Abstract
Background:
Meningioma firmness is a critical factor that influences ease of resection and risk, notably when operating on tumors intimate with neurovascular structures such as the mesial sphenoid wing. This study develops a predictive tool using preoperative magnetic resonance imaging (MRI) characteristics to determine meningioma consistency.
Methods:
101 patients with intracranial meningioma (50 soft/51 firm) were included. MRI characteristics of 38 tumors (19 soft/19 firm) were retrospectively reviewed to identify preoperative imaging features that were then correlated with intraoperative description of the tumor as either “soft and/or suckable” or “firm and/or fibrous”. Criteria were developed to predict consistency and then blindly applied to the remaining 63 meningiomas (31 soft/32 firm).
Results:
The overall sensitivities for detecting soft and firm consistency were 90% and 56%, respectively (95% CI = 73–97% and 38–73%; P < 0.001). Compared to gray matter, meningiomas that were T2 hypointense were almost always firm. Soft meningiomas were hyperintense on T2 and hypointense on T1. Soft meningiomas were slightly larger and less likely to be associated with edema. There was a slight preponderance of firm meningiomas in the infratentorial compartment. Grade of meningioma was not predictive. Contrast enhancement, diffusion restriction, changes in overlying bone, intratumoral cysts, and angiographic features were not predictable.
Conclusions:
This tool using T1 and T2 series predicts meningioma consistency. Such knowledge should assist the surgeon in preoperative planning and counseling.
Keywords: Consistency, firm, magnetic resonance imaging, meningioma, soft
INTRODUCTION
Meningioma was named by Harvey Cushing in 1922 and represents a dural-based tumor that arises from arachnoid cells surrounding the brain. The incidence is approximately 2.3 per 100,000 for benign meningiomas and 0.17 per 100,000 for malignant meningiomas, with an average age at presentation of 43 years in women and 52 years in men.[1,7] Regarding treatment, resection remains the treatment of choice in most cases with external beam radiotherapy, radiosurgery, arterial embolization, and chemotherapy serving more of an adjunct role when deemed necessary.[7] Some factors that reflect surgical risk include tumor size, location, and firm consistency. Little et al.[5] reported in their series of petroclival meningiomas that risk of cranial nerve deficits increased with a multitude of factors, one being a tumor with fibrous consistency. Meningioma consistency is particularly important with involvement of neurovascular structures near the mesial sphenoid wing.
Currently, there are no reliable imaging modalities that predict meningioma firmness. Thus, it is difficult for the surgeon to predict the intraoperative consistency of the meningioma; which may be described as “soft,” “firm,” or a combination of both. The purpose of our study was to develop a quick, systematic, and reliable tool that uses preoperative magnetic resonance imaging (MRI) characteristics to predict intraoperative meningioma consistency classified as either soft or firm.
MATERIALS AND METHODS
Following Institutional Review Board's approval at Mayo Clinic in Rochester, we identified 101 patients with intracranial meningioma resected at our institution from 1997 to 2010 where the meningiomas were classified as either “soft and/or suckable” or “firm and/or fibrous” by the surgeon in the operative report. We chose these terms as they are descriptive and somewhat standard nomenclature regarding intraoperative meningioma consistency. More meningiomas were resected at our institution during this time period. However, these were not included due to the surgeon not describing the tumor's consistency in the operative note using the chosen descriptive terms.Thirty-eight (19 soft and 19 firm) of the 101 meningiomas were randomly selected and reviewed by a neuroradiologist in an attempt to identify preoperative MRI characteristics that might assist in predicting soft or firm intraoperative consistency [Table 1]. The neuroradiologist was not blinded as to the consistency of the 38 meningiomas so that predictive criteria could be developed. The computed tomography (CT) density and appearance of the adjacent bone was also reviewed. Angiographic features if available were reviewed. These characteristics were then analyzed and used to formulate criteria to predict intraoperative consistency. We (JH, FM, JM) then blindly applied these criteria in an attempt to predict intraoperative consistency in the remaining 63 meningiomas (31 soft and 32 firm). The preoperative MRIs of the 63 meningiomas were reviewed, with the only identifiers being the patient's medical record number and the date of surgery. The three reviewers then studied the preoperative MRIs and collectively assigned T1 and T2 intensities to the 63 meningiomas. Statistical analyses of the developed criteria included sensitivity and 95% confidence intervals. Statistical significance was assessed with the Fischer's exact two-tail test and a P-value of <0.05 was considered statistically significant.
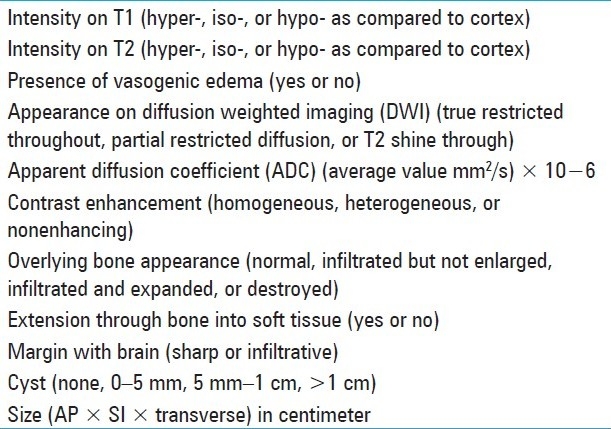
Table 1.
Characteristics assessed on preoperative MRI
RESULTS
There were 63 females and 38 males and their age range was 15–83 years with a mean age of 57 years. There were 50 soft and 51 firm meningiomas, with the mean age for soft and firm tumors being 58 and 56 years, respectively. Primary and recurrent (defined as surgically treated in the past) meningiomas were included in the study and consisted of 89 primary (87%) and 12 (13%) recurrent tumors. Three patients in the study had neurofibromatosis type II. All three of these meningiomas were described in the operative report as soft.
The location of each of the 101 meningiomas was described as either supratentorial or infratentorial. There were 67 supratentorial (66%) and 34 (34%) infratentorial meningiomas included in the study. Of the supratentorial meningiomas, 37 (55%) were soft and 30 (45%) were firm. Of the infratentorial meningiomas, 13 (38%) were soft and 21 (62%) were firm. The pathology type was recorded as WHO grade I, II, or III meningioma. There were 88 (87%) grade I, 11 (11%) grade II, and 2 (2%) grade III meningiomas included. The majority of the meningiomas were classified histologically as meningothelial, although other subtypes were present.
All references to intensity regarding T1- and T2-weighted imaging on MRI were in comparison with cerebral cortex. Soft meningiomas were slightly larger than firm meningiomas at an average volume of 31 ml compared with 23 ml. Surrounding vasogenic edema was present in 50% of soft meningiomas compared with 63% of firm meningiomas (P > 0.05). There was a tendency of infratentorial meningiomas to be firm. WHO grade did not affect the consistency, as of the grade I meningiomas there were 43 soft and 45 firm, of the grade II meningiomas 6 were soft and 5 were firm, and of the grade III ones 1 was soft and 1 was firm. Characteristics such as appearance on diffusion weighted imaging (DWI) or apparent diffusion coefficient (ADC), contrast enhancement, bone appearance, extension beyond bone, tumor-brain interface, cyst presence/size, CT density, and angiographic appearance when available were evaluated but not found to be predictive of consistency in this series. Age and gender also did not appear to be predictive of consistency.
What did have value was the appearance of soft and firm meningiomas on T1- and T2-weighted imaging. These are performed as standard series in almost all present-day MRIs. The ubiquitous nature of T1- and T2-weighted imaging makes them beneficial when gleaning important data from the preoperative MRI. Based on the appearance of soft and firm meningiomas, we were able to develop a table that allows the surgeon to more conclusively determine meningioma consistency [Table 2]. For example, if a meningioma appears hypointense on T2 and that is the only series reviewed to determine consistency, the surgeon can be almost always certain (100% in this study or +++++; P < 0.001) the tumor is firm [Figure 1]. Further, if any portion of the meningioma is T2 hypointense, in this study, all of those meningiomas were described as firm in the operative report. Aside from those meningiomas that are T2 hypointense, meningiomas that are heterogeneous in intensity should be classified based on their predominant appearance on T1 and/or T2. If the tumor is both T2 hyperintense and T1 hypointense, the surgeon can predict that the meningioma is highly likely soft (90% in this study or ++++; P < 0.01) [Figures 2 and 3]. Both the associations of T2 hypointensity with firmness and T2 hyerintensity and T1 hypointensity with softness were statistically significant. However, if the surgeon determines that the meningioma is T1 isointense, then the tumor is more likely to be firm (62% in this study or ++; P = 0.16) [Figure 4]. If the meningioma is T2 hyperintense and T1 isointense, it is more likely to be soft (56% in this study or +; P = 1.00) because of the slightly higher tendency of T2 hyperintensity to be associated with soft consistency than T1 isointensity being associated with firm consistency. Analyzing surrounding vasogenic edema for meningiomas that were T1 hypointense or isointense or T2 isointense or hyperintense in an attempt to improve the ability to predict soft or firm consistency was not beneficial. The overall sensitivities for detecting soft and firm consistency were 90% and 56%, respectively (95% CI = 73–97% and 38–73%; P < 0.001). Therefore, Table 2 is more effective at predicting soft meningioma consistency. However, in the case of meningiomas that are T2 hypointense and T1 isointense, one can predict these are almost always firm.
Table 2.
Association of meningioma appearance with intraoperative consistency (a higher number of + means greater tendency to be associated with that consistency)
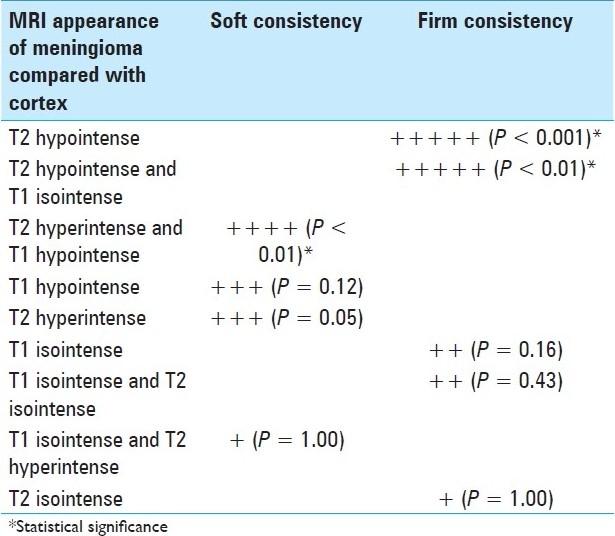
Figure 1.
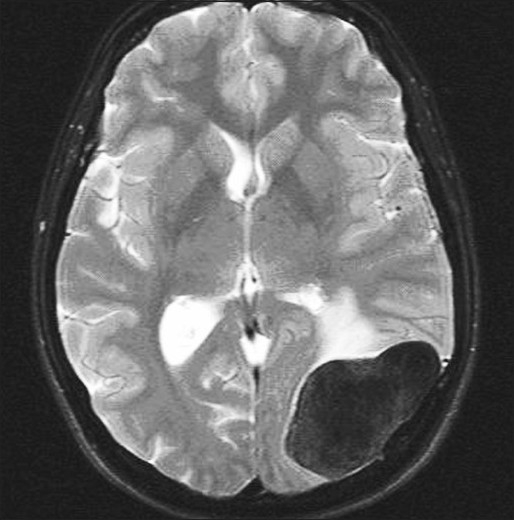
Firm meningioma: Axial FSE T2WI with fat saturation demonstrates marked hypointensity
Figure 2.
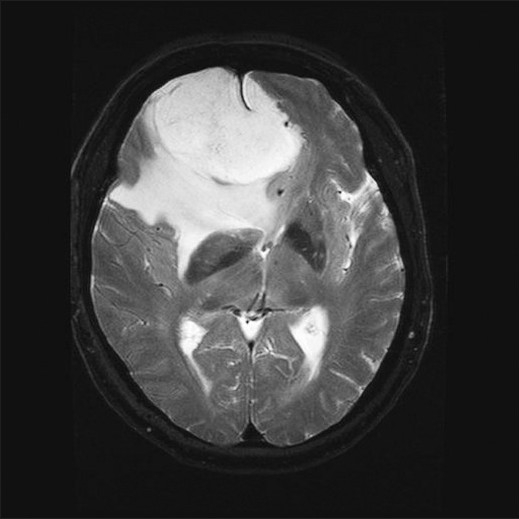
Soft meningioma: Axial FSE T2WI with fat saturation demonstrates T2 hyperintensity with surrounding vasogenic edema
Figure 3.
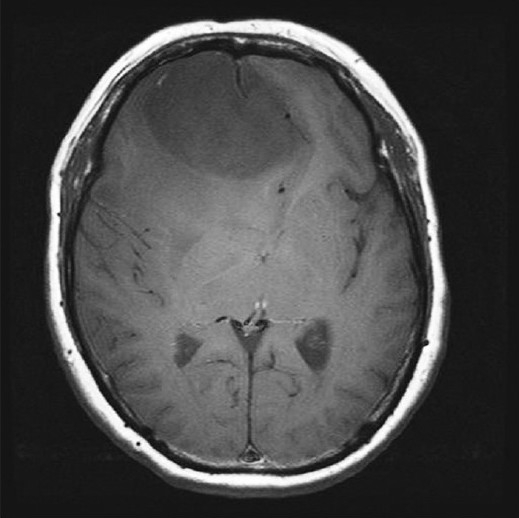
Soft meningioma: Axial T1WI demonstrates T1 hypointensity
Figure 4.
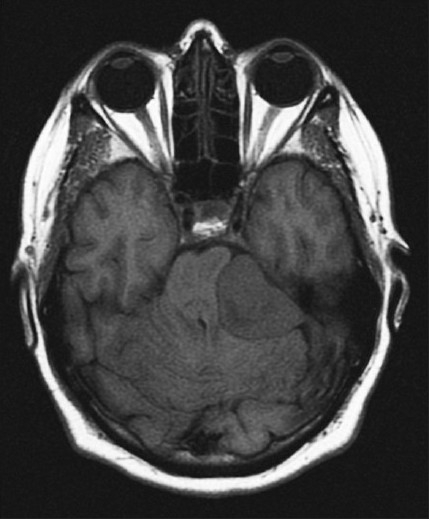
Firm meningioma: Axial T1WI demonstrates T1 isointensity to the adjacent gray matter
DISCUSSION
We sought to develop a quick, reliable, and valid schematic tool for determining meningioma consistency. In this series, soft meningiomas were predictably more often hyperintense on T2 and hypointense on T1, whereas firm meningiomas were more often hypointense on T2 and isointense on T1. The table serves to add more organization to this analysis. To that point, this schematic table appears to be more effective at detecting soft meningiomas than firm meningiomas. However, if the meningioma is T2 hypointense, it consistently predicts firm meningiomas with excellent accuracy. In our analysis, soft meningiomas had a slightly larger volume than firm meningiomas but were slightly less often associated with vasogenic edema although not statistically significant. In the infratentorial compartment, there was a predominance of firm meningiomas. It is our thinking that these data will help with surgical planning.
Our findings are consistent with those in the literature. Maiuri et al.[6] examined 35 intracranial meningiomas to determine intraoperative consistency. They found that meningiomas hyperintense to the cortex on T2 are usually soft, more vascular, and more frequently of syncytial or angioblastic subtypes. However, tumors hypointense or hypo-isointense on T2 tend to have a more hard consistency and are more often of fibroblastic or transitional subtypes. In their series, meningiomas that were T2 hypointense were mainly fibroblastic and T2 hyperintense tumors were mainly syncytial, angioblastic, and partly transitional. T2 isointense meningiomas were mainly transitional and partly fibroblastic and syncytial. The authors concluded that the signal intensity on MRI may be useful in the preoperative characterization of intracranial meningiomas.[6] Soyama et al.[10] reported on 40 surgically treated intracranial meningiomas, regarding T2 intensity and correlation with histological subtypes. They assigned signal intensity scores on T2 and found that while there was a moderate variation of signal intensity within a given histological subtype, the mean signal intensity scores on T2 of the fibrous type of meningioma were significantly lower than those of the other subtypes. The score correlated with the collagen content and they concluded that meningiomas significantly hypointense to cortex on T2 are composed primarily of fibrous elements.[10] Yrjänä et al.[11] reported on 21 surgically treated meningiomas that had been imaged using a low-field MRI and found that tissue hardness correlated best with relative intensity on T2-weighted images.
Application of more sophisticated series with MRI, such as fractional anisotropy (FA) and magnetic resonance spectroscopy (MRS), to analyze meningiomas has been done. Kashimura et al.[3] evaluated FA as measured by MRI to predict meningioma consistency. They found in 29 patients with intracranial meningiomas (11 firm and 18 soft) where diffusion tensor imaging was performed preoperatively that the calculated FA values of fibroblastic meningiomas were significantly higher than those of meningothelial meningiomas. Further, the FA values of hard tumors were significantly higher than those of soft tumors.[3] Pfisterer et al.[9] utilized MRS to differentiate meningioma grade in 68 resected meningiomas. Forty-six were WHO grade I, 14 were WHO grade II, and 8 were WHO grade III. Fifty-nine were primary meningiomas and nine were recurrences. Invasion of adjacent tissue was identified in 32 cases. They found that absolute concentrations of total alanine and creatine were decreased in high-grade compared with low-grade meningiomas, as was the ratio of glycine to alanine. Additionally, alanine and the glycine to alanine ratio data helped distinguished between primary and recurrent meningiomas. The absolute concentrations of alanine and creatine, and the glycine to alanine and choline to glutamate ratios were associated with rapid recurrence. Their data indicated that meningioma tissue can be characterized by metabolic parameters with MRS, which are not typically identified by histopathologic analysis alone. Further, creatine, glycine, and alanine may be used as markers of meningioma grade, recurrence, and the likelihood of rapid recurrence.[9]
Determination between WHO grade II and III meningiomas using DWI and ADC calculation in MRI has also been reported. Nagar et al.[8] found that irregular tumor margins, peritumoral edema, and adjacent bone destruction were present significantly more often in grade II and III meningiomas than in grade I. Further, they also reported that the ADC ratios in grade II and III meningiomas are significantly lower than in grade I and that a decrease in these ratios on follow-up imaging may suggest dedifferentiation to a higher grade.[8]
Another study assessing radiographic characteristics and pathology related to surrounding vasogenic edema from meningiomas was performed by Lee and colleagues.[4] Seventy-nine patients with meningioma were examined by MRI and cerebral angiography. The predictive factors possibly related to surrounding vasogenic edema, such as age, gender, tumor location, tumor size, peritumoral rim or CSF cleft, shape of tumor margin, intensity of tumor on T2, pial blood supply, and pathology, were evaluated. The investigators found that male sex, tumor size, signal intensity of tumor in T2, grade II and III tumor, and pial blood supply correlated with peritumoral edema on univariate analyses. However, in multivariate analyses, pial blood supply was statistically significant as a factor for peritumoral edema in meningioma, while male sex and hyperintensity on T2 might have statistical probability in peritumoral edema. Thus, the authors concluded in their results that male sex, hyperintensity on T2, and pial blood supply are all associated with peritumoral edema in meningioma and they influence the clinical prognosis of patients.[4] Hashiba et al.[2] reported that the presence of surrounding vasogenic edema, an ambiguous brain-tumor border, and irregular tumor shape were significantly correlated with a higher MIB-1 staining index value using a scoring system described in their study. Finally, the application of magnetic resonance elastography to further delineate meningioma consistency is an area of ongoing research and it is our hope that this tool might also add to the preoperative assessment of meningioma consistency.
CONCLUSIONS
It is our premise that this quick and easily applied system using T1 and T2 series will be used to predict the intraoperative consistency of cranial meningiomas on preoperative MRI. If the surgeon is able to more accurately predict consistency, it would be helpful when planning surgical techniques and for tools that may be needed during resection, such as suction in soft meningiomas versus ultrasonic aspiration or laser in firm meningiomas. Risks to nearby neurovascular structures encased by tumor may be better identified if tumor consistency can be more accurately determined preoperatively. Consistency of tumor, surgical accessibility, and degree of arachnoid plane violation are only some of the important considerations in meningioma surgery. This tool for predicting consistency might serve as an adjunct for the surgeon in preoperative counseling of the patient.
Contributor Information
Jason M. Hoover, Email: hoover.jason@mayo.edu.
Jonathan M. Morris, Email: morris.jonathan@mayo.edu.
Fredric B. Meyer, Email: meyer.fredric@mayo.edu.
REFERENCES
- 1.Cushing H, Eisenhardt L. Springfield, IL: Charles C; 1938. Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results; pp. 298–319. [Google Scholar]
- 2.Hashiba T, Hashimoto N, Maruno M, Izumoto S, Suzuki T, Kagawa N, et al. Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol. 2006;23:49–54. doi: 10.1007/s10014-006-0199-4. [DOI] [PubMed] [Google Scholar]
- 3.Kashimura H, Inoue T, Ogasawara K, Arai H, Otawara Y, Kanbara Y, et al. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg. 2007;107:784–7. doi: 10.3171/JNS-07/10/0784. [DOI] [PubMed] [Google Scholar]
- 4.Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK, et al. Peritumoral brain edema in meningiomas: Correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. 2008;69:350–5. doi: 10.1016/j.surneu.2007.03.027. discussion 355. [DOI] [PubMed] [Google Scholar]
- 5.Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T. Surgical management of petroclival meningiomas: Defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56:546–59. doi: 10.1227/01.neu.0000153906.12640.62. [DOI] [PubMed] [Google Scholar]
- 6.Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML. Intracranial meningiomas: Correlations between MR imaging and histology. Eur J Radiol. 1999;31:69–75. doi: 10.1016/s0720-048x(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MW, Wilson CB. Meningiomas. In: Youmans JR, editor. Neurological Surgery. 4th ed. Philadelphia: WB Saunders; 1996. pp. 2782–825. [Google Scholar]
- 8.Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, et al. Diffusion-weighted MR imaging: Diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008;29:1147–52. doi: 10.3174/ajnr.A0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfisterer WK, Nieman RA, Scheck AC, Coons SW, Spetzler RF, Preul MC. Using ex vivo proton magnetic resonance spectroscopy to reveal associations between biochemical and biological features of meningiomas. Neurosurg Focus. 2010;28:E12. doi: 10.3171/2009.11.FOCUS09216. [DOI] [PubMed] [Google Scholar]
- 10.Soyama N, Kuratsu J, Ushio Y. Correlation between magnetic resonance images and histology in meningiomas: T2-weighted images indicate collagen contents in tissues. Neurol Med Chir (Tokyo) 1995;35:438–41. doi: 10.2176/nmc.35.438. [DOI] [PubMed] [Google Scholar]
- 11.Yrjänä SK, Tuominen H, Karttunen A, Lahdesluoma N, Heikkinen E, Koivukangas J. Low-field MR imaging of meningiomas including dynamic contrast enhancement study: Evaluation of surgical and histopathologic characteristics. AJNR Am J Neuroradiol. 2006;27:2128–34. [PMC free article] [PubMed] [Google Scholar]


