Abstract
With the cases described, we strive to introduce single photon emission computerized tomography in combination with conventional computer tomography (SPECT/CT) to shoulder surgeons, illustrate the possible clinical value it may offer as new diagnostic radiologic modality, and discuss its limitations. SPECT/CT may facilitate the establishment of diagnosis, process of decision making, and further treatment for complex shoulder pathologies. Some of these advantages were highlighted in cases that are frequently seen in most shoulder clinics.
Keywords: Hemiarthroplasty, loosening, shoulder arthroplasty, SPECT/CT
INTRODUCTION
Single photon emission computerized tomography in combination with conventional computer tomography (SPECT/CT) pairs a high-resolution anatomical 3D-CT with a functional single photon emission tomography (SPECT).[1–5] Until recently, registration or image fusion of the functional (SPECT) with structural images (CT) has been difficult due to SPECT's poor spatial resolution (3-10 mm). Another important problem was the poor reproducibility in identifying the anatomical landmarks.[1,6,7] With the advent of integrated SPECT/CT machines in 1999, where the image data from SPECT are taken in the same coordinate frame as the CT scan, image fusion ceased to be an issue. Furthermore, hybrid SPECT/CT machines now provide superior SPECT data as the CT data can be used to derive the attenuation coefficients of the tissue.[8] With integrated machines, functional and anatomic information is provided in the same image, supporting diagnostic analysis of diseases where both types of data are vital. SPECT/CT has already proven useful as a diagnostic tool in areas such as endocrinology, cardiology, and neurosurgery.[1,9–16] In orthopedics, it has yet to be clinically validated and only a paucity of studies are available.[1–5,17–19] With the cases described, we strive to introduce SPECT/CT to shoulder surgeons, illustrate the possible clinical value it may offer as new diagnostic radiologic modality, and discuss its limitations.
Technical description
Hybrid SPECT/CT was performed in two centers using either a hybrid system, Symbia T16 (Siemens, Erlangen, Germany) or Hawkeye-4 (GE Healthcare). The systems are equipped with a pair of low-energy, high-resolution collimators and incorporate a dual-head gamma camera with an integrated, 16- or 4- slice CT scanner. Patients were injected intravenously with 10-20 mCi (340-740 MBq) of 99m-technetium-labelled diphosphonate or 99m-technetium-labelled anti-granulocyte antibodies (CIS Bio International Sur Yvette, France). Perfusion images are obtained in the initial 60 seconds post-injection followed by blood pool images 2 to 5 minutes later. SPECT was performed at 3 to 5 hours, followed immediately by a CT with the patient in the same position.
CASE REPORTS
Case 1
A 54-year-old male patient sustained a right-sided 4-part proximal humeral fracture, which was treated by open reduction and internal fixation with a Numelock polyaxial locking system (Stryker AG, Switzerland). One month later, he had to be revised due to a superficial wound infection with streptococcus. The infection resolved under antibiotic therapy with augmentin for 6 weeks. The plate was removed four months after initial fracture treatment.
At presentation to our clinic, the patient complained about activity-related pain and stiffness of his shoulder. Anterior-posterior and y-view radiographs revealed a valgus-impacted humeral head fragment. The healing of the fracture was in doubt and at least a partial humeral head necrosis was suspected [Figure 1a]. MRI showed wide intramedullary osteolytic zones, which were attributed either to a pseudoarthrosis or recurrence of infection. In addition, a severe osteoarthritis and a fatty degeneration of the supraspinatus muscle (Goutallier 2) were identified [Figure 1b]. To exclude a persistence of an infection, an anti-granulocyte SPECT/CT was performed. This showed absent tracer uptake of the humeral head fragment which was attributed to a humeral head necrosis. No signs of infection were present [Figure 1c]. The patient was scheduled for total shoulder arthroplasty.
Figure 1a.
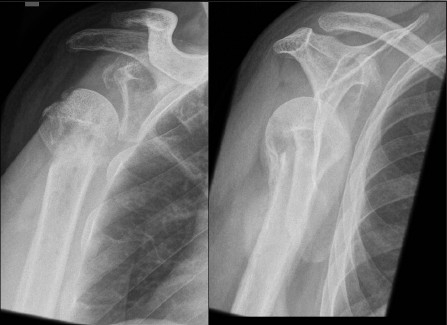
Anterior-posterior and y.view radiographs of the right shoulder at initial presentation indicating a valgus-impacted humeral head fragment and doubtful partial osteonecrosis of the humeral head
Figure 1.
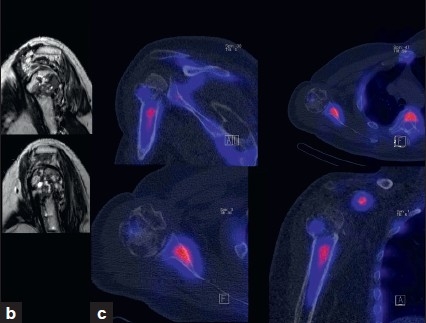
(b) MRI showing extensive cystic changes within the humeral head; (c) 99mTc.HDP.SPECT/CT showing decreased uptake of the humeral head fragment indicating a humeral head necrosis
Case 2
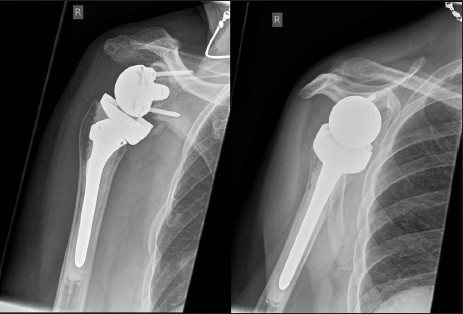
A 70-year-old female patient underwent an inverse shoulder arthroplasty (ANATOMICAL 3, Zimmer, Switzerland) due to a cuff arthropathy. Seven months later, she suffered from activity-related pain in her shoulder. Anterior posterior and y-view radiographs showed extensive osteolysis around the glenoid component [Figure 2a]. In SPECT/CT, increased tracer uptake between the glenoid component and the host bone was present [Figure 2b]. There was no increased tracer uptake around the stem indicating a well-fixed humeral stem component. At revision surgery, the glenoid component was loose. It was revised to a bipolar humeral head (custom-made, Zimmer, Switzerland) and the host bone defect was filled with autologous cancellous bone graft from the ipsilateral iliac crest. The stem was not revised, as it was well fixed [Figure 2c]. At three-month follow-up, the patient had not regained full range of motion, but presented with less in activities of daily living.
Figure 2a.
Anterior-posterior and y-view radiographs of the right shoulder showing wide radiolucent lines around the glenoid peg
Figure 2b.
99mTc-HDP-SPECT/CT showing increased tracer uptake at the glenoid component-host bone interface, which was interpreted as clear sign for mechanical loosening
Figure 2c.
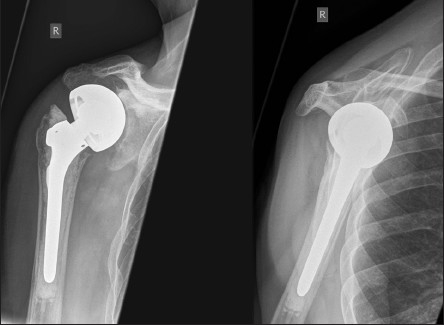
Anterior-posterior and y-view radiographs of the right shoulder after revision to a bipolar cup prosthesis
Case 3
A 59-year-old male patient underwent a shoulder hemiarthroplasty (EPOCA, Synthes, Switzerland) due to a 4-part proximal humeral fracture. The postoperative recovery was uneventful until 3 years postoperatively when shoulder pain increased. Anterior-posterior and y-view radiographs showed osteolytic zones around the humeral stem indicating aseptic loosening of the hemiarthroplasty [Figure 3a]. Since osteolytic zones and clinical symptoms often poorly correlate in shoulder arthroplasty, a SPECT/CT scan was performed. It showed increased tracer uptake only around the stem in all three phases, which was attributed to mechanical loosening [Figure 3b]. The hemiarthroplasty was revised (GLOBAL, DePuy, Switzerland) and a satisfying recovery was obtained.
Figure 3a.
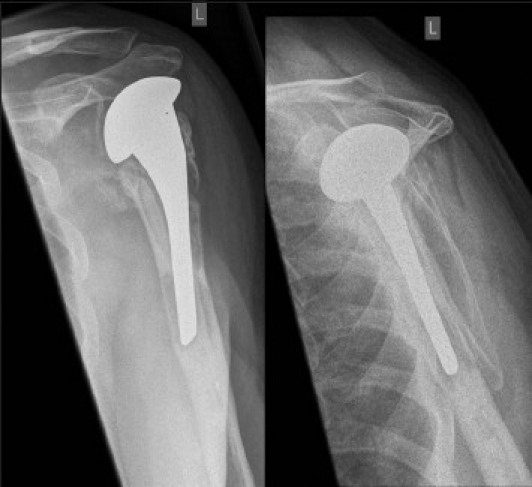
Anterior-posterior and y-view radiographs of the left shoulder indicating radiolucent lines around the humeral stem
Figure 3b.
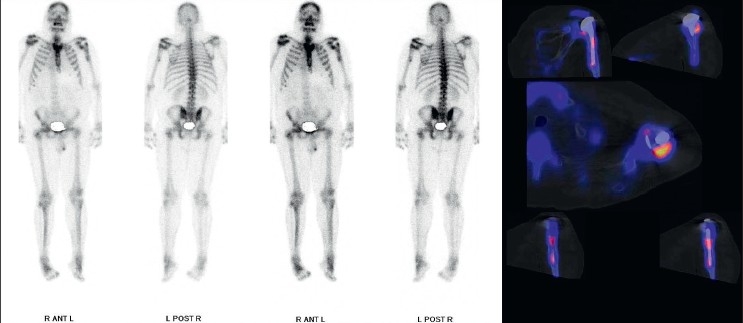
Planar scintigraphy and 99mTc-HDP-SPECT/CT showing increased tracer uptake around the humeral stem, which was caused by mechanical loosening
Case 4
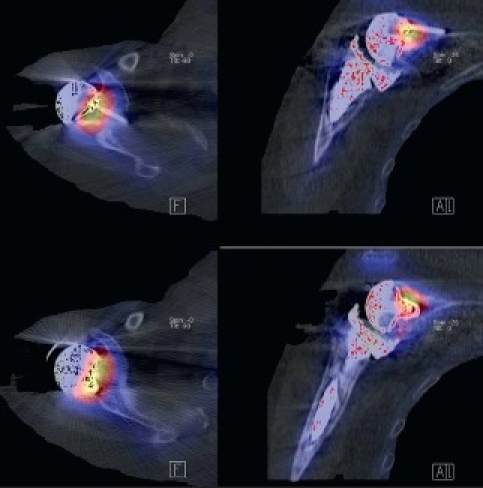
A 58-year-old patient presented with a painful right shoulder with reduced range of movement and a history of arthroscopy 3 months previously for a stiff painful glenohumeral joint. Given history of the arthroscopy and hence concern for infection, a two-phase bone scan supplemented with SPECT-CT (GE, Hawkeye-4) was performed. Accentuated blood pool activity was found on delayed planar scans with tracer uptake at the right humeral head. Although this finding supported osteonecrosis, the SPECT-CT [Figure 4] localized the activity more specifically at the glenohumeral joint interface. In addition, the CT showed radiographic erosive changes at both subarticular joint surfaces confirming a primary joint-based process [Figure 4]. A total shoulder replacement (TSR) was performed. No bacterial growth was confirmed in culture specimens. After a symptom-free period of 9 months, pain recurred. On examination, the pain was thought to be of subacromial origin and ultrasound-guided injections of the subacromial bursa brought partial relief. Continuing pain prompted the surgeon to consider prosthetic loosening. A CT scan in a modified position[20] showed no adverse features [Figure 4]. A SPECT/CT at 14 months after TSR showed no significant early blood pool phase activity mitigating against infection, whilst delayed planar images showed mild periprosthetic activity considered to be in the realm of osseous remodeling after TSR. More focal tracer activity was present at the acromion, raising this site as possible pain generators [Figure 4]. An arthroscopic subacromial decompression resulted in significant pain relief as assessed on clinical follow-up at one-month post-procedure.
Figure 4a.
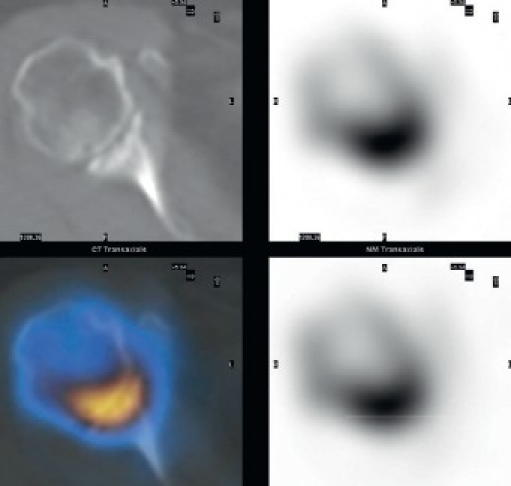
SPECT/CT showing erosive arthropathy with joint-based tracer uptake
Figure 4b.
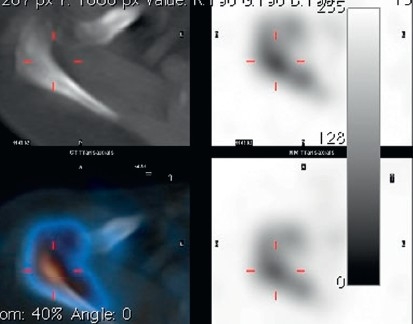
CT showing no significant periprosthetic lucency; planar scintigraphy indicated normal blood pool activity, delayed moderate periprosthetic tracer activity;SPECT/CT clearly found increased tracer activity of the acromion and acromioclavicular joint
DISCUSSION
The most important finding of the present case study was that SPECT/CT may facilitate the establishment of diagnosis, process of decision making, and further treatment for complex shoulder pathologies. Some of these advantages were highlighted in cases that are frequently seen in most shoulder clinics.
In case one, the anti-granulocyte SPECT/CT helped to noninvasively distinguish recurrence of infection from humeral head necrosis. 99m-Tc-labeled anti-granulocyte antibodies are directed against the concomitant infiltration of granulocytes in infected tissues and thereby these are helpful in diagnosing and localizing joint infections.[13,21] Recently, Graute et al. assessed the diagnostic sensitivity of scintigraphy, SPECT, and SPECT/CT. In relation to planar scintigraphy, SPECT/CT substantially improved the sensitivity and specificity for diagnosis and localization of suspected joint infections.[21] Although the authors only assessed infections of knee and hip arthroplasty, it could be speculated that similar results might be found in patients after shoulder arthroplasty.
The second case illustrates a common problem after shoulder arthroplasty, namely glenoid loosening. In patients after reversed shoulder prosthesis as well as after conventional arthroplasty, glenoid loosening represents one of the most common failure modes causing postoperative pain, limited function, and the need for revision surgery.[20] To date, detection and monitoring of glenoid fixation failure is considered to be difficult and shows a great inter- and intraobserver variability.[22] Radiolucent lines, particularly the width, are difficult to interpret in conventional radiographs. This might be due to inaccurate positioning of the patient and anatomical variation in glenoid version and mobility of the shoulder girdle.[22] Clearly, the radiolucent lines appeared to be smaller when retroversion was 10° or more and when anteversion was 15° or more.[22]
Nowadays, three-dimensional reconstructions of CT provide the surgeon with a better 3D understanding of patient's anatomy. In addition, the position and orientation of the prosthetic components after shoulder arthroplasty could be assessed in relation to standardized frames of reference. With SPECT/CT, it is not only possible to determine the width of radiolucencies and the localization of the metabolic tracer uptake, but also assess the orientation of the shoulder prosthesis, in particular the glenoid component. The orientation of the glenoid component has been reported to be a risk factor for early glenoid loosening.[23]
The potential benefit that SPECT/CT might provide for patients after shoulder arthroplasty is further highlighted by the third case where SPECT/CT identified osteolysis around the humeral stem and metabolic tracer uptake reflecting toggling of the component as cause of the patient's pain. SPECT-CT may be especially useful when MRI is contraindicated, equivocal, or prone to artefacts when metalwork is in situ, such as in patients after arthroplasty.
The fourth case highlights the potential clinical benefit that SPECT/CT might offer in patients with shoulder pain. As the shoulder girdle consists of several joints and different anatomical structures within, a small area pain can very often not be localized to distinct structures or joints. SPECT/CT might help to identify the pain generator, which was shown in the presented case.
Limitations
Using SPECT/CT in orthopedic patients, one should be aware of its limitations. As this was only a small case series, conclusions should be drawn with all due caution. To date, no sufficient information about the natural course of SPECT/CT after shoulder surgery has been established. The sensitivity and specificity of SPECT/CT for establishing the diagnosis in patients with shoulder problems has not been investigated to date as there is only a paucity of studies available in orthopedics.[2–4,17–19] The cost-effectiveness of SPECT/CT compared with other imaging has not been addressed so far.
CONCLUSIONS
SPECT/CT provides shoulder surgeons with a promising diagnostic tool, which may be particularly helpful in patients with shoulder pain, in which radiological diagnostic possibilities are currently limited, such as painful partial or total shoulder arthroplasty. The clinical value, the sensitivity and specificity of SPECT/CT in patients before and after shoulder surgery should be further investigated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bybel B, Brunken RC, DiFilippo FP, Neumann DR, Wu G, Cerqueira MD. SPECT/CT imaging: Clinical utility of an emerging technology. Radiographics. 2008;28:1097–113. doi: 10.1148/rg.284075203. [DOI] [PubMed] [Google Scholar]
- 2.Hirschmann MT, Iranpour F, Davda K, Rasch H, Huegli R, Friederich NF. Combined single-photon emission computerized tomography and conventional computerized tomography (SPECT/CT): Clinical for the knee surgeons? Knee Surg Sports Traumatol Arthrosc. 2010;18:341–5. doi: 10.1007/s00167-009-0879-9. [DOI] [PubMed] [Google Scholar]
- 3.Hirschmann MT, Iranpour F, Konala P, Kerner A, Rasch H, Cobb JP, et al. A novel standardized algorithm for evaluating patients with painful total knee arthroplasty using combined single photon emission tomography and conventional computerized tomography. Knee Surg Sports Traumatol Arthrosc. 2010;18:939–44. doi: 10.1007/s00167-010-1070-z. [DOI] [PubMed] [Google Scholar]
- 4.Mohan HK, Gnanasegaran G, Vijayanathan S, Fogelman I. SPECT/CT in imaging foot and ankle pathology- the demise of other coregistration techniques. Semin Nucl Med. 2010;40:41–51. doi: 10.1053/j.semnuclmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Scharf S. SPECT/CT imaging in general orthopaedic practice. Semin Nucl Med. 2009;39:293–307. doi: 10.1053/j.semnuclmed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad R, Kumar GS, Katam K, Dunlop D, Pozo JL. Significance of a “hot patella” in total knee replacement without primary patellar resurfacing. Knee. 2009;16:337–40. doi: 10.1016/j.knee.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Schillaci O. Hybrid SPECT/CT: A new era for SPECT imaging? Eur J Nucl Med Mol Imaging. 2005;32:521–4. doi: 10.1007/s00259-005-1760-9. [DOI] [PubMed] [Google Scholar]
- 8.Patton JA, Turkington TG. SPECT/CT physical principles and attenuation correction. J Nucl Med Technol. 2008;36:1–10. doi: 10.2967/jnmt.107.046839. [DOI] [PubMed] [Google Scholar]
- 9.Bailey E, HoShon I, Roach P. Additional benefit of SPECT/CT in bone scanning of metastatic renal cell carcinoma. Clin Nucl Med. 2007;32:411–4. doi: 10.1097/01.rlu.0000259641.90922.1f. [DOI] [PubMed] [Google Scholar]
- 10.Bockisch A, Freudenberg LS, Schmidt D, Kuwert T. Hybrid imaging by SPECT/CT and PET/CT: Proven outcomes in cancer imaging. Semin Nucl Med. 2009;39:276–89. doi: 10.1053/j.semnuclmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury FU, Scarsbrook AF. The role of hybrid SPECT/CT in oncology: Current and emerging clinical applications. Clin Radiol. 2008;63:241–51. doi: 10.1016/j.crad.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.D’Amico A, Szczucka K, Borys D, Gorczewski K, Steinhof K. SPECT/CT fusion: A new diagnostic tool for endocrinology. Endokrynol Pol. 2006;57:4. [PubMed] [Google Scholar]
- 13.Filippi L, Schillaci O. Usefulness of hybrid SPECT/CT in 99mTc-HMPAO-labeled leukocyte scintigraphy for bone and joint infections. J Nucl Med. 2006;47:1908–13. [PubMed] [Google Scholar]
- 14.Filippi L, Uccioli L, Giurato L, Schillaci O. Diabetic foot infection: Usefulness of SPECT/CT for 99m-Tc-HMPAO-labeled leukocyte imaging. J Nucl Med. 2009;50:1042–6. doi: 10.2967/jnumed.108.059493. [DOI] [PubMed] [Google Scholar]
- 15.Mahmarian JJ. Hybrid SPECT/CT: Integration of CT coronary artery calcium scoring and angiography with myocardial perfusion. Curr Cardiol Rep. 2007;9:129–35. doi: 10.1007/BF02938339. [DOI] [PubMed] [Google Scholar]
- 16.Van der Ploeg IM, Valdes Olmos RA, Kroon BB, Nieweg OE. The hybrid SPECT/CT as an additional lymphatic mapping tool in patients with breast cancer. World J Surg. 2008;32:1930–4. doi: 10.1007/s00268-008-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knupp M, Pagenstert GI, Barg A, Bolliger L, Easley ME, Hintermann B. SPECT/CT compared with conventional imaging modalities for the assessment of the carus and valgus malaligned hindfoot. J Orthop Res. 2009;27:1461–6. doi: 10.1002/jor.20922. [DOI] [PubMed] [Google Scholar]
- 18.Hirschmann MT, Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF. Clinical value of SPECT/CT for evaluation of patients with painful knees after total knee arthroplasty - a new dimension of diagnostics? BMC Musculoskelet Disord. 2011;12:36. doi: 10.1186/1471-2474-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papathanassiou D, Bruna-Muraille C, Jouannaud C, Gagneux-Lemoussu K, Eschard JP, Liehn JC. Single-photon emission computed tomography combined with computed tomography (SPECT/CT) in bone diseases. Joint Bone Spine. 2009;76:474–80. doi: 10.1016/j.jbspin.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Gregory T, Hansen U, Taillieu F, Baring T, Brassart N, Mutchler C, et al. Glenoid loosening after total shoulder arthroplasty: An in vitro CT-scan study. J Orthop Res. 2009;27:1589–95. doi: 10.1002/jor.20912. [DOI] [PubMed] [Google Scholar]
- 21.Graute V, Feist M, Lehner S, Haug A, Müller PE, Bartenstein P, et al. Detection of low-grade prosthetic joint infections using (99m)Tc-antigranulocyte SPECT/CT: Initial clinical results. Eur J Nucl Med Mol Imaging. 2010;37:1751–9. doi: 10.1007/s00259-010-1431-3. [DOI] [PubMed] [Google Scholar]
- 22.Havig MT, Kumar A, Carpenter W, Seiler JG., 3rd Assessment of radiolucent lines about the glenoid. An in vitro radiographic study. J Bone Joint Surg Am. 1997;79:428–32. [PubMed] [Google Scholar]
- 23.Farron A, Terrier A, Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15:521–6. doi: 10.1016/j.jse.2005.10.003. [DOI] [PubMed] [Google Scholar]


