Abstract
Background:
There has been longstanding controversy over the use of magnesium sulfate infusion in the medical management of aneurysmal subarachnoid hemorrhage (SAH). Several clinical trials evaluating the beneficial effects of magnesium on cerebral vasospasm and their poor outcome have been published. However, results from the majority of these studies have been inconclusive. This meta-analysis was performed to evaluate the effectiveness of magnesium on patient outcomes after aneurysmal SAH.
Materials and Methods:
PubMed and the Cochrane library were searched for controlled clinical trials assessing the efficacy of magnesium sulfate infusion after aneurysmal SAH. Eight studies consisting of 936 patients were included.
Results:
There was a decreased risk of poor outcome at 3–6 months after SAH in magnesium treatment groups when compared to placebo [0.78 (95% CI 0.66–0.93)]. Poor outcome was defined as severe disability, persistent vegetative state, or death, as measured by the Glasgow outcome scale (GOS), extended Glasgow outcome scale (GOSE) or modified Rankin scale (mRS). The risk of mortality after SAH was unaffected by magnesium treatment [RR 0.68 (95% CI 0.58–1.27)].
Conclusion:
Magnesium sulfate infusion decreases risk of poor outcome after aneurysmal SAH. Current studies in the literature do not suggest a role for magnesium sulfate in mortality reduction after SAH.
Keywords: Cerebral vasospasm, delayed cerebral ischemia, magnesium sulfate, subarachnoid hemorrhage
Introduction
Although spontaneous subarachnoid hemorrhage (SAH) accounts for a relatively small percentage of all strokes, it affects up to 30,000 Americans each year and carries a high mortality rate, with recently reported rates at 33–45%.[1–8] Patients surviving the initial hemorrhage are at high risk for developing cerebral vasospasm, a leading cause of morbidity and mortality in the post-hemorrhage period. Despite the advances made in early and effective surgical and endovascular aneurysm treatments, the incidences of both clinical and radiographic vasospasm still remain high. In this article, we review concepts that underlie cerebral vasospasm and the potential role of magnesium sulfate in treating this condition.
Cerebral vasospasm: Background and pathophysiology
Cerebral vasospasm is the luminal narrowing of large cerebral arteries after SAH, which leads to decreased cerebral blood flow (CBF) and ischemia in the affected vascular territories. Vessel narrowing typically begins 3–5 days after initial hemorrhage, with peak vasoconstriction occurring between days 5 and 14.[9,10] Vasospasm occurs due to several cascades of complex events in the affected blood vessels and neurons, which can be divided into three main categories: elevated levels of intracellular calcium (Ca2+), increased production of vasoactive substances, and structural injury to arterial vessel walls.[11]
Elevated intracellular Ca2+
After SAH, there is an increase of Ca2+ influx into smooth muscle and neuronal cells through voltage-gated Ca2+ channels and NMDA receptors. In addition, glutamate is increased to six times its baseline levels, causing hyperactivation of NMDA receptors and further Ca2+ influx, resulting in a high intracellular Ca2+ state. In smooth muscle cells, this causes increased binding of Ca2+ to calmodulin. Calmodulin activates myosin light chain kinase (MLCK) to phosphorylate myosin, which promotes myosin-actin interaction. This induces smooth muscle contraction and vessel wall constriction. In neuronal cells, high intracellular Ca2+ causes hyperactivation of enzymes such as proteases, endonucleases and phospholipases, which destabilizes the cell body and membrane, resulting in neuronal injury and death.[11]
Vasoactive compounds and vessel wall injury
During days 3–5 after SAH, the red blood cells (RBCs) in the subarachnoid space begin breaking down and releasing oxyhemoglobin. Oxyhemoglobin inhibits nitric oxide (NO), a physiologic vasodilator, and stimulates leukocytes to produce endothelin-1 (ET-1), a potent physiologic vasoconstrictor. The net effect is vessel vasoconstriction. In addition, as oxyhemoglobin is degraded, the reactive oxygen species and free iron that are released cause oxidative damage to blood vessel walls.[11] Furthermore, vasoactive compounds such as angiotensin II, serotonin, and norepinephrine are produced in varying amounts after SAH, further contributing to vessel constriction.[12]
Vasospasm prophylaxis and SAH management: Role of magnesium sulfate
Current therapies for prevention and treatment of cerebral vasospasm include Nimodipine, the only Class I, Level A supported therapy,[13] Simvastatin, induced hypertension, euvolemia, intra-arterial vasodilation, magnesium sulfate infusion, and various therapies currently being investigated in clinical trials. These include Clozosentan (an endothelin antagonist), Tirilizad (a free radical scavenger), albumin, and lumbar drainage.[14]
Magnesium sulfate is a physiologic Ca2+ antagonist widely used amongst many medical specialties. It is perhaps most well known for its use in obstetrics and was first implemented in the mid-1950s to treat eclampsia in pregnancy.[15] Since then, magnesium has also been used for the treatment of cardiac arrhythmias, hypertension, status asthmaticus, preterm labor, seizures, dyspepsia, and constipation.[16] Magnesium is an appealing therapy for cerebral vasospasm because it targets the vasospasm cascade at several different points as illustrated in [Figure 1].[11,12] It antagonizes voltage-gated Ca2+ channels and NMDA receptors, as well as prevents Ca2+ from binding calmodulin. This decreases smooth muscle contraction, blood vessel constriction, and neurotoxicity. Magnesium also inhibits leukocyte production of ET-1, decreases free radical formation, and attenuates the effect of several vasoconstrictive substances. Additional protective mechanisms of magnesium include decreasing production of inflammatory mediators, inhibiting platelet aggregation, increasing RBC deformability, and improving perfusion to the brain through increasing cardiac contractility.[11] In the mid-1990s, magnesium was shown to dilate spastic vessels in animal models of traumatic SAH.[17] Since then, it has gained much popularity for potentially producing the same effect in humans, and several clinical trials evaluating this effect have followed.
Figure 1.
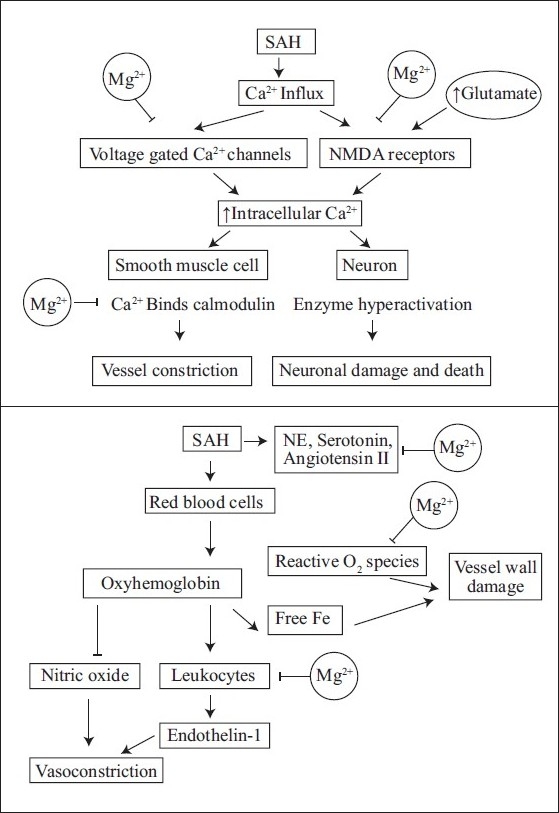
Effect of magnesium sulfate on the vasospasm cascade
Over the past decade, at least 11 clinical trials have` been performed assessing the efficacy of magnesium sulfate in SAH therapy.[12,18–27] Interestingly, although the majority of the trials have demonstrated a trend toward decreased incidence of vasospasm, delayed cerebral ischemia (DCI), and poor functional outcome with magnesium treatment,[12,18–27] only two studies have demonstrated a statistically significant difference in risk reduction of these outcome measures.[12,26] We attributed this largely to the limitations of small study population sizes and conducted a meta-analysis of these trials to further investigate magnesium's potential benefits.
Materials and Methods
Search strategy and criteria for study selection
PubMed and Cochrane library databases were searched from January 2000 to May 2010 using the subsequent terms: “magnesium sulfate” and “subarachnoid hemorrhage” or “vasospasm”. Only clinical trials were selected, and after review of each full text article, we found the definition of cerebral vasospasm to be highly varied across the trials. Some studies defined cerebral vasospasm as “clinical vasospasm”,[27] others defined it as “radiographic vasospasm”,[21,24–26] and some required the presence of both clinical and radiographic signs to meet the criteria for vasospasm.[12,22–23] Furthermore, measurement methods of radiographic vasospasm varied among the trials (i.e. by TCD, angiography, or CT scan).
Our inclusion criteria for selected studies were as follows: (1) randomized controlled trials comparing the efficacy of intravenous magnesium sulfate treatment versus placebo in patients with aneurysmal SAH and (2) studies reporting functional outcomes at 3 or 6 months as indicated by Glasgow Outcome Scale (GOS), extended Glasgow Outcome Scale (GOSE) or modified Rankin Scale (mRS). The primary outcome of this meta-analysis was poor outcome at 3–6 months after SAH, an outcome measure that was consistently defined.[12,18–27] Poor outcome was defined as severe disability, persistent vegetative state, or death, corresponding to GOS scores 1–3, GOSE scores 1–4, and mRS scores 5–6. An additional outcome evaluated was mortality rate.
Data extraction
The full text of each study was retrieved and reviewed, and only those meeting the study selection criteria were included in the analysis. Data were extracted by two investigators (Tsinsue Chen and Bob Carter) and the following information was obtained from each study: study design, number of patients, magnesium administration protocol, and incidence of primary and secondary outcomes.
Statistical analysis
The data were processed and meta-analysis was performed with fixed effects and random effects models using Stata/IC 11.0 Data Analysis and Statistical software by StataCorp. The difference in risk outcome was represented by the relative risk (RR) with 95% confidence intervals (95% CI) of outcome occurrence in treatment versus placebo group in each study. Statistical significance was set at P <0.05.
Results
Searches of PubMed, Cochrane Library, and bibliographies of pertinent manuscripts identified 11 clinical trials relevant to the topic of interest.[12,18–27] Eight of these met the study selection criteria and were included in the analysis. There were a total of 936 patients; 477 were treated with magnesium sulfate and 459 received placebo.[12,21–27] Characteristics of these eight trials are listed in [Table 1].
Table 1.
Trial characteristics and outcome results
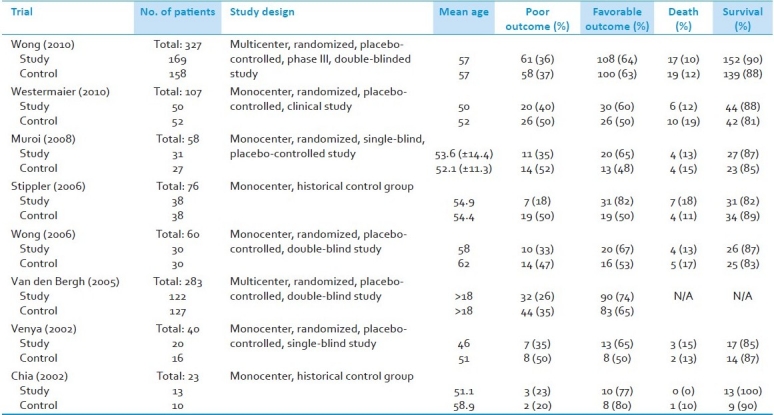
All treatment groups were administered intravenous magnesium sulfate within 5 days of initial SAH. A summary of infusion protocols is included in [Table 2]. Infusion dosing ranged between 12 and 48 g/day; initiation of treatment ranged from immediately after confirmation of SAH to 5 days post-hemorrhage; total length of treatment varied between 12 and 18 days post-hemorrhage. Five of the eight studies administered a one-time magnesium bolus prior to continuous infusion[22,24–27] and the other three studies began infusion without bolus.[12,21,23] Patients in all studies received standard intensive care unit (ICU) care and were simultaneously administered Nimodipine.[12,18–27]
Table 2.
Magnesium administration protocol and dose monitoring of included trials

All the eight studies provided data for the primary outcome, which was poor outcome at 3–6 months. Our analysis demonstrated that magnesium sulfate infusion decreases the risk of poor functional outcome at 3–6 months after SAH [RR 0.78 (95% CI 0.66–0.93)] [Figure 2]. There was a 20% RR reduction with treatment. Incidence of poor outcome was 32% in the treatment group and 40% in the control group. The secondary outcome of interest was mortality. All, but one, study provided mortality data; no results were available from the 2005 trial published by Van den Bergh et al. A total of 677 patients were included, and similar mortality rates were seen in both the groups (12% in the treatment group vs. 14% in the control group). Our analysis demonstrated that there was no effect of magnesium on mortality rate after SAH [RR 0.68 (95% CI 0.58–1.27)] [Figure 3].
Figure 2.
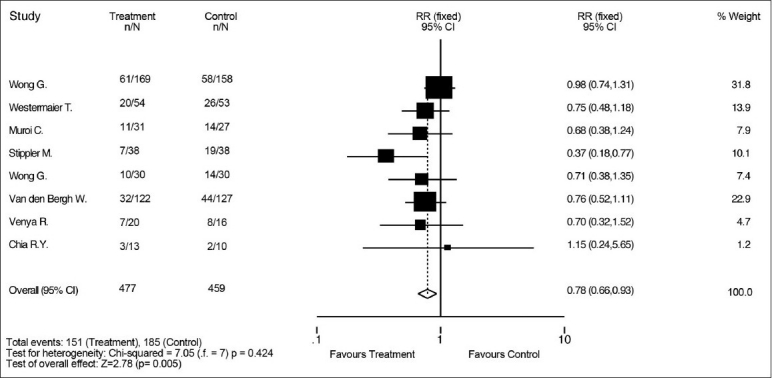
Effect of magnesium sulfate vs. control on risk of poor outcome 3-6 months after aneurismal subarachnoid hemorrhage. There is a decreased risk of poor outcome with magnesium treatment. Poor outcome is defined as death, vegetative state, or sever disability, as measured by Glasgow outcome scale, extended Glasgow outcome scale, and modified Rankin scale score
Figure 3.
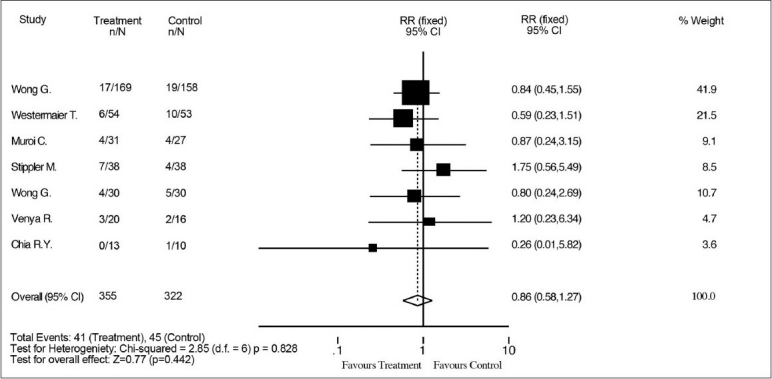
Effect of magnesium sulfate vs. control on risk of mortality after aneurismal SAH. Magnesium has no effect on risk of mortality after aneurismal SAH
Discussion
Magnesium's role in SAH management has been investigated in several clinical and experimental studies in the past decade. Our meta-analysis of eight selected trials (936 patients) shows that magnesium treatment confers a decreased risk of poor clinical outcome when compared to control and is a worthy therapy in SAH management. It is still uncertain, however, whether magnesium has a direct beneficial effect on decreasing the risk of clinical or radiographic vasospasm in the immediate post-hemorrhage period. Our results are consistent with findings from the analysis done by Zhao et al. on five controlled trials conducted from 2002 to 2006[28] and the study by Ma et al., which included 513 patients from 2002 to 2008 in a combined analysis of poor outcome. Ma et al.'s pooled data from five clinical trials also demonstrated a borderline statistically significant decrease in risk for clinical signs of DCI.[29]
An important point to consider is that patients may process and metabolize magnesium at different rates based on variations in genetics, co-morbidities (i.e. renal failure), and pre-hemorrhage nutritional status. The same daily dose of magnesium may result in different serum levels in each patient. Thus, it may be best to dose magnesium by targeting therapeutic serum ranges rather than administering a constant infusion rate.
Limitations of combining these eight studies include variations in the magnesium dosing protocols as well as the timing of measurement outcomes. Future studies should aim for a phase III trial design with population sizes large enough to evaluate the outcomes of interest at a statistically significant level. Though pooling trials through meta-analysis provides useful information, a larger trial with clearly defined study parameters would provide more powerful evidence assessing the efficacy of magnesium use. Rather than administering a universal dose of magnesium to all treatment groups, different groups should be formed based on target levels of magnesium. For example, one treatment group should have daily levels to target 3–4 mg/dL, another to target 4–5 mg/dL, and one to target 5–6 mg/dL. Categorizing results based on serum ranges allows for determination of target serum levels that correspond with the best patient outcomes. Outcome measures should include clinical vasospasm, radiographic vasospasm, poor outcome, and good outcome at both 3 and 6 months.
Magnesium sulfate in subarachnoid hemorrhage (MASH II) is a Netherlands-based, phase III, international multicenter trial currently underway. Its primary outcome measure is poor outcome at 3 months after SAH as measured by mRS 4–5. Secondary outcomes include no symptoms at 3 months and Rankin scores for all patients. Sample size is 1200 patients, as was determined to be necessary to reach statistical significance based on the previous MASH I study by the same investigators. Magnesium infusion protocol will remain unchanged from MASH I [treatment to be administered within 4 days of confirmed SAH, without bolus, at a rate of 64 mmol/day (16 g/day)]. Investigators report this dose to be safe and effective in maintaining serum levels of 1.0–2.0 mmol/day. As of 2008, over 430 patients had been enrolled from six international centers, and investigators anticipate results to be available this year.[30] Magnesium clearly plays a beneficial role in SAH management; however, larger trials are still needed to evaluate the extent of magnesium's positive effects. Results from MASH II will help clarify these effects and provide further evidence regarding the use of magnesium sulfate after SAH.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: Results from an international collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Huang CY, Chan FL, Yu YL, Woo E, Chin D. Cerebrovascular disease in Hong Kong Chinese. Stroke. 1990;21(2):230–235. doi: 10.1161/01.str.21.2.230. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Selvin S, Gress DR. The burden, trends and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–8. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Connoly ES, Batjer HH, Dacy RG, Dion JE, Diringer MN, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Graf CJ, Nibbelink DW. Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage: Report on a randomized treatment study. Stroke. 1974;5(4):557–601. doi: 10.1161/01.str.5.4.557. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 7.Cross DT, 3rd, Tirschwell DL, Clark MA, Tuden D, Derdeyn CP, Moran CJ, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. J Neurosurg. 2003;99:810–817. doi: 10.3171/jns.2003.99.5.0810. [DOI] [PubMed] [Google Scholar]
- 8.Keyrouz SG, Diringer MN. Clinical review: Prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11(4):220–230. doi: 10.1186/cc5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage: an update. Ann Neurol. 1983;14:599–608. doi: 10.1002/ana.410140602. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CM, Roberson GH, Ojemann RG. Cerebral vasospasm with ruptured saccular aneurysm: the clinical manifestations. Neurosurgery. 1977;1(3):245–248. doi: 10.1227/00006123-197711000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bergh WM, Dijkulzen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnes Res. 2004;17(4):301–13. [PubMed] [Google Scholar]
- 12.Stippler M, Crago E, Levy E, Kerr M, Yonas H, Horowitz M, et al. Magnesium infusion for vasospasm prophylaxis after subarachnoid haemorrhage. J Neurosurg. 2006;105:723–729. doi: 10.3171/jns.2006.105.5.723. [DOI] [PubMed] [Google Scholar]
- 13.Dorhout MS, Rinkel GJ, Feigin FL, Algra A, van den Berg WM, Vermeulen M, et al. Calcium anatagonists for aneurismal subarachnoid haemorrhage (Review) Cochrane Database Syst Rev. 2007;18(3):CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabenstein AA, Wijdicks EF, Lanzino G. Multidisciplinary management and emerging therapeutic strategies in aneurismal subarachnoid haemorrhage. Lancet Neurol. 2010;9(5):504–519. doi: 10.1016/S1474-4422(10)70087-9. [DOI] [PubMed] [Google Scholar]
- 15.Pritchard JA. The use of the magnesium ion in the management of eclamptogenic toxemias. Surg Gynecol Obstet. 1955;100(2):131–40. [PubMed] [Google Scholar]
- 16.Clinical Pharmacology. Magnesium sulfate: Indications. [Last accessed on 2010 Aug 9]. Available from: http://www.clinicalpharmacologyip.com/Forms/Monograph/monograph.aspx?cpnum=1415&sec=monoindi.
- 17.Ram Z, Sadeh M, Shacked I, Sahar A, Hadani M. Magnesium sulfate reverses experimental delayed cerebral vasospasm after SAH in rats. Stroke. 1991;22(7):922–927. doi: 10.1161/01.str.22.7.922. [DOI] [PubMed] [Google Scholar]
- 18.Yahia AM, Kirmani JF, Qureshi AI, Guterman LR, Hopkins LN. The Safety and Feasibility of Continuous Intravenous Magnesium Sulfate for Prevention of Cerebral Vasospasm in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. 2005;3:1616–23. doi: 10.1385/NCC:3:1:016. [DOI] [PubMed] [Google Scholar]
- 19.Prevedello DM, Cordeiro JG, Leite de Morais A, Saucedo NS, Chen IB, Araujo JC. Magnesium sulfate: Role as possible attenuating factor in vasospasm morbidity. Surg Neurol. 2006;66(1):110–117. doi: 10.1016/j.surneu.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Steiger H. Intravenous Magnesium versus Nimodipine in the Treatment of Patients with Aneurysmal Subarachnoid Hemorrhage: A Randomized Study. Neurosurgery. 2006;58(6):1054–1065. doi: 10.1227/01.NEU.0000215868.40441.D9. [DOI] [PubMed] [Google Scholar]
- 21.Chia RY, Hughes RS, Morgan MK. Magnesium: A useful adjunct in the prevention of cerebral vasospasm following aneurismal subarachnoid haemorrhage. J Clin Neurosci. 2002;9(3):279–281. doi: 10.1054/jocn.2001.1039. [DOI] [PubMed] [Google Scholar]
- 22.Venya RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, et al. Magnesium sulfate therapy after aneurismal subarachnoid hemorrhage. J Neurosurg. 2002;96:510–514. doi: 10.3171/jns.2002.96.3.0510. [DOI] [PubMed] [Google Scholar]
- 23.Van den Bergh WM. Magnesium Sulfate in Aneurysmal Subarachnoid Hemorrhage: A Randomized Controlled Trial. Stroke. 2005;36(5):1011–1015. doi: 10.1161/01.STR.0000160801.96998.57. [DOI] [PubMed] [Google Scholar]
- 24.Wong GK, Chan MT, Boet R, Poon WS, Gin T. Intravenous Magnesium Sulfate After Aneurysmal Subarachnoid Hemorrhage: A Prospective Randomized Pilot Study. J Neurosurg Anesthesiol. 2006;18:142–148. doi: 10.1097/00008506-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Muroi C, Terzic A, Fortunati M, Yonekawa Y, Keller E. Magnesium sulfate in the management of patients with aneruysaml subarachoid hemorrhage: A randomized, placebo-controlled, dose-adapted trial. Surg Neurol. 2008;69(1):33–39. doi: 10.1016/j.surneu.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Westermaier T, Stetter C, Vince G, Pham M, Tejon JP, Eriskate J, et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: A randomized, placebo-controlled, clinical study. Crit Care Med. 2010;38(5):1382–1384. doi: 10.1097/CCM.0b013e3181d9da1e. [DOI] [PubMed] [Google Scholar]
- 27.Wong GK, Poon W, Chan MT, Boet R, Gin T, Ng SC, et al. Intravenous Magnesium Sulphate for Aneurysmal Subarachnoid Hemorrhage (IMASH): A Randomized, Double-Blinded, Placebo-Controlled, Multicenter Phase III Trial. Stroke. 2010;41(5):921–926. doi: 10.1161/STROKEAHA.109.571125. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Zhou Y, Zhang X, Zhuang Z, Shi J. A meta analysis of treat subarachnoid hemorrhage with magnesium sulfate. J Clin Neurosci. 2009;16(11):1394–1397. doi: 10.1016/j.jocn.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Lui WG, Zhang JM, Chen G, Fan J, Shen HS. Magnesium sulphate in the management of patients with aneruysmal subarachnoid haemorrhage: A meta-analysis of prospective controlled trials. Brain Inj. 2010;24(5):730–735. doi: 10.3109/02699051003610516. [DOI] [PubMed] [Google Scholar]
- 30.Dorhout Mees SM. Magnesium in aneruysmal subarachnoid hemorrhage (MASHII) phase III clinical trial MASH-II study group. Int J Stroke. 2008;3(1):63–65. doi: 10.1111/j.1747-4949.2008.00168.x. [DOI] [PubMed] [Google Scholar]


