital subtraction angiography (DSA)][1] combined with the increasing sophistication of computed tomography (CT) and magnetic resonance (MR) technology has fueled the popularization of non-invasive angiographic imaging and has raised questions regarding the role of DSA in the diagnosis of cerebral aneurysms.[2] A busy cerebrovascular neurosurgeon at a high-volume referral center will often be asked to evaluate patients with a suspected aneurysm or other vascular anomaly as diagnosed by CT or MR angiography (CTA or MRA) and will have to make a decision about how to proceed with this data and best manage the patient. As non-invasive imaging technology steadily improves and ultimately approaches the accuracy of DSA for the detection of aneurysms, physicians will be expected to limit the number of catheter angiograms performed for diagnostic purposes. The current literature, however, seems to focus on the specificity of CTA/MRA in an idealized situation, where the highest quality equipment generates images that are interpreted by the most experienced neuroradiologists – a scenario that is admittedly rare in the everyday, neurovascular practice. Based on the perception that clinically meaningful discrepancies between the non-invasive imaging diagnosis and the findings on conventional angiography are more common than what might be expected from a reading of the literature, we sought to determine the proportion of patients for whom the additional information gleaned from conventional angiography led to a change in treatment plan.
MATERIALS AND METHODS
Using CPT billing codes, a list of all patients of the two senior authors (RAM, MJA), who had undergone digital subtraction cerebral angiography (DSA) at our institution in the period between January 2008 and December 2009, was generated. Institutional review board (IRB) approval was obtained for this retrospective chart review (#100769). From this list, we isolated those patients with a presumptive diagnosis of intracranial aneurysm based on either CTA or MRA performed within 90 days of initial DSA. In some of the patients, DSA was performed for follow-up purposes after aneurysm coiling, and in these cases, the initial diagnostic DSA was analyzed and compared with the non-invasive imaging at the time of first presentation. None of the initial angiographic studies included in this analysis dated back to before 2005.
These patients were then further subdivided based on whether there was clinical or radiological evidence of aneurysmal rupture or subarachnoid hemorrhage (SAH), a critical consideration in devising a management strategy. Aneurysm size was recorded as the greatest measurable diameter and location was noted, especially taking care to differentiate between cavernous and supraclinoid internal carotid artery (ICA) aneurysms. On a per-patient basis, non-invasive imaging findings were then compared to the “gold standard” 3D rotational DSA (3DRA) diagnosis to determine whether the new information gleaned from catheter angiography led to a change in management.
Patients with other presumptive diagnoses leading to the performance of DSA, such as intracranial arteriovenous malformation (AVM) or fistulae, were not considered in this study. Patients with a presumptive diagnosis of aneurysm based on contrast-enhanced CT or MR (as opposed to actual CTA or MRA) were also excluded, as were those who had undergone DSA at an outside hospital before transfer to our institution. In patients who underwent DSA, 3D rotational image acquisition (3DRA) was performed without exception in the region of the supposed anomaly found by CTA or MRA.
Of the SAH patients, most were emergently transferred to our institution and underwent in-house CT/CTA scanning which was read by one of our attending neuroradiologists. Of those without a history of hemorrhage, most were referred electively to neurosurgical clinic from an outside hospital with non-invasive imaging suggestive of intracranial aneurysm. Many of the patients in the latter group arrived to clinic either with actual films, a compact disc with digital images, or merely a printed radiological report. When only a printed report was available, every attempt was made to contact the referring hospital and request that actual images be sent for review. In the event that outside images were unobtainable, the presumptive diagnosis was recorded in whatever detail was included on the printed, outside hospital report.
In any patient with acute SAH as determined by clinical and radiological criteria, treatment rather than observation was recommended for any non-cavernous, intracranial aneurysm detected regardless of size. In the case of unruptured aneurysms, any aneurysm measuring less than 5 mm was deemed reasonable to observe, while treatment was generally recommended for any aneurysm that was 5 mm or greater. No patient with a cavernous aneurysm was offered treatment. No distinction was made between anterior circulation and posterior circulation aneurysms in terms of the decision to treat versus observe. The presence of daughter sacs or irregular morphology that might influence an interventionist to be more aggressive with treatment was not considered in this study.
RESULTS
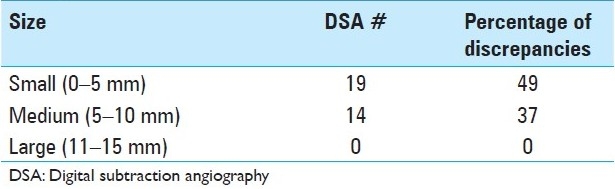
Three hundred sixty-one patients underwent DSA during the time period of the study (January 2008–December 2009). Of these, 163 patients had undergone CTA or MRA within 3 months prior to DSA, a requirement for inclusion in the study; these patients either suffered from acute SAH (65/163) or were seen electively in clinic for a presumptive diagnosis of “possible intracranial aneurysm.” Based on the new information gleaned from DSA, the plan of treatment was changed in 17/90 (18.9%) patients who had undergone CTA [Table 1] and 22/73 (30.1%) patients who had undergone MRA [Table 2]. Combining the results of both non-invasive imaging types, the plan of treatment was changed in 39/163 (23.9%) after performing DSA. Infundibuli, vessel loops, and fenestrations were among the benign anatomic variants erroneously read as “possible aneurysm” by non-invasive imaging. Vessel infundibuli were found in 3 (1.8%) of the patients in this study after performing 3DRA. Vessel loops and vessel fenestrations misinterpreted as aneurysms were found in 3 (1.8%) and 1 (0.6%) members of the total cohort, respectively. A discrepancy in largest aneurysmal dimension between DSA and non-invasive imaging was the most common reason for a change in treatment plan, especially in incidentally discovered aneurysms in which 5 mm was used as a size cut-off. Discrepancies between non-invasive imaging and DSA occurred most often at the supraclinoid ICA segment [Table 3] for aneurysms measuring 5 mm or less [Table 4].
Table 1.
Discrepancies leading to changes in management: Computed tomography angiography versus digital subtraction angiography
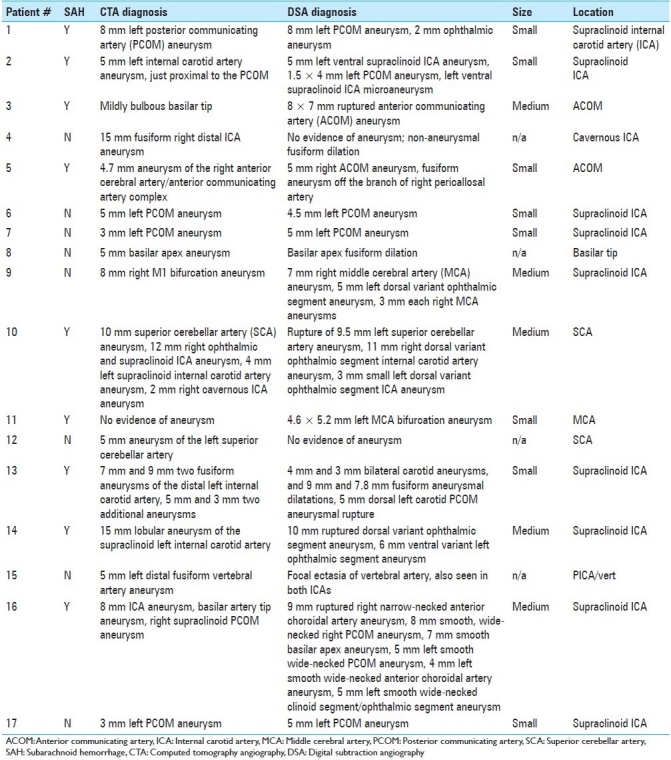
Table 2.
Discrepancies leading to changes in management: Magnetic resonance angiography versus digital subtraction angiography
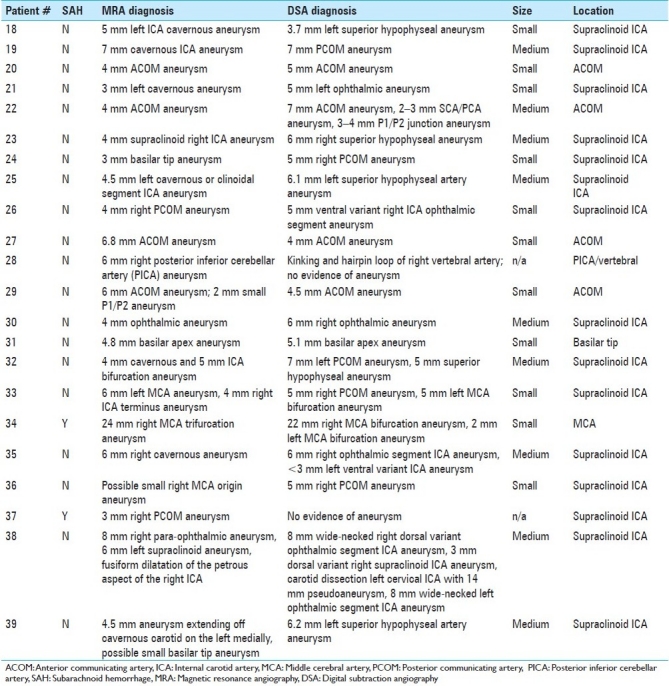
Table 3.
Location of discrepant aneurysms by magnetic resonance angiography and computed tomography angiography
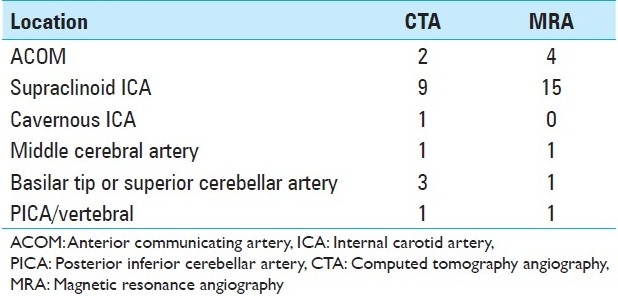
Table 4.
Percentage of discrepancies depending on aneurysm size
DISCUSSION
The overall sensitivity of single-slice CTA in the detection of intracranial aneurysms has typically been reported between 77% and 100% and the specificity between 79% and 100%,[3–6] with diagnostic accuracy decreasing significantly for small aneurysms less than 3 mm in diameter.[6,7,24] The relatively recent introduction of multidetector row, spiral CTA has led to improved resolution and prompted some claims that CTA achieves “equivalent diagnostic accuracy to that of conventional DSA in the detection of (some) aneurysms”.[8,9] However, even with modern, state-of-the-art, 64-slice multidetector CT scanners, the sensitivity for the detection of aneurysms less than 3 mm is still felt to be less than 90%.[10] For medium and large aneurysms, MRA has comparable sensitivity to CTA. However, for aneurysms less than 5 mm, most studies have shown CTA to be superior, with MRA sensitivity dropping off to 56%.[11,13,15] In a recent article by Schwab et al., significant discrepancies were found in nearly 60% of cases when comparing MRA to DSA in the detection of intracranial aneurysms. Most of the discordance between these imaging modalities occurred in the setting of small aneurysms (i.e. less than 5 mm) or for lesions located at the anterior communicating artery (ACOM) complex.[20]
While sensitivity and specificity have been the measure of diagnostic accuracy in numerous prior reports, we sought to analyze non-invasive imaging on the basis of whether or not the plan of treatment was changed in light of the additional information provided by DSA. We felt this analysis would be unique to other studies as it will provide practitioners with some practical information about how to confront patients who arrive to their clinic with a presumptive diagnosis of “possible intracranial aneurysm”. Acute SAH patients were also included in this analysis to increase the power of the study; however, there is significantly less controversy regarding the role for conventional angiography in these patients, for both diagnosis and treatment.
The decision of whether to treat an aneurysm is based on several patient factors (e.g. age, comorbidities, history of SAH) as well as features of the particular aneurysm (e.g. size, location, morphology). The procedural risk of securing the aneurysm must be weighed carefully against the natural history of the lesion.[16] For the purposes of providing a practical comparison between non-invasive imaging modalities and DSA, we simplified the decision-making process by considering only 1) presence of acute SAH, 2) aneurysm size, and 3) aneurysm location. Our decision-making algorithm deserves some explanation as it is somewhat simplified and cannot be used as a hard and fast guideline as much as a reasonable albeit somewhat arbitrary recommendation.
In terms of ruptured aneurysms, treatment was recommended for all aneurysms detected regardless of size. The most common scenario in which 3DRA provided additional information that changed the treatment plan was in the case of very small aneurysms (e.g. 1–3 mm) for which the sensitivity of CTA and MRA is known to be less than 90%.[1] In the setting of SAH, discrepancies in size between non-invasive modalities and DSA did not frequently lead to a change in treatment. If CTA detected a 3-mm aneurysm, for example, while 3DRA revealed a 5-mm aneurysm, treatment would be advocated in either case to prevent re-hemorrhage.
In the case of a patient with an unruptured aneurysm, size discrepancy was the most common reason for a change in treatment. According to our algorithm, a 3-mm unruptured aneurysm detected by CTA would reasonably be observed. If subsequent 3DRA, however, revealed that the aneurysm was indeed 5 mm or larger in greatest diameter, this new information would prompt a recommendation to treat. If however, a 2-mm aneurysm was detected with CTA or MRA, which on 3DRA was in fact revealed to be merely a vessel loop or infundibulum, this discrepancy would not actually change the recommendation as either diagnosis would be observed rather than treated. The use of a 5-mm-size cut-off, based on a recent paper by Solomon et al., is controversial and not meant to be taken as an absolute guideline.[17] It underscores the importance, however, of aneurysm size as one of the most critical factors in the determination of whether to treat or observe an asymptomatic, unruptured aneurysm. Naturally, the more precise we can be in our determination of aneurysm size, the better recommendations we can make for our patients.
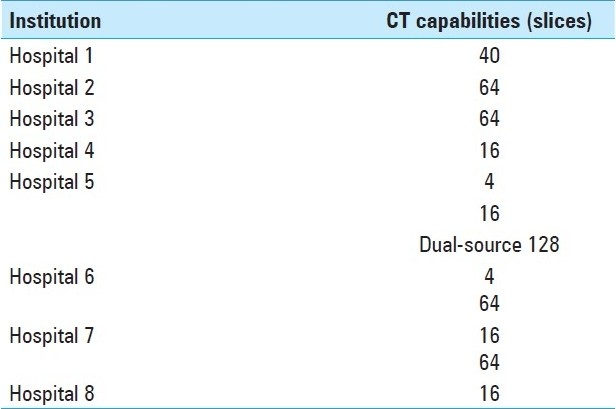
In addition to image quality, radiologist experience has been shown to be an important factor in the sensitivity and specificity of non-invasive imaging for the detection of aneurysms.[6] Regarding image quality, the referring hospitals in this study featured a range of CT scanning capabilities [Table 5]. Many of the hospitals that refer patients to our institution are small, regional hospitals with only a 16-slice multidetector CT scanner, and so it may be inappropriate to apply the same expectations of diagnostic accuracy to these scans as it would be to a state-or-the-art 64-slice multidetector CT scanner.[18,20] Of course, another option would be to repeat a CTA in a patient who presents with a low-quality scan and a presumptive diagnosis of “possible intracranial aneurysm.” At our institution, however, there is a bias toward performing DSA to settle equivocal cases. Furthermore, many of the outside reports were generated by a radiologist without special training in neuroradiology. Radiologist expertise has been shown to factor into the accuracy of aneurysm detection in a prior series.[6]
Table 5.
Top eight referring institution CT capabilities
Given the significant morbidity and mortality of SAH, missing the diagnosis of a cerebral aneurysm can have disastrous consequences for the patient. In many patients, the knowledge of harboring an untreated intracranial aneurysm led to anxiety and lower quality of life scores, particularly in psychosocial domains.[12] Failure to recognize a sentinel bleed or detect an intracranial aneurysm remains among the most common reasons for medical malpractice litigation in neurosurgery.[21–23] 3DRA continues to appeal to practitioners and patients alike when the possibility of an aneurysm has been raised both to avoid litigation and allay patient concerns.
Limitations
This retrospective chart review has a number of limitations and caution must be exercised in drawing firm conclusions from this data. First of all, in many of these cases, while the 3DRA was specifically focused on aneurysm detection, the non-invasive imaging may have been performed for other indications (e.g. MRI/MRA for headache). Naturally, we can expect a bias in favor of 3DRA image quality since the angiographer is specifically trying to rule in or rule out the diagnosis of aneurysm and is unlikely to terminate the procedure until adequate images have been obtained. Although 3DRA was generally performed at our institution to settle equivocal cases, repeating non-invasive imaging under idealized conditions (i.e. with state-of-the-art technology and neuroradiology expertise) may also have been a reasonable approach.
Secondly, it is not clear as to what extent the data may apply to specialists at other institutions. The decision to treat or observe an aneurysm is complex. Although a simple and rational algorithm was presented here, other specialists may use a different size cut-off or any number of other variables in making the decision to intervene.
There was a delay in up to 90 days between non-invasive imaging and 3DRA. Little is known about the growth rate of aneurysms, but it is certainly possible that some of the size discrepancies between imaging modalities could be attributed to this time delay.
Although a formal cost analysis was not performed as part of his study, the additional information provided by 3DRA image acquisition comes at a significant expense. At the authors’ institution, the “total research cost” for CTA is $773.28 (CPT 70496). MRA costs $760.88 (CPT 70544). The overall cost for DSA, however, is much greater, ranging from $7000 to $10,000 depending on the number of vessels catheterized. In the current healthcare environment, focused on cutting costs, these differences must be taken into consideration.
Cerebral angiography is not without complications, and thus, in order to justify such an intervention, the benefits of the procedure must significantly outweigh its risks.[14] Previously published data from our institution report a postoperative complication rate of 0.43% of the procedures performed. Only 0.04% of all procedural complications were considered to cause major permanent disability.[19] It is important to have a frank conversation with each patient to obtain informed consent and explain the justification for performing an invasive procedure.
CONCLUSIONS
Although reports of very high sensitivity and specificity are often quoted in the literature for the detection of cerebral aneurysms by non-invasive imaging, we found a significant proportion of patients for whom the additional information gleaned from DSA led to a change in treatment plan. Despite greater invasiveness and increased risk to the patient, 3DRA remains a useful and critically important tool in the detection and treatment of cerebral aneurysms.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/134/85607
Contributor Information
Luke Tomycz, Email: luke.tomycz@Vanderbilt.edu.
Neil K. Bansal, Email: neil.k.bansal@vanderbilt.edu.
Catherine R. Hawley, Email: catherine.r.hawley@Vanderbilt.edu.
Tracy L. Goddard, Email: tracey.goddard@Vanderbilt.edu.
Michael J. Ayad, Email: michael.ayad@Vanderbilt.edu.
Robert A. Mericle, Email: mericle@hwneuro.com.
REFERENCES
- 1.Alberico RA, Patel M, Casey S, Jacobs B, Maguire W, Decker R. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms. AJNR Am J Neuroradiol. 1995;16:1571–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas SW. Magnetic resonance imaging of intracranial aneurysms. Neuroimaging Clin N Am. 1997;7:709–20. [PubMed] [Google Scholar]
- 3.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke. 2009;40:994. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 4.Chapell ET, Moure FC, Good MC. Comparison of computed tomography angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: A meta-analysis. Neurosurgery. 2003;52:624–31. doi: 10.1227/01.neu.0000047895.82857.eb. discussion 630-1. [DOI] [PubMed] [Google Scholar]
- 5.Cloft HJ, Joseph GJ, Dion DE. Meta-analysis of risks of cerebral angiography in patients with subarachnoid hemorrhage, intracranial aneurysm and arteriovenous malformation: A meta-analysis. Stroke. 1999;30:317–20. doi: 10.1161/01.str.30.2.317. [DOI] [PubMed] [Google Scholar]
- 6.De Gast A, van Rooij W, Sluzewski M. Fenestrations of the anterior communicating artery: Incidence on 3D angiography and relationship to aneurysms. AJNR Am J Neuroradiol. 2008;29:296–8. doi: 10.3174/ajnr.A0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fager CA. Malpractice issues in neurological surgery. Surg Neurol. 2006;65:416–21. doi: 10.1016/j.surneu.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Hope JK, Wilson JL, Thomson FJ. Three-dimensional CT angiography in the detection and characterization of intracranial berry aneurysms. AJNR Am J Neuroradiol. 1996;17:439–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Huston J, 3rd, Nichols DA, Luetmer PH, Goodwin JT, Meyer FB, Wiebers DO, et al. Blinded prospective evaluation of sensitivity of MR angiography to known intracranial aneurysms: Importance of aneurysm size. AJNR Am J Neuroradiol. 1994;15:1607–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson DM, Trobe JD. The emerging role of MRA in the management of patients with third cranial nerve palsy. Am J Ophthalmol. 1999;128:94–6. doi: 10.1016/s0002-9394(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman MV, Mayo-Smith WW, Tung GA, Haas RA, Rogg JM, Mehta NR, et al. Detection of intracranial aneurysms: Multi-detector row CT angiography compared with DSA. Radiology. 2004;230:510–8. doi: 10.1148/radiol.2302021465. [DOI] [PubMed] [Google Scholar]
- 12.Komotar RJ, Mocco J, Solomon RA. Guidelines for the surgical treatment of unruptured intracranial aneurysms: The First Annual J.Lawrence Pool Memorial Research Symposium – Controversies in the management of cerebral aneurysms. Neurosurgery. 2008;62:183–94. doi: 10.1227/01.NEU.0000311076.64109.2E. [DOI] [PubMed] [Google Scholar]
- 13.Korogi Y, Takahashi M, Katada K, Ogura Y, Hasuo K, Ochi M, et al. Intracranial aneurysms: Detection with three-dimensional CT angiography with volume rendering: Comparison with conventional angiographic and surgical findings. Radiology. 1999;211:497–506. doi: 10.1148/radiology.211.2.r99ma02497. [DOI] [PubMed] [Google Scholar]
- 14.Leffers AM, Wagner A. Neurologic complications of cerebral angiography.A retrospective study of complication rate and patient risk factors. Acta Radiol. 2000;41:204–10. doi: 10.1080/028418500127345299. [DOI] [PubMed] [Google Scholar]
- 15.Marshman LA, Ward PJ, Walter PH, Dossetor RS. The progression of an infundibulum to aneurysm formation and rupture: Case report and literature review. Neurosurgery. 1998;43:1445–8. doi: 10.1097/00006123-199812000-00107. discussion 1448-9. [DOI] [PubMed] [Google Scholar]
- 16.McCollough CH, Zink FE. Performance evaluation of a multi-slice CT system. Med Phys. 1999;26:2223. doi: 10.1118/1.598777. [DOI] [PubMed] [Google Scholar]
- 17.McKinney AM, Palmer CS, Truwit CL, Karagulle A, Teksam M. Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:594–602. doi: 10.3174/ajnr.A0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocco J, Komotar R, Lavine S, Meyers P, Connolly E, Solomon R. The natural history of unruptured intracranial aneurysms. Neurosurg Focus. 2004;17:E3. doi: 10.3171/foc.2004.17.5.3. [DOI] [PubMed] [Google Scholar]
- 19.Ringer AJ, Lanzino G, Veznedaroglu E, Rodriguez R, Mericle RA, Levy EI, et al. Does angiographic surveillance pose a risk in the management of coiled intracranial aneurysms.A multicenter study of 2243 patients? Neurosurgery. 2008;63:845–9. doi: 10.1227/01.NEU.0000333261.63818.9C. discussion 849. [DOI] [PubMed] [Google Scholar]
- 20.Schwab K, Gailloud P, Wyse G, Tamargo R. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery. 2008;63:29–35. doi: 10.1227/01.NEU.0000335068.53190.46. [DOI] [PubMed] [Google Scholar]
- 21.Tipper G, U-King-Im JM, Price SJ, Trivedi RA, Cross JJ, Higgins NJ, et al. Detection and evaluation of intracranial aneurysms with 16-row multislice CT angiography. Clin Radiol. 2005;60:565–72. doi: 10.1016/j.crad.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Van der Schaaf I, Brilstra E, Rinkel G, Bossuyt P, van Gijn J. Quality of life, anxiety, and depression in patients with an untreated intracranial aneurysm or arteriovenous malformation. Stroke. 2002;33:440–3. doi: 10.1161/hs0202.102335. [DOI] [PubMed] [Google Scholar]
- 23.Westerlaan HE, Gravendeel J, Fiore D, Metzemaekers JD, Groen RJ, Mooij JJ, et al. Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology. 2007;49:997–1007. doi: 10.1007/s00234-007-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White PM, Wardlaw JM, Easton V. Can non-invasive imaging accurately depict intracranial aneurysms.A systematic review? Radiology. 2000;217:361–70. doi: 10.1148/radiology.217.2.r00nv06361. [DOI] [PubMed] [Google Scholar]


