Abstract
Aim
Exposure to road traffic and air pollution may be a trigger of acute myocardial infarction, but the individual pollutants responsible for this effect have not been established. We assess the role of combustion-derived-nanoparticles in mediating the adverse cardiovascular effects of air pollution.
Methods and results
To determine the in vivo effects of inhalation of diesel exhaust components, 16 healthy volunteers were exposed to (i) dilute diesel exhaust, (ii) pure carbon nanoparticulate, (iii) filtered diesel exhaust, or (iv) filtered air, in a randomized double blind cross-over study. Following each exposure, forearm blood flow was measured during intra-brachial bradykinin, acetylcholine, sodium nitroprusside, and verapamil infusions. Compared with filtered air, inhalation of diesel exhaust increased systolic blood pressure (145 ± 4 vs. 133 ± 3 mmHg, P< 0.05) and attenuated vasodilatation to bradykinin (P= 0.005), acetylcholine (P= 0.008), and sodium nitroprusside (P< 0.001). Exposure to pure carbon nanoparticulate or filtered exhaust had no effect on endothelium-dependent or -independent vasodilatation. To determine the direct vascular effects of nanoparticulate, isolated rat aortic rings (n= 6–9 per group) were assessed in vitro by wire myography and exposed to diesel exhaust particulate, pure carbon nanoparticulate and vehicle. Compared with vehicle, diesel exhaust particulate (but not pure carbon nanoparticulate) attenuated both acetylcholine (P< 0.001) and sodium-nitroprusside (P= 0.019)-induced vasorelaxation. These effects were partially attributable to both soluble and insoluble components of the particulate.
Conclusion
Combustion-derived nanoparticulate appears to predominately mediate the adverse vascular effects of diesel exhaust inhalation. This provides a rationale for testing environmental health interventions targeted at reducing traffic-derived particulate emissions.
Keywords: Air pollution, Diesel exhaust, Nanoparticles, Endothelium, Blood flow
See page 2613 for the editorial comment on this article (doi:10.1093/eurheartj/ehr200)
Introduction
Air pollution is increasingly recognized as an important and modifiable risk factor for cardiovascular disease.1 Acute exposure has been linked to a range of adverse cardiovascular events, including hospital admissions with angina,2 myocardial infarction,3 and heart failure,4 and long-term exposure increases the lifetime risk of death from coronary heart disease.5 Long-term residential exposure to air pollution is also associated with the extent of atherosclerosis in the carotid and coronary blood vessels.6–12 These associations are strongest for fine particulate matter (PM2.5) air pollution that arises from a variety of sources, including the combustion of diesel fuel by automobiles. This important source of PM2.5 is thought to explain the association between transient exposure to road traffic and the triggering of acute myocardial infarction.13
We have previously demonstrated that inhalation of dilute diesel exhaust impairs vascular function,14 and has pro-thrombotic effects in both healthy volunteers15 and patients with coronary heart disease.16 Diesel exhaust is a complex mixture of gases, particles, and volatiles, and our earlier studies preclude identification of the components responsible for these adverse effects. The recent American Heart Association statement highlights the need for a greater understanding of the role of these different components in mediating the cardiovascular effects of air pollution.1 Ultrafine particles, nitrogen dioxide, carbon monoxide, sulphur dioxide, and organics chemicals may all play a role. Transition metals and organic constituents, such as polyaromatic hydrocarbons, on the surface of particles are believed to be key mediators of the harmful actions of diesel exhaust.17 Understanding the role of individual pollutants is an important consideration in the science of emission control technology and is essential to guide public health and environmental policy.
Using complementary clinical and pre-clinical studies, our aim was to establish the role of combustion-derived particles in determining the adverse vascular effects of diesel exhaust inhalation. Using a specially designed human exposure chamber and particle filtration system, we compared the effects of dilute diesel exhaust with the gaseous components alone, and with ‘clean’ pure carbon nanoparticles. The direct vascular effects of diesel exhaust particles and carbon nanoparticles were compared in isolated rat aortic rings using myography.
Methods
Clinical studies
Subjects
Sixteen healthy male non-smokers aged between 18 and 32 years participated in these studies that were performed with the approval of the local research Ethics Committee, in accordance with the Declaration of Helsinki, and the written informed consent of all volunteers. Subjects taking regular medication and those with clinical evidence of atherosclerotic vascular disease, arrhythmia, diabetes mellitus, hypertension, renal or hepatic failure, asthma, significant occupational exposure to air pollution, or an inter-current illness likely to be associated with inflammation were excluded from the study. Subjects had normal lung function and reported no symptoms of respiratory tract infection for at least 6 weeks prior to or during the study. Routine measures of clinical haematology and biochemistry, including blood glucose, were normal.
Study design
Subjects attended on four separate occasions at least 2 weeks apart and receive a double-blind randomized cross-over exposure to filtered air, carbon nanoparticles, diesel exhaust and filtered diesel exhaust from which the particulate phase was removed. All subjects were subjected to all four exposures with at least 2-week washout between exposures. The order of the exposures was selected using a random allocation taken from a balanced block of 24 to account for all permutations. On each occasion, each subject was exposed for 2 h in a specially built exposure chamber. During each exposure, they performed moderate exercise (minute ventilation 25 L/min/m2) on a bicycle ergometer that was alternated with rest at 15-min intervals.
Based on previous exposure studies,14 vascular assessments were performed 6–8 h following each exposure. All subjects abstained from alcohol for 24 h and from food, tobacco, and caffeine-containing drinks for at least 4 h before each vascular study. Studies were carried out in a quiet, temperature-controlled room maintained at 22–24°C, with subjects lying supine. All subjects remained indoors between the exposure and vascular assessment to minimize additional exposure to particulate air pollution.
Exposures
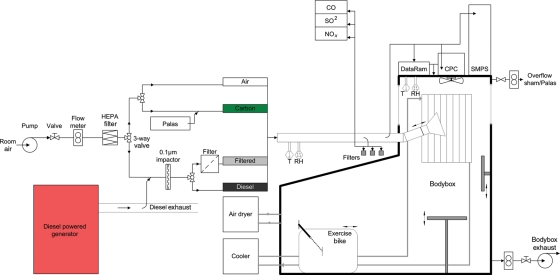
All exposures were delivered in a purpose-built exposure chamber (Figure 1). The air in the exposure chamber inlet was continuously monitored for nitrogen oxides [chemiluminescence NO-NO2-NOx analyser, model 40W, Thermo Environmental Instruments (TEI), USA], carbon monoxide (gas filter correlation CO analyser, model 48, TEI, USA), sulphur dioxide (pulsed fluorescence SO2 analyser, TEI, USA), and ozone (photometric analyser, monitor labs 9810O3, Measurement Controls Corporation, USA). Particle number was determined using a condensation particle counter (Model 3022A, Thermo Systems Incorporated, USA). Particle mass was continuously monitored using a DataRam nephelometer (Measuring Instruments for the Environment corporation, USA) to standardize diesel exposures, with the precise mass determined by gravimetric filter measurements (teflon 2.0 μm 4.7 mm, PALL Life Sciences, USA). Temperature and humidity in the chamber were measured with a thermometer and hygrometer (ETHG889, Oregon Scientific, Portland OR, USA).
Figure 1.
Exposure chamber and particle filtration system for clinical studies. Diesel exhaust was generated from an unloaded diesel engine using gas oil. More than 90% of the exhaust was shunted away, and the remaining part was diluted with air and fed at 75 L/min into the exposure chamber at steady-state concentration. Diesel exhaust particulate was removed for the control filtered exhaust exposure by passing dilute exhaust through a teflon filter. Carbon nanoparticles were generated using a Palas GFG 1000 spark discharge generator. NP, nanoparticles; T, temperature; RH, relative humidity; CO, carbon monoxide; SO2, sulphur dioxide; NOx, nitrogen oxides; CPC, condensation particle counter; SMPS, scanning mobility particle sizer.
Diesel exhaust was generated from an unloaded diesel engine (Deutz, 4 cylinder, 2.2 L, 500 rpm) using gas oil (Petroplus Refining Teesside Ltd, UK). More than 90% of the exhaust was shunted away, and the remaining part was diluted with air, passed through an impactor with a cutoff of 0.1 µm, and fed at 75 L/min into the exposure chamber at steady-state concentration of ∼300 µg/m3. Filtered exhaust was generated in an identical manner, but the exhaust was passed through a highly efficient TE38 Teflon filter (Schleicher & Schuell, Dassel, Germany) to remove particles before being fed into exposure chamber.
An aerosol of carbon nanoparticles was generated from graphite electrodes by an electric spark discharge generator (Palas CFG1000, Palas GmbH, Karlsruhe, Germany) in an atmosphere of argon. The output of the generator was mixed with filtered air, passed through an impactor with a cutoff of 0.1 µm, and fed at 75 L/min into the exposure chamber at steady-state concentration. The number concentration in the exposure chamber was maintained at 4000 × 103 particles/cm3 as this was associated with the maximum achievable mass concentration.
Vascular studies
All subjects underwent brachial artery cannulation with a 27-standard wire gauge steel needle under controlled conditions. Following a 30-min baseline saline infusion, acetylcholine at 5, 10, and 20 µg/min [endothelium-dependent vasodilator that does not release tissue plasminogen activator (t-PA); Merck Biosciences, Switzerland], bradykinin at 100, 300, and 1000 pmol/min (endothelium-dependent vasodilator that releases t-PA; Merck Biosciences, Switzerland), and sodium nitroprusside at 2, 4, and 8 µg/min (endothelium-independent vasodilator that does not release t-PA; David Bull Laboratories, UK) were infused for 6 min at each dose. The three vasodilators were separated by 20-min saline infusions and given in a randomized order. Verapamil at 10, 30, and 100 µg/min (endothelium and nitric oxide-independent vasodilator that does not release t-PA) was infused at the end of the study protocol.
Forearm blood flow was measured in the infused and non-infused arms by venous occlusion plethysmography using mercury-in-silastic strain gauges as described previously.20 Supine heart rate and blood pressure in the non-infused arm were monitored at intervals throughout each study using a semi-automated non-invasive oscillometric sphygmomanometer.
Venous cannulae (17-gauge) were inserted into large subcutaneous veins of the ante-cubital fossae of both arms. Blood (10 mL) was withdrawn simultaneously from each arm at baseline and during the infusion of each dose of bradykinin, and collected into acidified buffered citrate (Stabilyte tubes, Biopool International) for t-PA assays, and citrate (BD Vacutainer) for plasminogen activator inhibitor type 1 (PAI-1) assays. Samples were kept on ice before being centrifuged at 2000 g for 30 min at 4°C. Platelet-free plasma was decanted and stored at −80°C before assay. Plasma t-PA and PAI-1 antigen and activity concentrations were determined by enzyme-linked immunosorbant assays (t-PA Combi Actibind Elisa Kit, Technoclone, Vienna, Austria and Elitest PAI-1 antigen and Zymutest PAI-1 Activity, Hyphen Biomed, Neuville-Sur-Oise, France). Haematocrit was determined by capillary tube centrifugation at baseline and during infusion of bradykinin 1000 pmol/min.
Blood samples were taken immediately before, 2, 6, and 24 h after the exposure and analysed for total cells, differential count, and platelets using an autoanalyzer.
Pre-clinical studies
Preparation of particle suspensions
Suspensions of diesel exhaust particles and vascular tissues were performed as described previously.18 Briefly, diesel exhaust particles (SRM-2975; National Institute of Standards and Technology, Gaithersburg, USA) and carbon nanoparticles (Printex 90; Degussa, Frankfurt, Germany) were suspended in Krebs buffer at a stock concentration of 2 mg/mL, followed by vortexing and sonication.
The components of diesel exhaust particles were separated by serial washes in Krebs buffer (to remove constituents soluble in aqueous solutions) or dichloromethane (to remove organic constituents). For aqueous extraction, a suspension of diesel exhaust particles was prepared at a concentration of 2 mg/mL in Krebs buffer and agitated overnight at room temperature. The suspension was then centrifuged at 15 800 g for 10 min and the aqueous supernatant separated from the particle pellet. The pellet was resuspended in an identical volume of Krebs buffer and both solutions were recentrifuged and separated twice more to remove contaminants. The aqueous extract of diesel exhaust particles was passed through a syringe filter (0.22 μm; Millipore, Fisher Scientific, Loughborough, UK). Krebs buffer was added to the particle pellet to provide at a concentration of 2 mg/mL and resonicated for 15 min to ensure complete resuspension and disaggregation of the aqueous-washed diesel exhaust particles.
The aromatic organic constituents on the surface of diesel exhaust particles were separated through modification of the method used by Koike and Kobayashi.19 Briefly, particles were suspended in dichloromethane at 10 mg/mL in Krebs buffer and agitated for >24 h at room temperature. Suspensions were processed as for the aqueous separation; following three centrifugations, dichloromethane was completely evaporated (37°C for 4 h) from the particulate pellet and extract. The particulate pellet was resuspended at 2 mg/mL in Krebs buffer after 15 min of sonication (organic-washed diesel exhaust particles), and the residue from the evaporated extract was resuspended in dimethlysulphide at a concentration equivalent to that present in the original 10 mg/mL stock (organic extract from diesel exhaust particles). The final concentration of dimethyl sulphoxide in the biological assays was 1% and preliminary experiments showed that this concentration did affect vascular responses.
Myography
All experiments were performed according to the Animals (Scientific Procedures) Act 1986 (UK Home Office). Rings of thoracic aorta from adult male Wistar rats (n= 48 in total; 6–9 per experimental group) were mounted in a multi-myograph system (610M; Danish Myo Technology, Aarhus, Denmark) under a baseline tension of 14.7 mN. Vessels were pre-treated with diesel exhaust particles (100 μg/mL) or the aqueous and organic extracts from diesel exhaust particles, and carbon nanoparticles (100 μg/mL) 20 min prior to, and throughout, generation of concentration–response curves. Concentration–response curves to phenylephrine (1 nM–10 μM) were obtained, and a concentration that produced 80% maximum contraction (EC80; 0.1–1 μM) was chosen for each individual rat aortic ring. Following contraction, cumulative concentration–response curves were obtained for the endothelium-dependent vasodilator acetylcholine (1 nM–10 μM) and the endothelium-independent nitric oxide donor sodium nitroprusside (0.1 nM–1 μM). In some experiments rings were incubated with the superoxide scavenging enzyme—superoxide dismutase (SOD; 100 U/mL)—or the hydroxyl radical scavenger—mannitol (5 mM)—alone or via co-incubated with particles (10 or 100 μg/mL) 20 min prior to concentration–response curves.
Data analysis and statistics
Based on our previous studies of endothelial vasomotor function and endogenous fibrinolysis, to detect a 20% difference in forearm blood flow and a 16% difference in t-PA release, we require sample sizes of n = 16 at 80% power and two-sided P < 0.05.
Plethysmographic data were analysed as described previously.20 Estimated net release of t-PA antigen and activity was defined as the product of the infused forearm plasma flow (based on the mean haematocrit and the infused forearm blood flow) and the concentration difference between the infused and non-infused arms. Data from the myography studies were analysed as described previously,18 using two-way analysis of variance (ANOVA) to compare concentration–response curves and unpaired t-test to compare differences in maximum contraction. Vasodilator responses were expressed as a percentage of the pre-contraction to EC80 phenylephrine, where 100% relaxation represents a complete abolition of phenylephrine-induced tone. Continuous variables are reported as mean ± SEM. Statistical analyses were performed with GraphPad Prism (Graphpad Software, Inc., La Jolla, CA, USA) using ANOVA with repeated measures with Bonferroni post hoc tests of selected comparisons where appropriate. Statistical significance was taken at two-sided P < 0.05.
Results
Clinical studies
All studies were well tolerated with no adverse effects. Total leucocytes and neutrophils were increased 2 and 6 h following exposures (P< 0.001), but this was unaffected by the type of exposure (Table 1). Plasma concentrations of t-PA antigen and activity as well as PAI-1 antigen and activity also displayed circadian variation, but were not affected by exposure to diesel exhaust or pure carbon nanoparticles.
Table 1.
Systemic effects of exposure to filtered air, diesel exhaust, and carbon nanoparticles
| Filtered air |
Diesel exhaust |
Filtered exhaust |
Carbon |
ANOVA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, h | 0 | 2 | 6 | 0 | 2 | 6 | 0 | 2 | 6 | 0 | 2 | 6 | Time | Exposure |
| Leucocytes, ×109 cells/L | 5.3 ± 1.2 | 6.2 ± 1.6 | 6.4 ± 1.2 | 5.3 ± 1.2 | 6.2 ± 1.6 | 6.4 ± 1.2 | 5.3 ± 1.2 | 6.2 ± 1.6 | 6.4 ± 1.2 | 5.3 ± 1.2 | 6.2 ± 1.6 | 6.4 ± 1.2 | P = 0.020 | P = 0.640 |
| Neutrophils, ×109 cells/L | 2.8 ± 0.8 | 4.1 ± 1.6 | 4.4 ± 1.2 | 2.6 ± 0.8 | 4.3 ± 1.6 | 4.3 ± 1.2 | 2.7 ± 0.8 | 4.5 ± 1.2 | 4.5 ± 1.2 | 3.3 ± 1.2 | 4.9 ± 2.4 | 4.9 ± 1.6 | P < 0.001 | P = 0.292 |
| Lymphocytes, ×109 cells/L | 1.8 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.9 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.9 ± 0.8 | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.8 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | P = 0.019 | P = 0.076 |
| Platelets, ×109 cells/L | 222 ± 32 | 226 ± 32 | 232 ± 32 | 226 ± 36 | 236 ± 40 | 232 ± 40 | 230 ± 28 | 230 ± 24 | 234 ± 28 | 229 ± 40 | 231 ± 36 | 234 ± 36 | P = 0.928 | P = 0.062 |
| t-PA antigen, ng/mL | 5.8 ± 2.0 | 7.0 ± 2.0 | 5.4 ± 1.6 | 6.1 ± 2.4 | 7.4 ± 2.8 | 5.7 ± 1.6 | 6.0 ± 2.4 | 7.2 ± 2.4 | 5.5 ± 1.6 | 6.5 ± 2.8 | 8.0 ± 3.2 | 5.7 ± 2.0 | P = 0.010 | P = 0.394 |
| t-PA activity, ng/mL | 0.5 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 | 0.5 ± 0.4 | 0.9 ± 0.4 | 1.0 ± 0.4 | 0.5 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.6 ± 0.4 | 1.1 ± 0.4 | 0.9 ± 0.4 | P < 0.001 | P = 0.083 |
| PAI-1 antigen, ng/mL | 27 ± 16 | 15 ± 8 | 13 ± 8 | 24 ± 16 | 18 ± 8 | 12 ± 4 | 25 ± 12 | 17 ± 12 | 12 ± 8 | 25 ± 12 | 18 ± 8 | 12 ± 4 | P < 0.001 | P = 0.782 |
| PAI-1 activity, ng/mL | 1.2 ± 0.8 | 0.5 ± 0.4 | 0.4 ± 0.4 | 1.1 ± 1.2 | 0.5 ± 0.4 | 0.4 ± 0.4 | 1.4 ± 1.2 | 0.6 ± 0.8 | 0.4 ± 0.4 | 1.1 ± 0.8 | 0.4 ± 0.4 | 0.4 ± 0.4 | P < 0.001 | P = 0.316 |
Values are reported as mean ± standard deviation (n= 16); ANOVA with repeated measures.
Exposures
Particulate mass and number concentrations in dilute diesel exhaust were similar to our previous exposures14,15 at 348 ± 16 µg/m3 and 1198 × 103 particles/cm3, respectively (Table 2). Filtration reduced the number of particles by 1000-fold (2 × 103 particles/cm3), resulting in a 60-fold lower mass concentrations of 6 ± 4 µg/m3. Gaseous pollutants were not affected by particle filtration and neither temperature nor humidity varied between exposures.
Table 2.
Characterization of exposure conditions
| Filtered air | Diesel exhaust | Filtered exhaust | Carbon | |
|---|---|---|---|---|
| PM (teflon filter), µg/m3 | <1 | 348 ± 64 | 6 ± 16 | 70 ± 28 |
| Particle number, ×1000/cm3 | <1 | 1198 ± 204 | 2 ± 4 | 3865 ± 424 |
| Particle diameter, nm | – | 67 ± 4 | – | 37 ± 4 |
| Carbon monoxide, p.p.m. | 0.2 ± 0.4 | 3.5 ± 0.8 | 3.2 ± 0.4 | 0.1 ± 0.0 |
| Sulphur dioxide, p.p.m. | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.4 | 0.1 ± 0.0 |
| Nitric oxide [NO], p.p.m. | <0.01 | 0.4 ± 0.1 | 0.4 ± 0.1 | <0.01 |
| Nitrogen dioxide [NO2], p.p.m. | <0.01 | 0.2 ± 0.0 | 0.2 ± 0.0 | <0.01 |
| NOx [NO + NO2], p.p.m. | <0.01 | 0.6 ± 0.1 | 0.6 ± 0.1 | <0.01 |
| Temperature, °C | 18.2 ± 0.8 | 18.7 ± 0.4 | 18.6 ± 0.4 | 18.7 ± 0.8 |
| Relative humidity, % | 72 ± 8 | 62 ± 8 | 62 ± 4 | 64 ± 8 |
Values are presented as number or mean ± standard deviation (n= 16).
Despite delivering four-fold more carbon nanoparticles (3865 × 103 vs. 1198 × 103 particles/cm3) using the Palas generator, the mass concentration of particulate was only 70 ± 7 µg/m3. The difference in mass concentration between carbon and diesel exhaust exposures was due to the smaller size of carbon nanoparticles (median diameter 37 ± 1 vs. 67 ± 1 nm, respectively). Greater than 85% of diesel exhaust particles and 95% of carbon particles, by particle number, had an aerodynamic diameter of <100 nm.
Vascular function
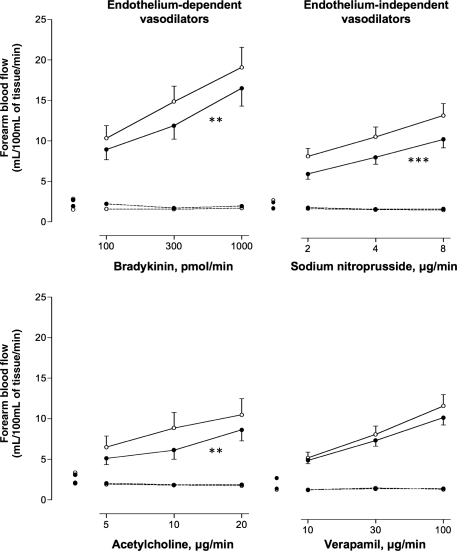
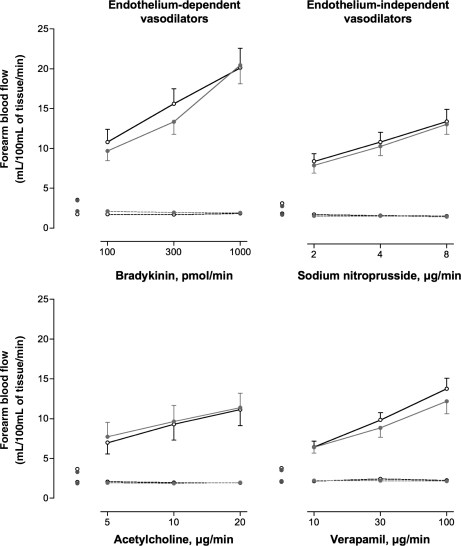
There were no differences in resting heart rate or basal forearm blood flow between exposures (Table 3). Inhalation of dilute diesel and filtered diesel exhaust increased systolic blood pressure (145 ± 4 and 144 ± 3 mmHg, respectively) compared with filtered air (133 ± 3 mmHg; P= 0.012). While there was a dose-dependent increase in blood flow with each vasodilator (P< 0.001 for all), this response was attenuated during bradykinin (P= 0.005), acetylcholine (P= 0.008), and sodium nitroprusside (P< 0.001) infusions following exposure to diesel exhaust compared with filtered air (Figure 2). Verapamil-induced vasodilatation was not significantly impaired following exposure to diesel exhaust (P= 0.08). Neither exposure to filtered diesel exhaust (Figure 3) nor pure carbon nanoparticles (data not shown) had any effect on endothelium-dependent and -independent vasodilatation. Bradykinin caused a dose-dependent increase in plasma t-PA antigen and activity concentrations (P< 0.001) that was not altered by any of the exposures compared with filtered air (data not shown).
Table 3.
Baseline haemodynamic variables from vascular assessment at 6 h
| Filtered air | Diesel exhaust | Filtered exhaust | Carbon | Significance | |
|---|---|---|---|---|---|
| Heart rate, b.p.m. | 68 ± 8 | 67 ± 12 | 65 ± 12 | 70 ± 8 | P = 0.475 |
| Systolic blood pressure, mmHg | 133 ± 12 | 145 ± 16* | 144 ± 12* | 139 ± 16 | P = 0.012 |
| Diastolic blood pressure, mmHg | 69 ± 8 | 69 ± 8 | 69 ± 4 | 71 ± 8 | P = 0.904 |
| Infused FBF, mL/100 mL tissue/min | 2.4 ± 0.8 | 2.1 ± 0.8 | 2.3 ± 1.6 | 2.0 ± 0.8 | P = 0.686 |
| Non-infused FBF, mL/100 mL tissue/min | 1.7 ± 0.8 | 1.9 ± 0.8 | 2.1 ± 1.2 | 2.2 ± 1.2 | P = 0.410 |
Values are reported as mean ± standard deviation. ANOVA with repeated measures. Bonferroni post test. FBF, forearm blood flow.
*P < 0.05 exposure vs. filtered air.
Figure 2.
Forearm blood flow 6–8 h after exposure to diesel exhaust or air. Infused (solid line) and non-infused (dashed line) forearm blood flow in healthy subjects, 6–8 h following diesel (filled circle) or air (open circle) exposure, during intra-brachial infusion of bradykinin, acetylcholine, sodium nitroprusside, or verapamil: for all dose responses P < 0.0001. For diesel exposure (filled circle) vs. air (open circle); bradykinin (**P= 0.005), acetylcholine (**P= 0.008), sodium nitroprusside (***P< 0.001), and verapamil (P= 0.08).
Figure 3.
Forearm blood flow 6–8 h after exposure to filtered diesel or air. Infused (solid line) and non-infused (dashed line) forearm blood flow in healthy subjects 6–8 h, following filtered diesel (grey circle) or air (open circle) exposure, during intra-brachial infusion of bradykinin, acetylcholine, sodium nitroprusside, or verapamil: for all dose responses P< 0.0001. For filtered diesel exposure (grey circle) vs. air (open circle); bradykinin, acetylcholine, sodium nitroprusside, and verapamil (P> 0.05 for all comparisons).
Pre-clinical studies
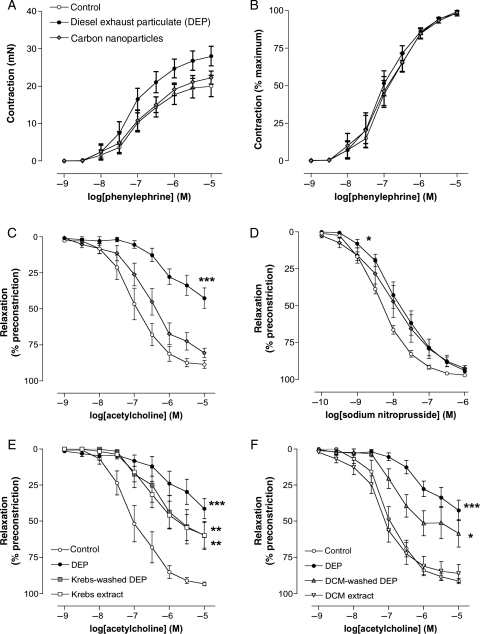
In vitro exposure of rat aortic rings to diesel exhaust particles produced a small increase in the maximum contractile response to phenylephrine (P= 0.03, unpaired t-test; Figure 4A), without affecting the sensitivity to phenylephrine. Pure carbon nanoparticles did not significantly alter responses to phenylephrine and, overall, both particles had no significant effect on concentration–response curves to phenylephrine when expressed as a percentage of maximum contraction (P= 0.64 and P= 0.86, respectively; two-way ANOVA; Figure 4B). Compared with vehicle control, vasorelaxation to acetylcholine was inhibited following exposure to diesel exhaust particles (P< 0.001; Figure 4C). Although there was an apparent rightward shift in the concentration–response curve to acetylcholine with carbon nanoparticles, vasorelaxation was not significantly affected (P= 0.139; Figure 4C). Diesel exhaust particles caused a small but significant inhibition of relaxation to sodium nitroprusside (P= 0.019) compared with control (Figure 4D), whereas carbon nanoparticles did not (P= 0.224).
Figure 4.
Direct vascular effects of particles in rat aortic rings in vitro. Effect of diesel exhaust particles (filled circle), carbon nanoparticles (u) (both 100 µg/mL), or vehicle (open circle) (Krebs buffer) on (A) phenylephrine contraction in mN, (B) phenylephrine contraction as a percentage of maximum contraction (C) acetylcholine, and (D) sodium-nitroprusside-induced vasodilatation. Diesel exhaust particles did not affect contraction to phenylephrine (P= 0.64), but inhibited vasorelaxation to acetylcholine (***P< 0.001) and sodium nitroprusside (*P= 0.019) compared with control. Although there was an apparent rightward shift in the dose–response curve to acetylcholine and sodium nitroprusside with carbon nanoparticles, vasorelaxation was not significantly affected (P= 0.139 and P = 0.224, respectively). Effect of (E) aqueous and (F) organic separation of diesel exhaust particles on acetylcholine responses. (E) Aqueous-washed diesel exhaust particles (grey square), and aqueous extract from diesel exhaust particles (open square), inhibit vasodilation to acetylcholine (**P< 0.01 for both), with the combined effects of both constituents being equal to that of untreated particles (filled circle). (f) Organic-washed diesel exhaust particles (s) inhibited acetylcholine induced vasodilatation (*P= 0.010); the magnitude of this effect was not different from that of untreated diesel exhaust particles (filled circle) (P= 0.103). The organic extract of diesel exhaust particles (inverted triangle) had no effect on vasorelaxation to acetylcholine (P= 0.645). n= 6 for all groups.
Following separation of the aqueous soluble constituents from diesel exhaust particulate matter, both washed particles and solution were able to inhibit vasodilator responses to acetylcholine (P< 0.01 for both), with the combined effects of both constituents being equal to that of untreated particles (Figure 4E). Dichloromethane-washed diesel exhaust particles markedly inhibited acetylcholine-induced vasodilatation (P= 0.010); the magnitude of which was not statistically different from untreated diesel exhaust particles (P= 0.103; Figure 4F). Accordingly, the organic extract of diesel exhaust particles removed by dichloromethane had no effect on relaxation to acetylcholine (P= 0.645).
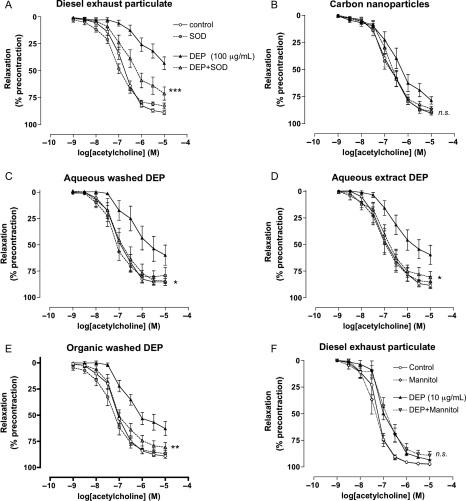
The superoxide free radical scavenger, superoxide dismutase (SOD), partially reversed the inhibitory effects of diesel exhaust particles on acetylcholine responses (P< 0.001; Figure 5A). Superoxide dismutase completely reversed the action of Krebs-washed diesel particles (P= 0.03), the Krebs extract (P= 0.03), and dichloromethane-washed diesel particles (P= 0.009), as well as the response to carbon nanoparticles (P= 0.18), although this effect was not statistically significant due to the modest inhibitory effect of these particles alone (Figure 5B–E). The hydroxyl radical scavenger, mannitol, however, had no effect on the action of diesel particles (10 μg/mL) on acetylcholine responses (P= 0.84; Figure 5F).
Figure 5.
Reversal of the effects of particles in vitro by superoxide dismutase. (A) Diesel exhaust particles, (B) carbon nanoparticles, (C) Krebs-washed diesel exhaust particles, (D) soluble extract from Krebs-washed diesel exhaust particles, and (E) dichloromethane-washed diesel exhaust particles. Effect of (filled triangle) particles (100 µg/mL), (grey circle) superoxide dismutase (100 U/mL), (grey triangle) particles + superoxide dismutase together, or vehicle (open circle) (Krebs buffer) on acetylcholine-induced vasodilatation. Superoxide dismutase reversed the actions of all particles (*P< 0.05, **P< 0.01, ***P< 0.001) compared with particles alone, except nCB where the reversal by superoxide dismutase was not significantly affected (P= 0.18); n= 6–9 for all groups. (f) The hydroxyl radical scavenger mannitol had no effect on the action of diesel exhaust particles on acetylcholine-induced relaxation (nsP= 0.84: mannitol + diesel exhaust particles compared with diesel exhaust particles alone). (filled triangle) Diesel exhaust particles (10 µg/mL), (diamond) mannitol (5 mM), (inverted triangle) diesel exhaust particles + mannitol together, or vehicle (open circle) (Krebs buffer); n= 6 for all groups.
Discussion
In a complementary series of clinical and pre-clinical studies, we have attempted to identify the components responsible for the adverse cardiovascular effects of air pollution. When assessed in the clinic, we showed that the vascular dysfunction associated with diesel exhaust inhalation is prevented by particle filtration. However, the nature of the particles appears to be critical since a pure carbon nanoparticulate exposure alone had no discernible effect on vascular function. Investigating this further, we were able to demonstrate that combustion-derived diesel exhaust nanoparticulate causes direct vascular dysfunction in vitro and this appears to be attributable to both soluble and insoluble fractions present on the surface of the particulate. Taken together, our findings suggest that the adverse vascular effects of diesel exhaust inhalation are predominantly mediated by combustion-derived nanoparticulate. This provides a rationale for testing environmental health interventions targeted at reducing traffic-derived particulate emissions.
Several pollutants in diesel exhaust emissions are considered harmful to human health including fine particles, nitrogen dioxide, sulphur dioxide, and volatile organic compounds.1,21 While we have previously shown that exposure to nitrogen dioxide, at levels in excess of those found in the present study, does not alter vascular function,22 it would not be possible to assess the importance of all the components of diesel exhaust individually and such a strategy would not account for potentially important synergistic interactions between pollutants. Therefore, our present study was designed specifically to determine the role of nanoparticulate emissions, and whether the organic and inorganic surface compounds, or the carbonaceous particles themselves, are the main arbiter of the adverse cardiovascular effects.
Exposure to dilute diesel exhaust for 2 h impaired vasomotor vascular function with reduced vasodilatation in response to both endothelium-dependent and -independent agonists. The exposures are standardized to ensure a particle concentration of 300 μg/m3. These concentrations are found on a regular basis in heavy traffic, occupational settings, and in the world's most-polluted cities. Exposure to 300 µg/m3 for 1 h increases a person's average exposure during a 24-h period by only 12 µg/m3 and changes of this magnitude occur in even the least polluted of cities on a daily basis.14 Depletion of particles in diesel exhaust using a highly efficient teflon filter reduced concentrations by 1000-fold, and did not alter concentrations of the gaseous pollutants of volatile organic species. We did not observe any impairment of vascular function following exposure to filtered exhaust, suggesting that the particles are essential in mediating the adverse effects of diesel exhaust. While it is possible that the non-particulate components of diesel exhaust have a synergistic role in the presence of particulates, it is clear from our complementary in vitro studies that diesel exhaust particles themselves have the capacity to inhibit vascular function directly.
In the clinical studies, exposure to pure carbon nanoparticles did not cause significant vascular dysfunction. In the pre-clinical experiments, in vitro exposure to carbon nanoparticles caused a modest inhibition of acetylcholine-induced relaxation, although this effect was not statistically significant. Although carbon nanoparticles cause pro-inflammatory effects in vitro,23 the only previous study to address the vascular effects of exposure to carbon nanoparticles in man identified changes in circulating leucocyte expression of adhesion molecules,24 but no consistent effect on systemic vascular function.25 These findings suggest that while exposure to ‘pure’ carbon nanoparticles can exert some systemic effects within the cardiovascular system, their vascular actions are relatively small in comparison with particles from vehicle exhaust. These observations are in keeping with our previous studies where exposure to concentrated ambient particles, low in combustion-derived particulate, did not affect vascular function in either healthy subjects or patients with coronary heart disease.26 Taken together, these studies suggest that particle composition is an important determinant of the health effects of air pollution exposure.
The particles in diesel exhaust are laden with surface organic compounds from unburned hydrocarbon fuels, and coated with oxidized transition metals added to the fuels to improve efficiency.27 Experimental studies have established that diesel exhaust particles induce cellular oxidative stress and up-regulate pro-inflammatory pathways.28 Particle size, surface area, and surface chemistry are thought to be important determinants of these cellular effects. Whether inhaled nanoparticulate is capable of translocation into the circulation in humans remains uncertain,29,30 but many of the chemical species on the surface of these particles are hydrophilic, could diffuse across tight junctions into the pulmonary interstitium, and be released into the circulation to affect vascular function directly. In particular, the organic species absorbed on the surface of combustion-derived particles are chemically active and potentially harmful to human health.17,31,32
Interestingly, removing the soluble and organic constituents from the surface of diesel exhaust particles did not completely abolish the effect on acetylcholine-mediated relaxation in vitro. While dichloromethane removes aromatic components, such as polyaromatic hydrocarbons, it is unclear to what extent neutral non-polar organics (aliphatics) or the polar organics, such as quinones, are effected by this treatment. It is possible that these components are more important than aromatic hydrocarbons in determining the adverse vascular effects of diesel exhaust particles. At present, it is not possible to speculate further on the identity of these harmful surface constituents, but a better understanding of the detrimental components of diesel exhaust particulate will be necessary for the future evaluation of technologies designed to modify vehicle exhaust emissions.
Vasomotor dysfunction was associated with an increase in arterial pressure that was not present in our previous studies.14,16 In our present study, subjects were exposed to each of the four conditions for 2 h, where previously the exposures were limited to 1h. It is possible that the longer duration of exposure results in a more marked vascular pertubation with effects on systemic vascular resistance and arterial pressure. Against this hypothesis, there were no differences in basal vascular tone between exposures, and systolic blood pressure was also raised following exposure to filtered exhaust, perhaps suggesting that the vasomotor and pressor effects are mediated by different mechanisms or components of the exhaust. There is a substantial body of evidence to support a direct effect of air pollution on blood pressure,33–36 and the mechanisms that underpin this response are beginning to emerge. Experimental studies suggest an important role for vascular oxidative stress and the up-regulation of Rho-kinases.37
The cellular pathways underlying the vascular impairment by diesel particle exposure are important for understanding the mechanism of action of particles and their expected impact on cardiovacular system. The pattern of vascular dysfunction induced by exposure to diesel exhaust was similar to that reported in our previous studies:14 reduced vasodilatation in response to endothelium-dependent agonists and the nitric oxide donor sodium nitroprusside. Together with the preserved response to verapamil, this would suggest decreased bioavailability of nitric oxide, or a decrease in the sensitivity of smooth muscle cells to nitric oxide, mediates the effect of diesel particles. While decreases in the activity of nitric oxide synthase appear to be at least partially involved in the impaired vasodilator responses to endothelium-dependent agonists,38,39 vascular oxidative stress may also contribute to the reduced nitric oxide bioavailability.40 Indeed, in vitro studies provide support for this mechanism, where diesel exhaust particles intrinsically generate free radicals, are able to quench nitric oxide, and inhibit acetylcholine-mediated relaxation of aortic ring preparations.18 The involvement of superoxide free radicals in the action of diesel particles (or their extracts) on vascular function is further supported here by the ability of superoxide dismutase (but not the hydroxyl radical scavenger mannitol) to reverse the vascular impairment. The incomplete reversal of the actions of whole diesel exhaust particles may suggest that downstream pathways, such as alterations in calcium sensitivity or up-regulation of rho-kinases,37 may be partially involved in the action of diesel exhaust particles. However, we note that pathways downstream of soluble guanylate cyclase do not appear to be affected by in vitro exposure to urban particulate in general.41 Further studies are required to more fully establish the underlying mechanism for the vascular impairment and determine the effects of exposure on the l-arginine-nitric oxide pathway in more detail.
Fibrinolytic function was not altered by any of the exposure conditions. In previous work, we have demonstrated that exposure to diesel exhaust generated from an idlling automobile engine impaired endothelial t-PA release from the forearm of both healthy subjects14 and patients with coronary heart disease.16 We used a different exposure system in our current study, and it is possible that differences between the exposures themselves could account for this inconsistency. The electrical generator used in the present study was running on commercially available gas oil as opposed to the heavier diesel oil used previously. Combustion was perhaps more efficient from the generator with a greater proportion of diesel exhaust particles containing elemental carbon rather than organics from unburnt fuel. Exposure conditions were also associated with five-fold lower levels of both carbon monoxide and nitrogen oxides than were present in our previous studies.15,16,42 The release of t-PA from granules in the endothelium is not dependent on nitric oxide and down-regulation of fibrinolytic function may be dependent on changes in protein synthesis. We have previously found divergence in the effects of exposure on vasomotor and fibrinolytic function at different time points, with the effects on vasodilatation being more prominent immediately after the exposure.
Study limitations
There are a number of potential limitations of this study that merit consideration. We had intended to use a versatile aerosol concentration enrichment system (VACES)26,43 to expose subjects to diesel exhaust particles in isolation. Unfortunately, it was not possible to reduce the concentration of gaseous co-pollutants sufficiently to perform a useful comparison with unmodified dilute diesel exhaust. We therefore elected to use a particle filtration system to assess the effects of the gaseous co-pollutants in isolation, and as such can only infer from our findings that diesel exhaust particles are responsible for the adverse vascular effects described in vivo.
To address this potential limitation, we undertook complementary in vitro studies. We acknowledge that the direct application of particles to the vasculature in vitro makes a number of assumptions regarding the feasibility of particle translocation, the numbers of particles likely to translocate into the cardiovascular system and changes to the nature of the particulate during this transit.18 Because of the vast number of potentially active constituents of diesel exhaust particles, and the chemical processes needed to fractionate these constituents, predictive in vitro assays are essential to explore the mechanism of action (and interaction) of different particle components. The concentrations of diesel exhaust particulate employed here are high, and it is unlikely that nanoparticles would reach these concentrations in the systemic circulation without a means of accumulation over a prolonged period. However, the similarity of the vasodilator response in vitro to that of the clinical studies is striking, both in the previous investigations14,18 and the present findings, suggesting that this methodology is representative of the vascular pathways activated in vivo. We believe that this combined approach is justified and provides additional insight into the components responsible for the adverse vascular effects of diesel exhaust inhalation in man.
While we demonstrate that particle filtration abrogates the effects of exposure to diesel exhaust, we used a highly efficient teflon filter in our experimental system that would not readily be applicable to the filtration of vehicle emissions or in air conditioning systems. It would be important, therefore, to verify the benefits of particle filtration using commercially available filtration systems before these findings can guide environmental health policy decisions on the use of retrofit particle traps.
Conclusions
Inhalation of dilute diesel exhaust (but not the associated gaseous pollutants or carbon nanoparticles alone) impairs vascular function in man. These findings suggest that the adverse vascular effects of diesel exhaust are predominately mediated by combustion-derived particles and provide a rationale for testing environmental health interventions to reduce particulate emissions to establish whether they can reduce the incidence of cardiovascular events.
Funding
This work was supported by a British Heart Foundation Programme Grant [PG/10/009]. NLM was supported by an Intermediate Clinical Research Fellowship from the British Heart Foundation [FS/10/024] and Programme Grant (PG/05/003), UK. Dutch Ministry of Housing, Spatial Planning and Environment (VROM), the Netherlands. Funding to pay the Open Access publication charges for this article was provided by the British Heart Foundation.
Conflict of interest: No conflicts of interest to disclose. The study complies with the Declaration of Helsinki and has been approved by all relevant local (UK) Ethics Committees.
Acknowledgements
We thank Pamela Dawson, Daan Leseman, Finny Paterson and all the staff at the Wellcome Trust Clinical Research Facility, Edinburgh, for their assistance with the studies. We also acknowledge the support of the British Heart Foundation Centre of Research Excellence (CoRE) award and UK National Health Service (NHS) Research Scotland (NRS), through NHS Lothian and the Chief Scientist Office.
References
- 1.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HJ, Cho SH, Nyberg F, Pershagen G. Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. Epidemiology. 2001;12:413–419. doi: 10.1097/00001648-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Moebus S, Möhlenkamp S, Dragano N, Nonnemacher M, Fuchsluger M, Kessler C, Jakobs H, Memmesheimer M, Erbel R, Jöckel KH, Hoffmann B HNR Study Investigative Group. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56:1803–1808. doi: 10.1016/j.jacc.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann B, Moebus S, Dragano N, Möhlenkamp S, Memmesheimer M, Erbel R, Jöckel KH Heinz Nixdorf Recall Investigative Group. Residential traffic exposure and coronary heart disease: results from the Heinz Nixdorf Recall Study. Biomarkers. 2009;14(Suppl. 1):74–78. doi: 10.1080/13547500902965096. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann B, Moebus S, Dragano N, Stang A, Möhlenkamp S, Schmermund A, Memmesheimer M, Bröcker-Preuss M, Mann K, Erbel R, Jöckel KH. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009;117:1302–1308. doi: 10.1289/ehp.0800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jöckel KH Heinz Nixdorf Recall Study Investigative Group. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann B, Moebus S, Stang A, Beck EM, Dragano N, Möhlenkamp S, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jöckel KH Heinz Nixdorf RECALL Study Investigative Group. Residence close to high traffic and prevalence of coronary heart disease. Eur Heart J. 2006;27:2696–2702. doi: 10.1093/eurheartj/ehl278. [DOI] [PubMed] [Google Scholar]
- 11.Künzli N, Jerrett M, Garcia-Esteban R, Basagaña X, Beckermann B, Gilliland F, Medina M, Peters J, Hodis HN, Mack WJ. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 2010;5:e9096. doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, Löwel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 14.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 15.Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Cassee FR, Donaldson K, Boon NA, Badimon JJ, Sandstrom T, Blomberg A, Newby DE. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- 16.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Söderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 17.Wichmann HE. Diesel exhaust particles. Inhal Toxicol. 2007;19(Suppl. 1):241–244. doi: 10.1080/08958370701498075. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Borthwick SJ, Shaw CA, MacLean SG, McClure D, Mills NL, Duffin R, Donaldson K, Megson IL, Hadoke PW, Newby DE. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ Health Perspect. 2009;117:611–616. doi: 10.1289/ehp.0800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koike E, Kobayashi T. Organic extract of diesel exhaust particles stimulates expression of Ia and costimulatory molecules associated with antigen presentation in rat peripheral blood monocytes but not in alveolar macrophages. Toxicol Appl Pharmacol. 2005;209:277–285. doi: 10.1016/j.taap.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 21.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 22.Langrish JP, Lundbäck M, Barath S, Söderberg S, Mills NL, Newby DE, Sandström T, Blomberg A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal Toxicol. 2010;22:192–198. doi: 10.3109/08958370903144105. [DOI] [PubMed] [Google Scholar]
- 23.Barlow PG, Clouter-Baker A, Donaldson K, Maccallum J, Stone V. Carbon black nanoparticles induce type II epithelial cells to release chemotaxins for alveolar macrophages. Part Fibre Toxicol. 2005;2:11. doi: 10.1186/1743-8977-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frampton MW, Stewart JC, Oberdorster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang LS, Utell MJ. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AP, Pietropaoli AP, Frasier LM, Speers DM, Chalupa DC, Delehanty JM, Huang LS, Utell MJ, Frampton MW. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ Health Perspect. 2008;116:375–380. doi: 10.1289/ehp.10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, Freney EJ, Heal MR, Donovan RJ, Blomberg A, Sandström T, MacNee W, Boon NA, Donaldson K, Newby DE, Cassee FR. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect. 2008;116:709–715. doi: 10.1289/ehp.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levsen K. The analysis of diesel particulate. Frenzius Z Anal Chem. 2002;331:467–478. [Google Scholar]
- 28.Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, de la Fuente JM, Cassee FR, Boon NA, MacNee W, Millar AM, Donaldson K, Newby DE. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 30.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 31.Biswas S, Verma V, Schauer JJ, Cassee FR, Cho AK, Sioutas C. Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ Sci Technol. 2009;43:3905–3912. doi: 10.1021/es9000592. [DOI] [PubMed] [Google Scholar]
- 32.Cheung KL, Polidori A, Ntziachristos L, Tzamkiozis T, Samaras Z, Cassee FR, Gerlofs M, Sioutas C. Chemical characteristics and oxidative potential of particulate matter emissions from gasoline, diesel, and biodiesel cars. Environ Sci Technol. 2009;43:6334–6340. doi: 10.1021/es900819t. [DOI] [PubMed] [Google Scholar]
- 33.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Barragán-Mejía G, Garrido-García L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya H, Villarreal-Calderón R, Torres-Jardón R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 2007;115:1248–1253. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherng TW, Campen MJ, Knuckles TL, Gonzalez Bosc L, Kanagy NL. Impairment of coronary endothelial cell ETB receptor function after short-term inhalation exposure of whole diesel emissions. Am J Physiol Regul Integr Comp Physiol. 2009;297:R640–R647. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Pespect. 2011;119:98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills NL, Tornqvist H, Robinson SD, Gonzalez MC, Söderberg S, Sandström T, Blomberg A, Newby DE, Donaldson K. Air pollution and atherothrombosis. Inhal Toxicol. 2007;19(Suppl. 1):81–89. doi: 10.1080/08958370701495170. [DOI] [PubMed] [Google Scholar]
- 41.Courtois A, Andujar P, Ladeiro Y, Baudrimont I, Delannoy E, Leblais V, Begueret H, Galland MA, Brochard P, Marano F, Marthan R, Muller B. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: comparison of urban particulate matter and manufactured nanoparticles. Environ Health Perspect. 2008;116:1294–1299. doi: 10.1289/ehp.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, MacNee W, Donaldson K, Söderberg S, Newby DE, Sandström T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 43.Freney EJ, Heal MR, Donovan RJ, Mills NL, Donaldson K, Newby DE, Fokkens PH, Cassee FR. A single-particle characterization of a mobile versatile aerosol concentration enrichment system for exposure studies. Part Fibre Toxicol. 2006;3:8. doi: 10.1186/1743-8977-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]