Abstract
Malaria control can be improved by rapid, sensitive, low-cost detection of infection. Several such strategies are being pursued. Rapid diagnostic tests can detect infections at parasite densities above 200 μL−1. Polymerase chain reaction methods can detect low parasite densities, but are slow and prone to contamination under field conditions. Methods that detect hemozoin presence in blood have been proposed as alternatives for rapid detection of infection. In this study, we used a benchtop nuclear magnetic resonance (NMR) device to detect hemozoin. This device could be deployed in malaria-endemic settings. We measured synthetic hemozoin in phosphate-buffered saline and malaria parasites in human blood. The NMR detected hemozoin in suspensions of 4 ng μL−1 and parasites at densities of 8,000–10,000 μL−1 (0.2% parasitemia). Thus, our preliminary NMR approach, although providing very rapid measurements, is unlikely to achieve the required sensitivity and specificity for malaria diagnosis, unless a preliminary concentration step is performed.
Although a reduced incidence of malaria has been achieved in many regions through up scaled distribution of insecticide-treated bed nets and effective treatment with artemisinin combination therapies, malaria infection remains a major global health problem.1 A significant challenge in malaria case management is difficulty in obtaining a reliable diagnosis. In most endemic areas, light microscopic examination of blood films remains the only means of parasite detection but the availability of microscopy is variable. Rapid diagnostic tests (RDTs) represent an alternative, but they cannot detect low parasitemia, and storage of the kits at elevated temperatures may cause deterioration in their performance.2 Thus, presumptive treatment is often given based on clinical features alone.
In programs that aim for malaria elimination or eradication, mass drug administration is not currently considered feasible.3 Therefore; there is a need for high-throughput, ultrasensitive, on-site, same-day diagnosis to detect asymptomatic carriers. Well-equipped research sites may have polymerase chain reaction (PCR) facilities that could support large-scale screening but there are delays in obtaining the results. An alternative potential technique that could provide rapid results is measurement of parasite-produced hemozoin. For this purpose, a novel “magneto-optic technology test” (MOT-test) was developed and evaluated recently.4 This test can measure hemozoin concentrations in a blood sample down to 5 ng μL−1 or less.4,5 This corresponds to a hemozoin-containing parasite density of about 8,000–9,000 μL−1 and is above the detection limit of the RDT, thin blood film (200 μL−1), thick blood film (5–50 μL−1), and PCR (0.1–1 μL−1).6–8 It is surprising that alternative established methods for magnetic measurements, especially nuclear magnetic resonance (NMR), have not yet been investigated for their potential to detect hemozoin. The aim of this study was, therefore, to investigate whether a benchtop NMR device could be used as a convenient, sensitive, and efficient diagnostic tool in a field setting.
Synthetic hemozoin was prepared using the method of Jaramillo with slight modifications.9 Fourier transform infrared spectroscopy and transmission electron microscopy confirmed the chemistry and morphology of hemozoin.9 Plasmodium falciparum parasites were grown in vitro using a modification of methods developed by Trager and Jensen.10 Sorbitol synchronization was used to narrow parasite stage distribution and measurements were conducted ∼24 h after synchronization. Twofold dilutions of synthetic hemozoin in phosphate-buffered saline (PBS) were prepared. Similarly, 2-fold parasite dilution series were prepared in human red blood cells at 40% hematocrit. Samples of the dilutions (1 mL) were transferred into acid-washed, sterilized glass NMR tubes, cooled to 4°C, and measured within a few hours. The parasite samples were frozen and thawed three times after the first measurement to induce erythrocyte lysis. Thereafter, the samples were measured again in the lysed state. Proton relaxometry measurements were made on a Bruker mq-60 minispec (Bruker, Ettlingen, Germany) operating at 1.4 T. Samples were placed in an aluminum block within a water bath at 37.5°C for at least 15 minutes before relaxometry measurements. A Carl-Purcell-Meiboom-Gill (CPMG) spin echo sequence was used to measure T2 using 2000 echoes, an echo spacing (TE) of 1 ms, and a repetition time of 5 s. After the measurements, samples were acid digested and inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was used to determine total iron concentration in the samples.
Figure 1 shows the detection of synthetic hemozoin material in PBS (pH 7.4). Reliable detection down to 4 ng μL−1 was possible similar to the previously published MOT-test. As a comparison: In a sample where all parasites have converted 50% of erythrocyte hemoglobin to hemozoin this concentration would correspond to 7,400 parasites/μL of blood or ∼0.15% parasitemia if commonly used conversions are applied. (i.e., 8,000 white blood cells/μL of blood, 5 × 106 red blood cells/μL of blood, and 3 × 108 hemoglobin molecules/red blood cell).11–13
Figure 1.
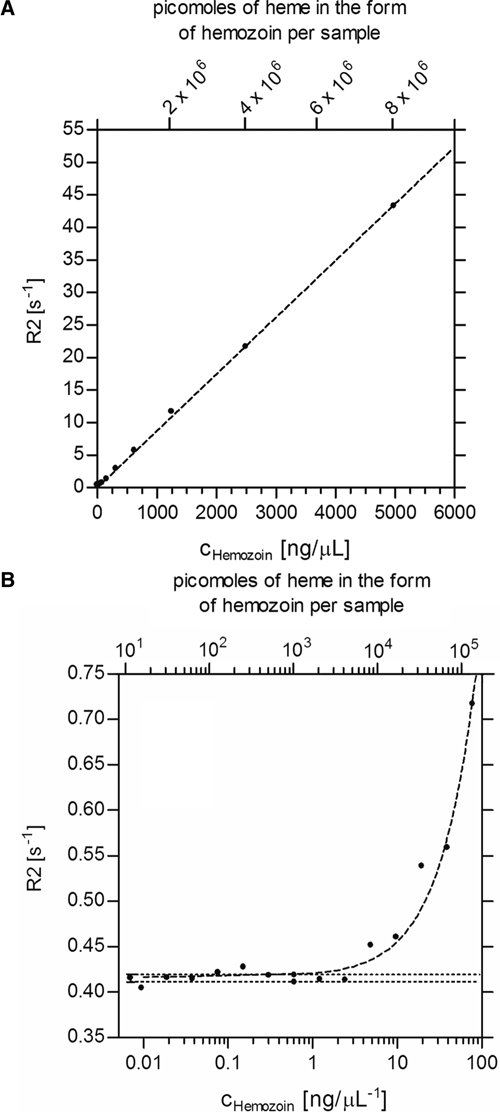
Dilution series and detection limit for synthetic hemozoin in phosphate-buffered saline (PBS). Panel A: Two-fold dilution series of synthetic hemozoin material in PBS (pH = 7.4) for higher concentrations confirms linearity of the standard curve. Panel B: The linear regression curve indicates that concentrations of hemozoin above 4 ng μL−1 are sufficient to measure R2 values above the noise level using the 60 MHz, 1.4 T NMR spectrometer. This corresponds to a theoretical parasite density of about 7,400 μL−1 or an equivalent parasitemia of 0.15%.
Figure 2 shows the detection limit achieved when using parasitized cells in suspensions with normal, uninfected red blood cells. Because these samples were prepared for measurement 24 h after synchronization, the parasites were late trophozoites and schizonts. The ICP-AES analysis showed that the parasites had converted an average of ∼25% hemoglobin to hemozoin at this stage. For intact parasitized cells (Figure 2; Panel A), the detection limit was ∼15,000 parasites/μL of blood. After cell lysis (Panel B) the detection limit was 8,000–10,000 parasites/μL of blood, an improved value that may be caused by the better dispersion of the hemozoin in the sample after its release from the parasites.
Figure 2.
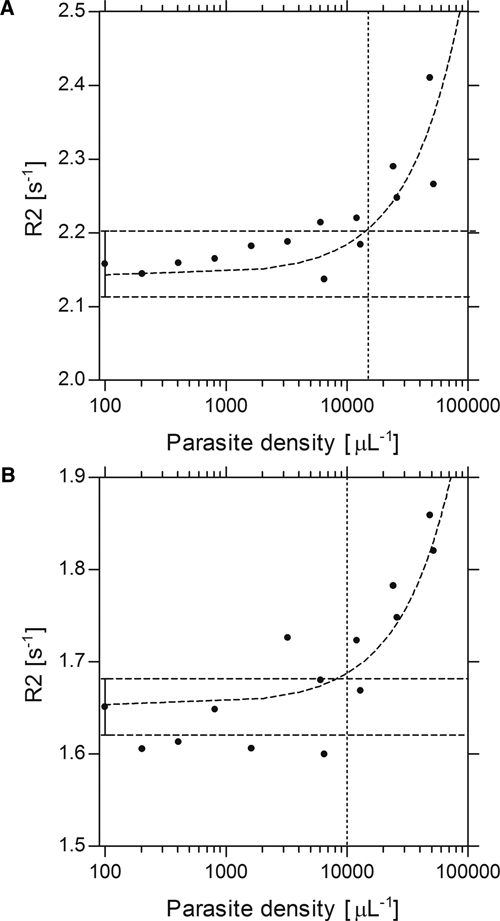
NMR detection limit for parasitized cells. Panel A: The detection limit for intact parasites measured using the 60 MHz, 1.4 T NMR is ∼15,000 μL−1. Panel B: The detection limit for lysed parasites measured using the 60 MHz, 1.4 T NMR is ∼8,000–10,000 μL−1 (the same samples are shown in Panels A and B). Note that 1 mL of blood was used for each experiment.
This study shows that the detection limit of hemozoin using a 1.4 T 60 MHz NMR device that has potential field application is approximately equivalent to that produced by 107 Plasmodium-infected red blood cells. In a 1 mL blood sample as used in this study, this corresponds to a hemozoin containing parasite density of 8,000–10,000 μL−1 (0.2% parasitemia). This is well above the limits of detection achieved with light microscopy, RDTs, and PCR methods.
Diagnostic techniques for malaria that rely solely on the detection of the total amount of hemozoin in blood samples have several limitations. First, vascular sequestration of hemozoin-containing late stages of Plasmodium falciparum efficiently removes these cells from the peripheral blood. Circulating parasite forms are relatively low in hemozoin and so diagnostic sensitivity is further impaired. The present methods deliberately used relatively late-stage, hemozoin-containing parasites but the sensitivity was still poor. Second, hemozoin detection on its own can only yield an indirect picture of infection status because hemozoin-containing leukocytes and free hemozoin may be present in peripheral blood of previously infected patients and may cause false positive results. Infection with schistosomes, which also generate hemozoin, could in theory lead to misdiagnosis, although this seems unlikely given the small amounts of hemozoin produced by Schistosoma. Third, Plasmodium speciation is not possible using NMR or other methods that only detect hemozoin, because hemozoin production is not species-specific.
In this study, we have characterized the sensitivity of a benchtop NMR with a magnetic field strength of 1.4 T for the detection of hemozoin. The diagnostic sensitivity of this technique and others based on total hemozoin detection in blood samples is low compared with other established methods, including light microscopy.4,5 Other methods that exploit hemozoin presence for malaria diagnosis such as birefringence observation by polarized light, magnetic fractionation (MF) before light microscopy, laser flow cytometry (LFC), and laser desorption mass spectrometry (LDMS) have been shown to exhibit much higher sensitivities using less sample volume for detection of hemozoin containing parasites in vitro and in mouse models (by LDMS), hemozoin containing leukocytes (by LFC), and gametocytes (by MF).14–18
Footnotes
Authors' addresses: Stephan Karl, Lucia Gutierrez, Michael J. House, and Tim G. St. Pierre, School of Physics, The University of Western Australia, Crawley, Australia, E-mails: Stephan.karl@physics.uwa.edu.au, lucia@cyllene.uwa.edu.au, mhouse@physics.uwa.edu.au, and stpierre@physics.uwa.edu.au. Timothy M. E. Davis, School of Medicine and Pharmacology, The University of Western Australia, Fremantle Hospital, Fremantle, WA, Australia, E-mail: tdavis@cyllene.uwa.edu.au.
References
- 1.Hommel M. Towards a research agenda for global malaria elimination. Malar J. 2008;7((Suppl 1)):S1. doi: 10.1186/1475-2875-7-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig MH, Bredenkamp BL, Williams CH, Rossouw EJ, Kelly VJ, Kleinschmidt I, Martineau A, Henry GF. Field and laboratory comparative evaluation of ten rapid malaria diagnostic tests. Trans R Soc Trop Med Hyg. 2002;96:258–265. doi: 10.1016/s0035-9203(02)90092-1. [DOI] [PubMed] [Google Scholar]
- 3.Baird JK. Eliminating malaria–all of them. Lancet. 2011;376:1883–1885. doi: 10.1016/S0140-6736(10)61494-8. [DOI] [PubMed] [Google Scholar]
- 4.Newman DM, Heptinstall J, Matelon RJ, Savage L, Wears ML, Beddow J, Cox M, Schallig HD, Mens PF. A magneto-optic route toward the in vivo diagnosis of malaria: preliminary results and preclinical trial data. Biophys J. 2008;95:994–1000. doi: 10.1529/biophysj.107.128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mens PF, Matelon RJ, Nour BY, Newman DM, Schallig HD. Laboratory evaluation on the sensitivity and specificity of a novel and rapid detection method for malaria diagnosis based on magneto-optical technology (MOT) Malar J. 2010;9:207. doi: 10.1186/1475-2875-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejon P, Andrews L, Hunt-Cooke A, Sanderson F, Gilbert SC, Hill AV. Thick blood film examination for Plasmodium falciparum malaria has reduced sensitivity and underestimates parasite density. Malar J. 2006;5:104. doi: 10.1186/1475-2875-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamara DT, Thomson JM, Kasehagen LJ, Zimmerman PA. Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J Clin Microbiol. 2004;42:2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaramillo M, Bellemare MJ, Martel C, Shio MT, Contreras AP, Godbout M, Roger M, Gaudreault E, Gosselin J, Bohle DS, Olivier M. Synthetic Plasmodium-like hemozoin activates the immune response: a morphology—function study. PLoS ONE. 2009;4:e6957. doi: 10.1371/journal.pone.0006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 11.Krantz A. Red cell-mediated therapy: opportunities and challenges. Blood Cells Mol Dis. 1997;23:58–68. doi: 10.1006/bcmd.1997.0119. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. White blood cell counts and malaria. J Infect Dis. 2005;192:323–330. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DW. The molecular biology of mammalian hemoglobin synthesis. Ann Clin Lab Sci. 1980;10:116–122. [PubMed] [Google Scholar]
- 14.Hanscheid T, Egan TJ, Grobusch MP. Hemozoin: from melatonin pigment to drug target, diagnostic tool, and immune modulator. Lancet Infect Dis. 2007;7:675–685. doi: 10.1016/S1473-3099(07)70238-4. [DOI] [PubMed] [Google Scholar]
- 15.Karl S, David M, Moore L, Grimberg BT, Michon P, Mueller I, Zborowski M, Zimmerman PA. Enhanced detection of gametocytes by magnetic deposition microscopy predicts higher potential for Plasmodium falciparum transmission. Malar J. 2008;7:66. doi: 10.1186/1475-2875-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karl S, Davis TM, St-Pierre TG. A comparison of the sensitivities of detection of Plasmodium falciparum gametocytes by magnetic fractionation, thick blood film microscopy, and RT-PCR. Malar J. 2009;8:98. doi: 10.1186/1475-2875-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romagosa C, Menendez C, Ismail MR, Quintó L, Ferrer B, Alonso PL, Ordi J. Polarization microscopy increases the sensitivity of hemozoin and Plasmodium detection in the histological assessment of placental malaria. Acta Trop. 2004;90:277–284. doi: 10.1016/j.actatropica.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Scholl PF, Kongkasuriyachai D, Demirev PA, Feldman AB, Lin JS, Sullivan DJ, Jr, Kumar N. Rapid detection of malaria infection in vivo by laser desorption mass spectrometry. Am J Trop Med Hyg. 2004;71:546–551. [PubMed] [Google Scholar]


