Abstract
Context:
GH secretion peaks at puberty and continues to be secreted in adulthood, albeit at a declining rate. Profound GH deficiency (GHD) in adults with pituitary disease is associated with symptoms that improve with GH substitution, but it is important to tailor the GH dose to avoid overtreatment. Measurement of serum IGF-I levels is an important clinical tool in this regard, but it is well recognized that some patients receiving GH treatment do not show an increase in IGF-I.
Objective:
The objective of the study was to identify novel serum biomarkers of GH treatment in adults with GHD.
Design and Patients:
Eight patients with profound GHD as a consequence of a pituitary adenoma or its treatment were evaluated before and 3 months after GH replacement therapy (0.2–0.4 mg/d).
Main Outcome Measures:
Serum proteomic changes were studied using two-dimensional gel electrophoresis and mass spectrometry. Protein profiles were analyzed and compared in serum samples obtained before and after GH treatment.
Results:
The levels of six serum protein spots were significantly altered after GH substitution. These proteins were identified as five isoforms of haptoglobin (decreased in posttreatment samples) and one isoform of apolipoprotein A-I (increased in posttreatment samples). Importantly, changes in the levels of the identified proteins were associated with decreases in fat mass and increases in lean mass in all patients. These results were independent of serum IGF-I levels.
Conclusions:
Evaluation of the identified proteins provides a novel alternative to traditional markers of GH status, such as serum IGF-I levels, to assess GH therapy in GH deficient adults.
Management of patients with pituitary mass lesions frequently involves surgical debulking (1, 2). GH deficiency (GHD) is a frequent occurrence in these patients, which may be caused by either tumor compression or the surgical procedure (1, 2). GHD in adults is a recognized syndrome including reduced lean body mass, reduced exercise capacity, abdominal obesity, and elevated blood lipid levels (3, 4). Many of these abnormalities are reversed or improved by GH substitution, which is an approved indication for this condition (5, 6). Adverse effects such as insulin resistance and fluid retention develop unless the GH dose is tailored according to age and gender (7, 8). The clinical tools recommended to monitor GH substitution include anthropometric measurements [viz. body weight, body mass index (BMI), body composition], serum IGF-I measurements, blood lipid profiles, and routine assessment of glycemic control such as hemoglobin A1c levels (9). Clinical studies have shown that the effects of GH therapy vary with onset of GHD, age, gender, treatment duration, and dose (7). In addition, preexisting metabolic abnormalities such as obesity are confounding factors in measuring the benefits of GH replacement therapy (9). Thus, establishing alternative biochemical markers to predict the effects of GH therapy would meet a clinical demand. This in turn could be used for both the diagnosis and treatment of GH deficiency in adults and the identification of the physiological effects of GH as a function of age.
In recent years, clinical proteomics has become of increasing importance in the discovery of disease-specific biomarkers (10–13). In this regard, serum and plasma proteomics have revealed potential targets for diagnosis and treatment of several pathological states including cancer (14, 15). Thus, we applied to the analysis of the serum protein profile of GHD adults. Serum samples from eight recently diagnosed GHD patients were obtained before and after GH replacement treatment and analyzed by two-dimensional gel electrophoresis (2DE) and mass spectrometry. The level of six proteins was significantly different in posttreatment serum samples. The identified proteins represent potential markers to evaluate the systemic impact of GH replacement treatment in adults.
Subjects and Methods
Subjects
Eight patients (three females and five males) with GHD due to a clinically nonfunctioning pituitary adenoma were included in this study. Individuals were 38–64 yr of age (52.8 ± 3.34 yr, height 1.76 ± 0.03 m, weight 82.16 ± 3.63 kg, mean ± sem). Seven patients had undergone transsphenoidal surgery because of compression of the optic chiasm, whereas one patient developed a pituitary apoplexia and subsequent hypopituitarism on the basis of a pituitary adenoma, which was not operated. GHD was diagnosed with an insulin tolerance test with a mean ± se peak GH level of 1.56 ± 0.51 μg/liter. The insulin tolerance test was performed 9 ± 2 months after surgery (n = 7). One patient had isolated GHD; the remaining seven patients were diagnosed with ACTH deficiency (n = 6), TSH deficiency (n = 6), and FSH/LH deficiency (n = 3), which was substituted with hydrocortisone, levothyroxine, and sex steroids, respectively. The substitution therapy for these deficits was initiated before the diagnosis of GHD and was continued in an unchanged dose during GH replacement. All subjects gave a written informed consent before participating in the study, which was approved by the Ethical Committee of Central Region Denmark (2007-0243) in adherence to the Declaration of Helsinki. The protocol also was approved by the Ohio University Institutional Review Board.
Study design
Patients were treated daily with GH sc injections (dose 0.2–0.4 mg/d, depending on age and gender). Blood samples were collected before GH treatment and after 3 months of daily GH administration. The patients arrived at the laboratory in the morning after an overnight fast, and blood samples were collected. After incubation for 30–60 min at room temperature, the samples were centrifuged at 3500 × g for 10 min at 4 C. Serum was removed and stored at −20 C until shipping to Athens, OH.
Anthropometric measurements
Physical examinations included measurements of weight, height, BMI, and body composition before and after GH treatment. Lean mass and fat mass were measured using dual-energy x-ray absorptiometry (QDR-2000; Hologic Inc., Waltham, MA).
Biochemical assessment
Total IGF-I was determined in acid ethanol-extracted serum using an in-house time-resolved immunofluorometric assay as previously described (16, 17) [intraassay coefficient of variation (CV) <5% and interassay CV <10%]. Insulin was analyzed by a commercial time-resolved immunofluorometric assay (Auto DELFIA; PerkinElmer, Turku, Finland) with an intraassay CV of 3.4% and an interassay CV of 3.8%. Insulin resistance was calculated by the homeostasis model assessment index for insulin resistance (HOMA-IR) as insulin (milliunits per liter) × plasma glucose (millimoles per liter)/22.5 using single fasting samples (18). Free fatty acids were analyzed by a commercially available kit (intraassay CV 2–4% and interassay CV 3–6%, detection limit 0.02 mmol/liter) (Wako Chemicals, Neuss, Germany). Total haptoglobin levels were determined by Cobas c-systems, an immunoassay system (Roche Diagnostics, Mannheim, Germany). Serum levels of iodothyronines [total T4 (TT4) and total T3 (TT3)] were measured routinely at the Department of Clinical Biochemistry at Aarhus University Hospital. The reference ranges (nanomoles per liter) for TT4 and TT3 are 60–120 and 1.1–2.5, respectively.
Sample preparation for proteomic analysis
Serum samples were shipped frozen on dry ice from Aarhus, Denmark, to Athens, OH, and stored frozen at −80 C until further analysis. All our proteomic procedures have been described previously (11–13, 19–21). Briefly, serum protein concentrations were determined by the Bradford method (Bio-Rad Laboratories Inc., Hercules, CA). Serum albumin and IgG were depleted using a ProteoPrep Blue albumin and IgG depletion kit (Sigma, St. Louis, MO) following the manufacturer's instructions. Three hundred micrograms (0.3 mg) of each sample were then diluted in sample buffer containing 7 m urea, 2 m thiourea, 1% (wt/vol) SB 3–10, 3% (wt/vol) [(3-cholamidopropyl) dimethyl-ammonio]-1-propane-sulfonate, 0.25% (vol/vol) Bio-Lyte 3/10 ampholytes (Bio-Rad Laboratories), and 1.5% (vol/vol) protease inhibitor cocktail (Sigma). Disulfide bonds were reduced and alkylated with tributylphosphine and iodoacetamide, respectively.
Two-dimensional gel electrophoresis
Samples were subjected to 2DE following procedures previously described (11–13, 19–21). Serum samples were transferred to individual wells of an isoelectric focusing (IEF) tray (Bio-Rad Laboratories) using 17 cm immobilized pH gradient strips (pH 3–10 linear; Bio-Rad Laboratories). IEF was run in a PROTEAN IEF cell (Bio-Rad Laboratories), in which strips were rehydrated at 50 V for 12 h, after which proteins were separated at 10,000 V for 60,000 V/h. After this procedure, the inositol phosphoglycan strips were treated with 2 ml of equilibration buffer (6 m urea; 2% sodium dodecyl sulfate; 375 mm Tris-HCl, pH 8.8; 20% glycerol). Next, 4.5 cm from each end of the 17-cm strips were cut and the resulting 8-cm strips (∼pH 5–8) were loaded onto a 15% polyacrylamide gel for SDS-PAGE. Proteins were separated in a Mini-PROTEAN 3 cell (Bio-Rad Laboratories) at 50 mA per gel for 250 V/h. The gels were then stained using SYPRO Orange (Invitrogen, Carlsbad, CA). the gel images were obtained using a PharosFX Plus molecular imager (Bio-Rad Laboratories) with an excitation wavelength of 488 nm and emission detected at 605 nm. Gel image analysis was performed using the software PDQuest Advanced version 8.0 (Bio-Rad Laboratories). Protein spots that were differentially expressed before and after treatment (P < 0.05) were manually excised from the gels and sent to ProteaBioscience, Inc. (Morgantown, WV) for identification by mass spectrometry (MS) and tandem MS (MS/MS) as described below.
MS analysis
The procedures used for MS and MS/MS have been previously described (11, 12, 22). Protein spots displaying significant (P < 0.05) intensity changes before and after GH treatment were analyzed by MS and MS/MS using matrix-assisted laser desorption/ionization (MALDI)-time of flight (TOF) and MALDI-TOF-TOF. Briefly, acrylamide gel plugs containing individual protein spots were dehydrated and then rehydrated with acetonitrile and 50 mm ammonium bicarbonate, respectively. Proteins were then reduced with 250 mm dithiothreitol and alkylated with 650 mm iodoacetamide. After this procedure, proteins were digested with 500 ng trypsin. Extraction of peptides was performed using 5% formic acid in 50% acetonitrile (dehydration), followed by rehydration with 50 mm ammonium bicarbonate. Peptides were then lyophilized, reconstituted in 10 mm acetic acid, and relyophilized to yield a purified protein digest extract. For MS and MS/MS analyses, the protein digest solution was loaded onto a C18 ProteaTip (Protea Biosciences, Inc., Morgantown, WV). The sample was spotted directly onto a MALDI target that was prespotted with 0.6 μl MALDI matrix (α-cyano-4-hydroxycinnamic acid) using 1 μl of an elution solution (0.1% trifluoroacetic acid/90% acetonitrile). Mass spectra were acquired on an ABI 4800 MALDI TOF/TOF analyzer (PE Applied Biosystems Inc., Warrington, UK). MS spectra were acquired in reflector-positive ion mode. Peptide masses were acquired for the range from 850 to 4000 Da. MS spectra were summed from 400 laser shots. Internal calibration was performed using a minimum of three trypsin autolysis peaks. For MS/MS, spectra were acquired until at least four peaks in the MS/MS spectra achieved a signal to noise ratio equal to 70.
Protein database searching with MS- and MS/MS-generated peak lists
Protein identities were further verified by using the MS and MS/MS data obtained and the online software Mascot (http://www.matrixscience.com) (11–13, 19–21). Our search parameters were the following: MS database, NCBInr; taxonomy, Homo sapiens; enzyme, trypsin; missed cleavages allowed, one; fixed modifications, none; protein mass, not specified; peptide tolerance, ±1.2 Da; mass values, positive ion mode; monoisotopic/average, monoisotopic; and the MS/MS database, NCBInr; taxonomy, Homo sapiens; enzyme, trypsin; missed cleavages allowed, one; fixed modifications, none; quantitation, none; peptide tolerance, ±1.2 Da; MS/MS tolerance, ±0.6 Da; peptide charge, 1+; monoisotopic/average, monoisotopic; precursor m/z, not specified; instrument, MALDI-TOF-TOF. Variable modifications that were included in separate and combined submissions for both MS and MS/MS were acetyl (K), carbamidomethyl (C), deamidated (NQ), oxidation (M), phospho (ST), phospho (Y), sulfo (S), sulfo (T), sulfo (Y). The general criteria used for assessment of protein identity were a significant match of at least two MS/MS fragments.
Western blot analysis
Western blotting was performed to confirm the identity of haptoglobin and apolipopritein (apo) A-1. For one-dimensional Western blots, 0.05 mg of each sample was loaded onto SDS-PAGE gels and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). For the two-dimensional (2-D) Western blots, 0.3 mg of each sample was subject to 2DE and transferred to polyvinylidene difluoride membranes. Membranes were then blocked in 5% nonfat dry milk and probed for 2 h with primary antibody. Antibodies against haptoglobin (rabbit antihaptoglobin of human origin, 1:500 dilution) and apoA-I (mouse anti-apoA-I of human origin, 1:500) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Membranes were subsequently identified with a horseradish peroxidase-conjugate secondary antibody (1:5000 dilution) and Pierce ECL Western blotting substrate (Thermo Scientific, Rockford, IL). The resulting blots were scanned using a Pharos FX Plus imaging system (Bio-Rad Laboratories).
Statistical analysis
All protein spot intensities were analyzed for normality (Shapiro-Wilk test) and variance [homogeneity test for two dependent samples (23)]. Protein spots displaying normal distributions and equal variances were compared between the two groups using a two-tailed paired t test (corresponding t and P value is reported; see significant spot B). The nonparametric Wilcoxon signed-ranks test was used to analyze the remaining spots (z and P values are reported; see spots A, C, D, E, and F). Anthropometric and biochemical parameters were analyzed using a two-tailed paired t test. All tests were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL). The levels of significance were set at P < 0.05.
Results
Anthropometric measurements after GH treatment
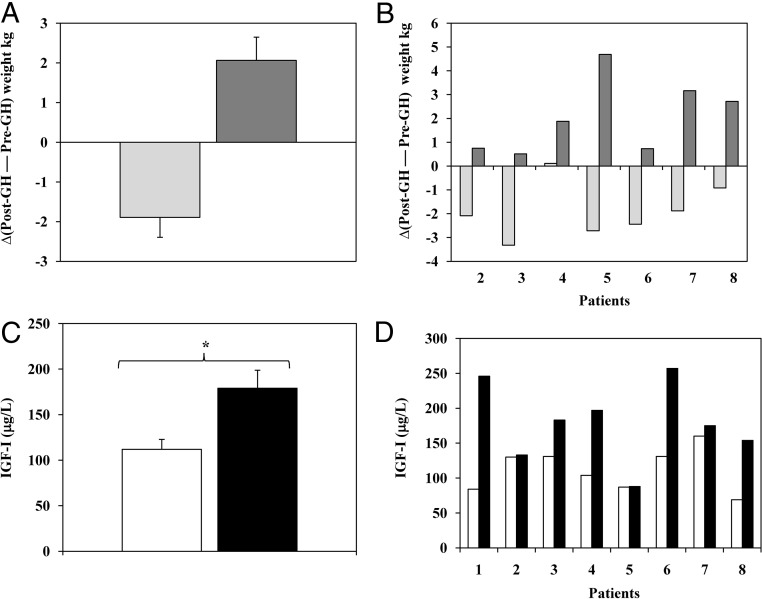
No significant changes in body weight and BMI were observed after 3 months of daily GH administration (Table 1). However, changes in body fat and lean mass were significantly different after GH treatment (Table 1). The mean and individual changes in fat and lean mass are presented in Fig. 1, A and B. Each patient demonstrated an increase in lean mass and a reduction in fat mass after GH therapy.
Table 1.
Anthropometric measurements, IGF-I values, and metabolic markers before and after GH treatment
| Before GH | After GH | P value | |
|---|---|---|---|
| Weight (kg) | 82.16 ± 3.63 | 82.46 ± 3.11 | 0.65 |
| BMI (kg/m2) | 25.74 ± 1.25 | 25.26 ± 1.15 | 0.91 |
| Fat mass (kg) | 27.35 ± 2.40 | 25.46 ± 2.57 | 0.01a |
| Lean mass (kg) | 52.21 ± 4.17 | 54.27 ± 3.91 | 0.01a |
| IGF-I sd | −0.46 ± 0.43 | 1.27 ± 0.76 | 0.02a |
| IGF-I (μg/liter) | 112 ± 11 | 179 ± 20 | 0.01a |
| Free fatty acids (mEq/liter) | 0.37 ± 0.19 | 0.42 ± 0.22 | 0.50 |
| Glucose (mmol/liter) | 5.09 ± 0.21 | 5.11 ± 0.09 | 0.89 |
| Insulin (pmol/liter) | 30.47 ± 4.7 | 33.62 ± 6.63 | 0.60 |
| HOMA-IR | 1.06 ± 0.18 | 1.04 ± 0.24 | 0.22 |
Results are shown as mean ± sem. P values were calculated by two-tailed paired t test.
P ≤ 0.05 was considered significant.
Fig. 1.
A, Change (after GH treatment to before GH treatment) in fat (light gray bars) and lean mass (dark gray bars) as measured by dual-energy x-ray absorptiometry (mean ± sem). B, Changes in fat and lean mass for each patient (2–8) after treatment. Light gray bars, Fat mass; dark gray bars, lean mass. No body composition data for patient 1 was available for analysis. C, Mean ± sem serum levels of IGF-I at pre- (white bars) and post-GH substitution (black bars). *, P < 0.05 in a two-tailed paired t test. D, IGF-I serum level changes before and after GH treatment in individual patients (1–8).
IGF-I values and metabolic markers after GH treatment
Total serum IGF-I levels (micrograms per liter) increased significantly after treatment with GH (Table 1). The increase in IGF-I expressed as sd scores was also significant (Table 1). IGF-I values are presented as mean ± sem in Fig. 1C, and level changes for each patient after treatment are shown in Fig. 1D. Note that patients 2 and 5 did not show an increase in serum IGF-1 after GH treatment. In contrast, positive changes in body composition were observed in all patients (Fig. 1, A and B).
No statistically significant changes in free fatty acids, glucose, or insulin were found after GH treatment (Table 1). Moreover, no differences in HOMA-IR were found after the treatment (Table 1). Serum levels of TT4 and TT3 remained within the normal range and did not change after GH treatment [TT4 (nanomoles per liter): 98 ± 15 (before) vs. 94 ± 13 (after) (P = 0.47); and TT3 (nanomoles per liter): 1.57 ± 0.14 (before) vs. 1.78 ± 0.18 (after) (P = 0.19)].
Serum proteome changes after GH treatment
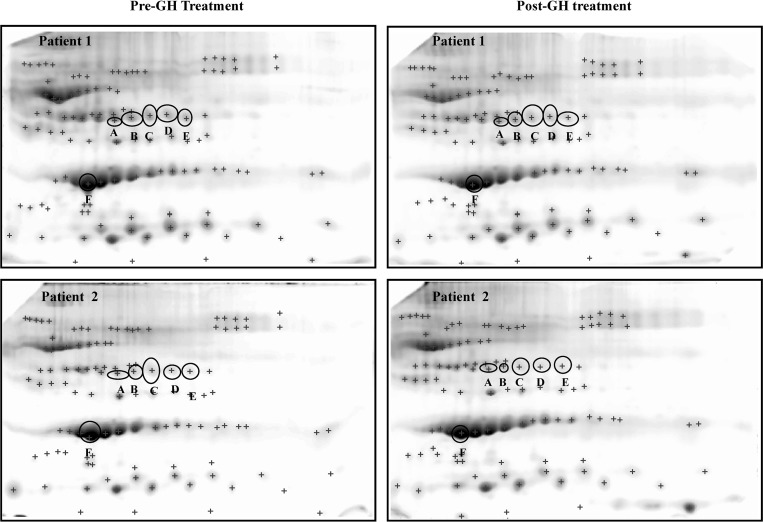
The proteomic profiles of all serum samples were evaluated in each subject before and after GH treatment. Protein profiles were reproducible (Fig. 2). A total of 111 protein spots were detected and matched in all gels (a total of 16 gels). Six protein spots were significantly altered (P < 0.05); five decreased (A–E) and one increased (F) after GH treatment (Fig. 2).
Fig. 2.
Representative 2DE gels showing their spot patterns. A total of 111 spots were analyzed [plus (+) signs]. Images belong to two different patients (see labels) and correspond to the pre- and post-GH substitution treatment. Six protein spots displayed significant changes after the treatment (labeled A–F).
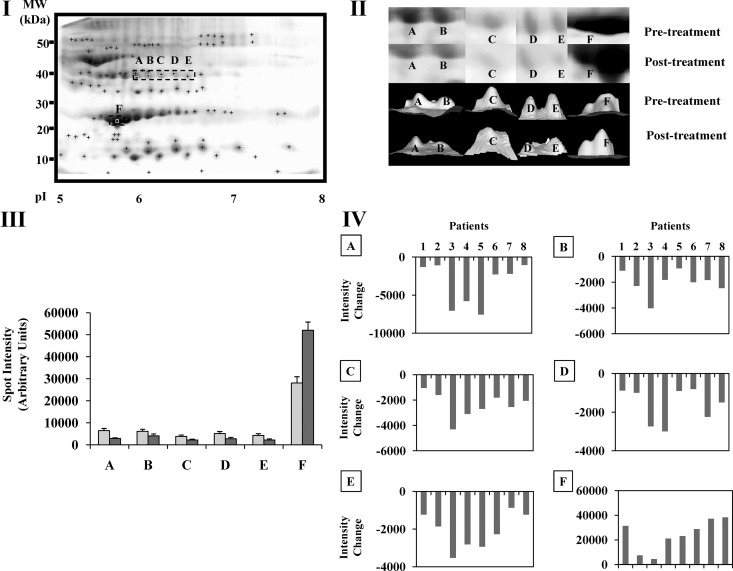
The intensity change for protein spots A–F before and after GH treatment is shown in Fig. 3 (III-IV).
Fig. 3.
I, Representative 2DE serum gel showing the five protein spots identified by MS as haptoglobin isoforms (labeled A–E and squared by a dotted outline) and apoA-I (labeled F and squared by a dotted outline). II, Three-dimensional (3D) view of protein spots A–F displaying intensities before (upper panel) and after treatment (lower panel) for each protein spot. Images were generated using PDQuest software version 8.0 (Bio-Rad Laboratories). Upper and lower images belong to the same patient. III, Intensity values (mean ± sem) before (gray) and after GH treatment (dark gray) for protein spots A–F. IV, Spot intensity changes (intensity after GH treatment minus intensity before GH treatment) for each individual subject (1–8) for each of the six spots (A–F). Letters above each panel correspond to the protein spot shown in each graph.
Protein spot identification by MS and MS/MS
The identities of spots A–F (Fig. 2) were determined by MS and MS/MS (Table 2). Among these proteins, five located at approximately 40 kDa (A–E) were identified as isoforms of haptoglobin [Fig. 3 (I)]. All were significantly down-regulated after GH treatment (spot A: z = 2.1, P = 0.03; spot B: t =1.02, P = 0.002; spot C: z = 2.5, P = 0.01; spot D: z = 2.38, P = 0.01; and spot E: z = 2.5, P = 0.01) [Fig. 3 (II–IV)]. Protein spot F was identified as apoA-1 (∼ 28 kDa) [Fig. 3 (I)] and increased (z = 1.96, P = 0.04) after GH administration in all patients [Fig. 3 (II–IV)].
Table 2.
Mass spectrometry for spots that changed significantly post-GH treatment
| Spot | Gel pI/MM | Identity match | Accession no. | MS Scorea | Maximum sequence coverage (%) | Matched fragments | MS/MS scoreb | Maximum sequence coverage (%) | Matched fragments |
|---|---|---|---|---|---|---|---|---|---|
| A | 5.9/40 | Haptoglobin | gi|3337390 | 71 | 39 | 10/47 | 394 | 22 | 6/59 |
| B | 6.2/40 | Haptoglobin | gi|3337390 | 69 | 42 | 11/51 | 338 | 25 | 7/65 |
| C | 6.3/40 | Haptoglobin | gi|3337390 | 98 | 39 | 10/51 | 355 | 22 | 6/66 |
| D | 6.4/40 | Haptoglobin | gi|3337390 | 65 | 29 | 9/41 | 124 | 17 | 4/53 |
| E | 6.5/40 | Haptoglobin | gi|3337390 | 75 | 33 | 9/48 | 97 | 11 | 3/58 |
| F | 5.7/28 | Apolipoprotein A-1 | gi|90108664 | 153 | 53 | 16/39 | 294 | 26 | 6/53 |
MM, Molecular mass.
A minimum MS score greater than 64 represents a statistically significant match (P < 0.05).
A minimum of two significant MS/MS peptide fragment matches was required to assign an identification for a spot.
Total haptoglobin levels and Western blot analyses before and after GH treatment
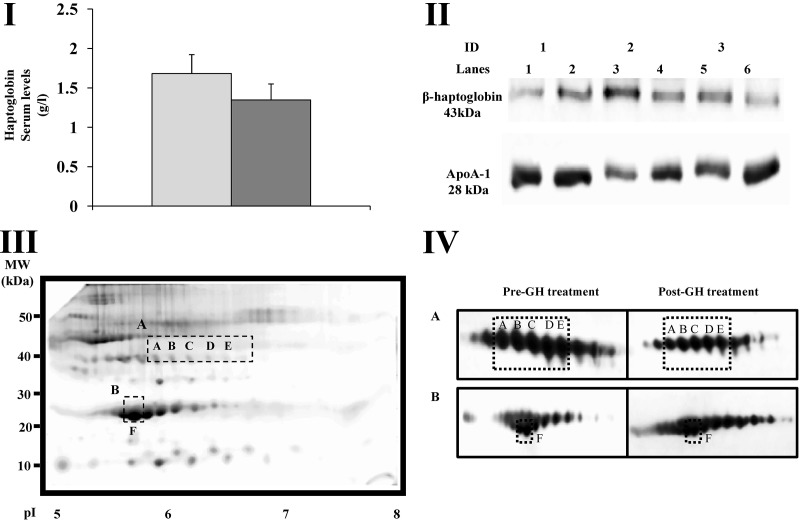
Haptoglobin serum levels were quantified, and although levels tended to decrease after GH treatment, no significant differences (P > 0.05) were found after GH administration [Fig. 4 (I)]. In agreement with these results, only slight differences in the total haptoglobin levels were found after Western blotting [Fig. 4 (II)]. Similarly, only slight decreases were found in the total apoA-I levels by Western blotting [Fig. 4 (II)].
Fig. 4.
I, Mean ± sem serum levels of haptoglobin before GH treatment (gray) and after treatment (dark gray). II, Western blots of haptoglobin and apoA-I in serum samples. Representative images of three different patients (patients 1, 2, and 3) before (lanes 1, 3, and 5) and after surgery (lanes 2, 4, and 6). Equal amounts of total protein were loaded (0.05 mg) and separated by SDS-PAGE. III, Representative 2-D gel showing the location of protein spots (A–F) (dashed squares). Molecular mass markers are indicated to the left of the panels and pI markers are shown on the bottom of the panels. IV, Representative 2-D blots showing different isoforms of haptoglobin (A) and apoA-I (B) identified by this technique. The isoforms corresponding to protein spots A–F are indicated by dashed squares.
Western blotting confirmation of haptoglobin and apoA-I
Haptoglobin and apoA-I were subjected to 2-D Western blot analysis and were found to be consistent with the MS identification [Fig. 4 (III-IV)]. Hp isoforms were identified at a molecular mass of approximately 40 kDa and an isoelectric point range of approximately 5.7–7.9 [Fig. 4 (III)]. Five of the identified isoforms correspond to spots A–E (molecular mass ∼40, pI ∼5.9–6.6) (Table 2). In agreement with our MS results, the isoforms corresponding to protein spots A–E decreased after GH treatment [Fig. 4 (IV)]. Several apoA-I isoforms were located at a molecular mass of approximately 28 kDa and pI ranging from approximately 5.2 to approximately 7.8 [Fig. 4 (III–IV)]. One of these isoforms corresponds to protein spot F (molecular mass ∼28, pI ∼5.8) (Table 2). As shown in Fig. 4 (IV), the apoA-I isoform corresponding to protein spot F is more prominent after GH treatment.
Discussion
The present study focused on the analysis of the serum proteome of adult GHD patients with nonfunctional pituitary adenomas before and after short-term GH replacement therapy. GHD in adults is associated with recognized adverse effects on several features including body composition and physical fitness, most of which is improved after GH replacement therapy (24). However, dose-dependent side effects, in particular fluid retention and glucose intolerance, are not uncommon (8). The clinical tools used to monitor the therapy include anthropometric measurements and serum IGF-I quantification (9).
Although IGF-I is a good biochemical marker of GH action, normal IGF-I levels do not necessarily rule out GHD and/or an inadequate response to GH therapy (9). As an example, in this study we found that after 3 months of daily GH administration, IGF-I levels were normalized in six of eight patients. In contrast, two patients (2 and 5, Fig. 1) showed no increases in serum IGF-I values. Interestingly, despite their IGF-I status, all patients presented significant differences in body composition, with decreased fat mass and increased lean mass. These results are consistent with the concept that serum IGF-I levels are regulated by several factors other than GH. Preexisting metabolic abnormalities, such as obesity, influence IGF-I values (9). For example, studies have shown that GH levels are differentially regulated at the extremes of the weight spectrum, and these effects may not reflect IGF-I status (25, 26). In addition, in vitro and in vivo studies have shown that GH has lipolytic effects, independent of IGF-I (27, 28). Therefore, given the lack of change in IGF-I levels in patients 2 and 5 with the positive effects of GH on body composition suggest that GH actions and the benefits of GH replacement therapy may not be accurately reflected by IGF-I serum levels.
It is also noteworthy that no significant changes in body weight were observed in any of the patients, despite changes in body composition, which is in accordance with the published results in experiments with a comparable duration of GH replacement (29). In addition, no differences in glucose, insulin, and fatty acid levels were seen after 3 months of GH replacement therapy. Moderate but overall significant elevations in fasting serum levels of insulin and glucose after GH replacement therapy have previously been reported in a metaanalysis, but the GH dose used in the majority of the included trials was significantly higher compared with this study (7). In the present study, we did not detect significant changes in serum levels of iodothyronines, but it is well known that GH stimulates the peripheral conversion of TT4 to TT3 (30). The reason that no significant change in iodothyronine levels was observed could reflect the low GH replacement dose, but it is also noteworthy that the thyroid function tests were done on a routine basis. It is therefore possible that a moderate GH-induced elevation in T3 levels did occur, which could have contributed to the observed changes in the serum proteome. It is, however, important to note that any substitution therapy apart from GH was unchanged during the study.
In terms of the proteomic results, we identified six protein spots that were consistently altered in all patients after GH treatment.
Spots A–E were identified as isoforms of haptoglobin. Haptoglobin is an acute-phase plasma protein, which consists of two α-subunits and two β-subunits joined by interchain disulfide bonds (31–33). Recent proteomic studies from our group using serum samples from acromegalic patients showed decreased expression of one isoform of haptoglobin-α2 (molecular mass ∼20, pI ∼6.2) after transsphenoidal surgery (12). In the present study, we found that the protein expression of four isoforms of haptoglobin (molecular mass ∼40, pI ∼5.9–6.5) was markedly down-regulated after GH treatment in GHD patients.
Supporting these results, 2-D Western blots showed the presence of several haptoglobin isoforms at a molecular mass of approximately 40 and in an approximate pH range of 5.7–7.9. Five of these isoforms correspond to protein spot A–E (molecular mass ∼40, pI ∼5.9–6.5), confirming the protein identity assigned by MS.
The expression of different isoforms of the same protein may be explained by the presence of posttranslational modifications (i.e. glycosylation and phosphorylation) that may alter charge and/or size of the protein (http://ca.expasy.org/swiss-2dpage/viewer). No significant differences in the total concentration of haptoglobin were found by conventional techniques such as ELISA or Western blotting. These results suggest that total haptoglobin levels may not necessarily reflect the systemic GH status. Future studies to identify the chemical nature of the posttranslational modifications and subsequent establishment of assays to evaluate the levels of specific haptoglobin isoforms will provide a more accurate approach to monitor and quantify the effects of GH replacement treatment in GHD patients. These data may also add to the knowledge of GH's physiological effects.
Spot F corresponded to one isoform of apoA-I (molecular mass ∼28, pI ∼5.7). GH's effects on the regulation of lipoprotein metabolism are controversial (34–37). Early studies reported that GH replacement therapy in GHD children did not significantly alter total apoA-I levels (36, 37). On the other hand, additional studies showed increases in apoA-I serum levels in acromegalic patients after treatment with somatostatin analogs (38). In agreement with previous results, no significant differences in total apoA-I levels were found by one-dimensional Western blots.
Proteomic studies from our laboratory have shown that several factors such as GH state (deficiency, normal, or increased levels), age, and duration of the treatment may lead to up-regulation or down-regulation of specific apoA-I isoforms (11–13). For example, a study by Sackmann-Sala et al. (13) showed decreases in the levels of an isoform of apoA-I (molecular mass ∼28, ∼pI 6.3) in serum samples from healthy adult males treated with a long-acting GHRH analog. On the other hand, a recent study using serum samples from acromegalic patients showed a down-regulation of two isoforms of apoA-I (molecular mass ∼10, pI ∼5.0–5.4) after transsphenoidal surgery. In the present study, we found a significant increase in the expression of an isoform of apoA-I (molecular mass ∼28, pI ∼5.7) in response to GH replacement treatment. These results suggest that the identified apoA-I isoform may represent a sensitive marker for GH effects in GHD adults.
To confirm the identity of protein spot F as apoA-I and to show the presence of different protein isoforms in the system, 2-D Western blots were performed. Several apoA-I isoforms were identified at a molecular mass of approximately 28 kDa in a pH range of 5.2–7.8. One of the resolved isoforms corresponded to spot F, confirming our MS data. Futures studies need to be conducted to reveal the posttranslational modifications associated with this apoA-I isoform.
In summary, in this study we found that the serum levels of six protein spots were significantly altered after GH administration. These protein spots were identified by MS as five isoforms of haptoglobin and one isoform of apoA-1. Although the chemical identification of the differences between protein isoforms and the clinical and physiological implications of our findings remain to be further investigated, the identified proteins represent potential biomarkers of GH action in adult GHD.
Acknowledgments
We acknowledge Juan Ding, Ph.D. (Edison Biotechnology Intitute, Ohio University, Athens, OH), for her helpful discussions regarding the mass spectrometry data.
This work was supported by a grant from World Anti-Doping Agency and the State of Ohio's Eminent Scholars Program that includes a gift by Milton and Lawrence Goll. Also, J.J.K. is supported by the following grants: NIHR15DK075436, NIHR01AG019899, and 1P01AG031736-01A1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- apo
- Apolipoprotein
- BMI
- body mass index
- CV
- coefficient of variation
- 2-D
- two-dimensional
- 2DE
- two-dimensional gel electrophoresis
- GHD
- GH deficiency
- HOMA-IR
- homeostasis model assessment index for insulin resistance
- IEF
- isoelectric focusing
- MALDI
- matrix-assisted laser desorption/ionization
- MS
- mass spectrometry
- MS/MS
- tandem MS
- TOF
- time of flight
- TT3
- total T3
- TT4
- total T4.
References
- 1. Corneli G, Baldelli R, Di Somma C, Rovere S, Gaia D, Pellegrino M, Gasco V, Durante C, Grottoli S, Colao A, Tamburrano G, Lombardi G, Ghigo E, Aimaretti G. 2003. Occurrence of GH deficiency in adult patients who underwent neurosurgery in the hypothalamus-pituitary area for non-functioning tumour masses. Growth Horm IGF Res 13:104–108 [DOI] [PubMed] [Google Scholar]
- 2. Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. 1991. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer 68:860–866 [DOI] [PubMed] [Google Scholar]
- 3. Bates AS, Van't Hoff W, Jones PJ, Clayton RN. 1996. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab 81:1169–1172 [DOI] [PubMed] [Google Scholar]
- 4. Cuneo RC, Salomon F, McGauley GA, Sonksen PH. 1992. The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf) 37:387–397 [DOI] [PubMed] [Google Scholar]
- 5. Jorgensen JO, Vahl N, Hansen TB, Thuesen L, Hagen C, Christiansen JS. 1996. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: increase in exercise capacity and normalization of body composition. Clin Endocrinol (Oxf) 45:681–688 [DOI] [PubMed] [Google Scholar]
- 6. Jørgensen JO, Thuesen L, Müller J, Ovesen P, Skakkebaek NE, Christiansen JS. 1994. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur J Endocrinol 130:224–228 [DOI] [PubMed] [Google Scholar]
- 7. Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. 2004. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199 [DOI] [PubMed] [Google Scholar]
- 8. Rosenfalck AM, Maghsoudi S, Fisker S, Jørgensen JO, Christiansen JS, Hilsted J, Vølund AA, Madsbad S. 2000. The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab 85:4173–4181 [DOI] [PubMed] [Google Scholar]
- 9. Ho KK. 2007. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157:695–700 [DOI] [PubMed] [Google Scholar]
- 10. Chung L, Clifford D, Buckley M, Baxter RC. 2006. Novel biomarkers of human growth hormone action from serum proteomic profiling using protein chip mass spectrometry. J Clin Endocrinol Metab 91:671–677 [DOI] [PubMed] [Google Scholar]
- 11. Christensen B, Sackmann-Sala L, Cruz-Topete D, Jørgensen JO, Jessen N, Lundby C, Kopchick JJ. 2011. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol 110:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. 2011. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol 164:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. 2009. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res 19:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chatterjee SK, Zetter BR. 2005. Cancer biomarkers: knowing the present and predicting the future. Future Oncol 1:37–50 [DOI] [PubMed] [Google Scholar]
- 15. Mauri P, Scigelova M. 2009. Multidimensional protein identification technology for clinical proteomic analysis. Clin Chem Lab Med 47:636–646 [DOI] [PubMed] [Google Scholar]
- 16. Jørgensen JO, Feldt-Rasmussen U, Frystyk J, Chen JW, Kristensen LØ, Hagen C, Ørskov H. 2005. Cotreatment of acromegaly with a somatostatin analog and a growth hormone receptor antagonist. J Clin Endocrinol Metab 90:5627–5631 [DOI] [PubMed] [Google Scholar]
- 17. Frystyk J, Dinesen B, Orskov H. 1995. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul 5:169–176 [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 19. List EO, Berryman DE, Palmer AJ, Qiu L, Sankaran S, Kohn DT, Kelder B, Okada S, Kopchick JJ. 2007. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics 7:1140–1149 [DOI] [PubMed] [Google Scholar]
- 20. Qiu L, List EO, Kopchick JJ. 2005. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics 4:1311–1318 [DOI] [PubMed] [Google Scholar]
- 21. Ding J, List EO, Okada S, Kopchick JJ. 2009. Perspective: proteomic approach to detect biomarkers of human growth hormone. Growth Horm IGF Res 19:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. 2010. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299:H529–H540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheskin D. 1997. Handbook of parametric and nonparametric statistical procedures. Boca Raton, FL: CRC Press [Google Scholar]
- 24. Hansen TB, Brixen K, Vahl N, Jorgensen JO, Christiansen JS, Mosekilde L, Hagen C. 1996. Effects of 12 months of growth hormone (GH) treatment on calciotropic hormones, calcium homeostasis, and bone metabolism in adults with acquired GH deficiency: a double blind, randomized, placebo-controlled study. J Clin Endocrinol Metab 81:3352–3359 [DOI] [PubMed] [Google Scholar]
- 25. Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, Ott M, Herzog DB, Johnson ML, Klibanski A. 2003. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 88:5615–5623 [DOI] [PubMed] [Google Scholar]
- 26. Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, Herzog DB, Misra M, Klibanski A. 2010. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab 95:4889–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campbell RM, Scanes CG. 1988. Inhibition of growth hormone-stimulated lipolysis by somatostatin, insulin, and insulin-like growth factors (somatomedins) in vitro. Proc Soc Exp Biol Med 189:362–366 [DOI] [PubMed] [Google Scholar]
- 28. Simpson HL, Jackson NC, Shojaee-Moradie F, Jones RH, Russell-Jones DL, Sönksen PH, Dunger DB, Umpleby AM. 2004. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab 89:425–432 [DOI] [PubMed] [Google Scholar]
- 29. Jörgensen JO, Pedersen SA, Thuesen L, Jörgensen J, Ingemann-Hansen T, Skakkebaek NE, Christiansen JS. 1989. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1:1221–1225 [DOI] [PubMed] [Google Scholar]
- 30. Jørgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. 1989. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab 69:1127–1132 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Q, Wang J, Dong R, Yang S, Zheng S. 2011. Identification of novel serum biomarkers in child nephroblastoma using proteomics technology. Mol Biol Rep 38:631–638 [DOI] [PubMed] [Google Scholar]
- 32. Koch W, Latz W, Eichinger M, Roguin A, Levy AP, Schömig A, Kastrati A. 2002. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem 48:1377–1382 [PubMed] [Google Scholar]
- 33. Van Vlierberghe H, Langlois M, Delanghe J. 2004. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 345:35–42 [DOI] [PubMed] [Google Scholar]
- 34. Yildiz M, Arslanoglu I, Yildiz N, Nartop F, Isik K, Isguven P. 2000. Effects of growth hormone replacement therapy on lipids, lipoproteins and apolipoproteins: is the increased apolipoprotein A-1/B ratio the only benefit? Pediatr Int 42:151–154 [DOI] [PubMed] [Google Scholar]
- 35. Lanes R, Soros A, Gunczler P, Paoli M, Carrillo E, Villaroel O, Palacios A. 2006. Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J Pediatr 149:324–329 [DOI] [PubMed] [Google Scholar]
- 36. Schaefer GB, Greger NG, Fesmire JD, Blackett PR, Wilson DP, Frindik JP. 1994. Lipids and apolipoproteins in growth hormone-deficient children during treatment. Metabolism 43:1457–1461 [DOI] [PubMed] [Google Scholar]
- 37. Blackett PR, Weech PK, McConathy WJ, Fesmire JD. 1982. Growth hormone in the regulation of hyperlipidemia. Metabolism 31:117–1120 [DOI] [PubMed] [Google Scholar]
- 38. Delaroudis SP, Efstathiadou ZA, Koukoulis GN, Kita MD, Farmakiotis D, Dara OG, Goulis DG, Makedou A, Makris P, Slavakis A, Avramides AI. 2008. Amelioration of cardiovascular risk factors with partial biochemical control of acromegaly. Clin Endocrinol (Oxf) 69:279–284 [DOI] [PubMed] [Google Scholar]