Abstract
All the records from the Spanish information system for hospital data of patients diagnosed with leishmaniasis during a 12-year period (1997–2008) were studied. The 2,028 individuals were hospitalized because of leishmaniasis, as indicated by the principal diagnostic code. The average hospitalization rate was 0.41/100,000 inhabitants. One-third of them were co-infected with human immunodeficiency virus (HIV). The incidence of hospitalization in the adult population with leishmaniasis co-infected with HIV increased with age, peaked at 35–39 years of age and subsequently declined. In the pediatric population, all leishmaniasis cases occurred in HIV-negative children. Incidence of hospitalizations was highest in Madrid and in the Mediterranean coast. The cost per inpatient hospital care was $9,601 corresponding to an annual direct cost of more than $1.5 million for inpatient care alone. The economical burden of leishmaniasis is not neglectable and in the 12-year study period it represented more than $19 million.
Introduction
Leishmaniasis is a group of parasitic diseases caused by two dozen species of protozoa belonging to the genus Leishmania and spread by the bite of the sand fly. Two major clinical forms are known: cutaneous leishmaniasis, affecting the skin causing scars and eventually disfiguration, and systemic or visceral leishmaniasis that can lead to deadly complications if untreated.1 Leishmaniasis is hypoendemic in the south of Europe. It is caused by Leishmania infantum, the dog being the main reservoir host. Leishmaniasis treatment switched from antimonials (20 mg/kg daily intramuscular for 28 days) to liposomal amphoterin B (3–5 mg/kg/daily dose by infusion given over a 3–6 days period, up to a total dose of 18–21 mg/kg) around 15 years ago. Between February 1982, date when leishmaniasis was declared a notifiable disease, and December 1995, a total of 1,574 accumulated cases were reported.2 In July 1996 a new decentralized surveillance system based in the political structure of the autonomous regions was implemented, but leishmaniasis is no longer a notifiable disease in all the 17 regions of Spain.
The human immunodeficiency (HIV) pandemic has modified the epidemiology of leishmaniasis. The World Health Organization (WHO) data show that coinfection with Leishmania and HIV is higher in Spain compared with other Mediterranean countries.3 Although coinfection by Leishmania and HIV can enhance their respective signs and symptoms, clinical manifestations of leishmaniasis in HIV-positive patients in the Mediterranean basis are not much different from those of immunocompetent patients.4 The introduction of highly active antiretroviral therapy (HAART) in Europe in 1997, however, has improved considerably the quality of life of the patients reducing the number of relapses, decreasing significantly the notification of co-infected cases, and lowering mortality.4–7
The 60th World Health Assembly (WHA) recognized leishmaniasis as one of the most neglected tropical diseases.8 The lack of accurate information on the epidemiology of the disease as suggested by the high variability in the incidence and mortality rates reported in different studies slows down efforts of disease control.9
This epidemiological retrospective survey, using the Spanish Centralized Hospital Discharge Database as an alternative data source to the national surveillance system, aimed to provide a population-based estimate of the burden of hospitalization caused by leishmaniasis and coexistent Leishmania/HIV coinfection in the Spanish population during a 12-year period (1997–2008). It was assumed that the great majority of leishmaniasis cases included here is caused by visceral leishmaniasis, because cutaneous leishmaniasis is usually treated in the outpatient clinics.
Material and Methods
This retrospective study used the National Information System for Hospital Data (Conjunto Mínimo Básico de Datos; CMBD) maintained by the Ministry of Health. It provides a complete record of all hospitalizations and is not subject to the limitations of outpatient surveillance systems, such as underdiagnosis or deficiencies in reporting. This system uses clinical codes for the Spanish version of the 9th International Classification of Diseases (Modificación Clínica Clasificación Internacional de Enfermedades; CIE-9-MC) and covers ∼98% of the public hospitals in Spain. Compulsory health insurance covers an estimated 99.5% of the Spanish population, although persons not covered by health insurance can receive treatment in public hospitals.10–12 This database has been shown to be a reliable tool for estimating cases of other illnesses that required hospitalization.13
All hospital discharges for leishmaniasis (ICD 9 CM 085.0-085.9; first diagnosis position) reported during a 12-year period (January 1, 1997 through December 31, 2008) were reviewed. For each entry, the following data were collected: age, sex, average length of hospitalization, diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD 9 CM]), and outcome. For patients with multiple entries to the hospitals, only the first episode of leishmaniasis was included in the study.
Statistical methods.
The average number of hospitalizations per year, annual incidence of hospital admissions (per 100,000 populations), average length of stay in the hospital, rate of death (per 100,000 populations), and case-fatality rate (%) were calculated. As a denominator, we used data on the population covered by the hospitals included in the CMBD information system, adjusted for population figures obtained from the Spanish municipal registers. It was assumed that the distribution by age of the population covered by these public hospitals was equal to the general population. Differences in proportions were assessed by the χ2 test and 95% confidence intervals (95% CI) were calculated. Student's t test was used to compare differences in means. We used two-sided tests and P < 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for Social Sciences (PASW for windows, version 17.0; Chicago, IL) and R (version 2.11.1).
The cost to the health care system of these hospitalizations was calculated by considering the diagnostic cost group, the total cost, and the number of discharges. The diagnostic cost group was based on the Diagnosis Related Groups (DRG) for hospitalized patient depending on discharge ICD classification, age, sex, and resources consumption. Each group has similar weight in hospital costs and can be applied to each related patient. The DRGs calculations were made with Core Grouping System Software (3M, St. Paul, MN).14
Results
The 6,585 hospitalizations events where leishmaniasis was included in any diagnostic position were found within the period of 1997–2008 (Table 1). These hospitalizations correspond to 3,745 individuals that were hospitalized at least once during the study period (1.76 admissions/patient). In 2,028 (54.1%) out of 3,745 subjects leishmaniasis was the principal diagnostic code. Among them, the majority was men (73%), on average 33.5 years of age, and one-third of them were HIV positive. More than 82% of the hospitalizations during the study period were related to visceral leishmaniasis and 68 (3%) of them died in the hospital.
Table 1.
Hospitalizations related to leishmaniasis in Spain (1997–2008)*
| Hospitalization, any diagnostic position | Incident hospitalization, any diagnostic position | Incident hospitalization, first diagnostic position | |||
|---|---|---|---|---|---|
| All | HIV-positive | HIV negative | |||
| n | 6,585 | 3,745 | 2,028 | 683 | 1,345 |
| Sex (male) | 5,290 (80%) | 2,894 (77%) | 1,479 (73%) | 579 (85%) | 900 (67%) |
| Age (SD) (years) | 36.85 (17.13) | 36.01 (19.09) | 33.46 (22.62) | 37.65 (8.19) | 31.32 (26.91) |
| HIV (%) | 4,356 (66%) | 2,097 (56%) | 683 (34%) | ||
| Hospitalization stay (days) | 15.98 (17.18) | 19.93 (18.57) | 17.25 (13.72) | 18.80 (15.30) | 16.47 (12.78) |
| Exitus | 405 (6%) | 250 (7%) | 68 (3%) | 24 (4%) | 44 (3%) |
| 085.0 Leishmaniasis visceral (kala-azar) | 5,347 (81%) | 2,896 (77%) | 1,667 (82%) | 585 (86%) | 1,082 (80%) |
| 085.1 Cutaneous leishmaniasis urban | 58 (1%) | 40 (1%) | 18 (1%) | 4 (1%) | 14 (1%) |
| 085.2 Cutaneous leishmaniasis asian desert | 6 (0%) | 5 (0%) | 1 (0%) | 0 (0%) | 1 (0%) |
| 085.3 Cutaneous leishmaniasis ethiopian | 8 (0%) | 4 (0%) | 2 (0%) | 1 (0%) | 1 (0%) |
| 085.4 Cutaneous leishmaniasis american | 20 (0%) | 16 (0%) | 12 (1%) | 2 (0%) | 10 (1%) |
| 085.5 Mucocutaneous leishmaniasis (american) | 79 (1%) | 49 (1%) | 34 (2%) | 4 (1%) | 30 (2%) |
| 085.9 Leishmaniasis unspecified | 1,067 (16%) | 735 (20%) | 294 (14%) | 87 (13%) | 207 (15%) |
HIV = human immunodeficiency virus.
Among the 2,028 individuals hospitalized because of leishmaniasis, as indicated by the principal diagnostic code, 683 (37%) were HIV positive. HIV-positive patients were significantly older (mean age: 37.65 years; SD: 8.19) than those HIV negative (N = 1,345; mean age 31.32 years; SD: 26.91, t = −7.93; P < 0.001). Gender distribution was also significantly different among the two groups: HIV positives (85% male) versus HIV negatives (67%), χ2 = 72.27, P < 0.001. The number of hospitalization days was found also higher in the HIV-positive group (18.8 days [SD: 15.3] versus 16.5 days [SD: 12.8], t = −3.42, P < 0.001).
Although the proportion of patients with visceral leishmaniasis was slightly higher among the HIV positives, there were no differences in mortality associated with the first episode of leishmaniasis adjusted for age (HIV positives = 24, HIV negatives 44, χ2MH = 1.24, P = 0.26).
Table 2 shows the development of the hospitalization, case-fatality, and mortality rate over the 12-year study period for the first hospitalization event caused by leishmaniasis, as identified by the first diagnostic position (incident hospitalized leishmaniasis). During the entire study period the hospitalization average was 0.41/100,000 inhabitants (95% CI [0.39; 0.42]). There was no significant increase in trend for first incident hospitalization caused by leishmaniasis over the study period (χ2 = 3.0529, P = 0.08059).
Table 2.
Epidemiology of first attack of hospitalized leishmaniasis in Spain (1997–2008)*
| Year | Population of Spain | No. cases† | Hospitalization rate (×100,000) | 95% CI | Case-fatality rate (%) | 95% CI | Mortality rate (×100,000) | 95% CI | Re-admission rate |
|---|---|---|---|---|---|---|---|---|---|
| 1997 | 38,734,929 | 161 | 0.42 | 0.351; 0.48 | 3.11 | 0.43; 5.79 | 0.01 | 0.002; 0.024 | 1.17 |
| 1998 | 39,055,598 | 117 | 0.3 | 0.245; 0.354 | 2.56 | −0.30; 5.43 | 0.01 | −0.001; 0.016 | 1.09 |
| 1999 | 39,398,117 | 111 | 0.28 | 0.229; 0.334 | 4.50 | 0.65; 8.36 | 0.01 | 0.002; 0.024 | 1.19 |
| 2000 | 39,689,795 | 151 | 0.38 | 0.32; 0.441 | 3.97 | 0.86; 7.09 | 0.02 | 0.003; 0.027 | 1.21 |
| 2001 | 40,294,505 | 202 | 0.5 | 0.432; 0.57 | 3.96 | 1.27; 6.65 | 0.02 | 0.006; 0.034 | 1.30 |
| 2002 | 41,001,136 | 177 | 0.43 | 0.368; 0.495 | 4.52 | 1.46; 6.65 | 0.02 | 0.006; 0.033 | 1.36 |
| 2003 | 41,862,723 | 191 | 0.46 | 0.392; 0.521 | 4.19 | 1.35; 7.03 | 0.02 | 0.006 ; 0.032 | 1.48 |
| 2004 | 42,333,730 | 204 | 0.48 | 0.416; 0.548 | 2.94 | 0.62; 5.26 | 0.01 | 0.003; 0.026 | 1.29 |
| 2005 | 43,226,359 | 182 | 0.42 | 0.36; 0.482 | 1.65 | −0.20; 3.50 | 0.01 | −0.001; 0.015 | 1.40 |
| 2006 | 43,814,785 | 192 | 0.44 | 0.376; 0.5 | 2.60 | 0.35; 4.86 | 0.01 | 0.001; 0.021 | 1.47 |
| 2007 | 44,296,722 | 203 | 0.46 | 0.395; 0.521 | 3.94 | 1.26; 6.62 | 0.02 | 0.006; 0.031 | 1.47 |
| 2008 | 45,234,666 | 137 | 0.3 | 0.252; 0.354 | 2.19 | −0.26; 4.64 | 0.01 | −0.001; 0.014 | 1.64 |
| TOTAL | 498,943,065 | 2,028 | 0.41 | 0.389; 0.424 | 3.35 | 2.57; 4.14 | 0.01 | 0.01; 0.017 | 1.35 |
95% CI = 95% confidence interval.
Cases: number of individuals hospitalized with leishmaniasis in the first diagnostic position for the first time during the study period (incident hospitalization).
The incidence of hospitalizations caused by leishmaniasis by age group and HIV coinfection condition is shown in Figure 1. In the adult population, the incidence of hospitalization increased with age, peaked at 35–39 years of age with 0.72/100,000 inhabitants (95% CI, 0.64; 0.80) and subsequently declined. The distribution of hospitalizations in HIV-positive patients was different from HIV-negative patients. The peak in hospitalizations caused by leishmaniasis at middle age could only be observed in HIV-positive patients, although HIV-negative patients showed a modest and linear increase in the hospitalization rate with age. In the HIV-negative patients, one-third of all leishmaniasis cases occurred in children < 10 years of age, getting the highest incidence in children up to 5 years of age: 1.90 (CI 95%, 1.73; 2.08)/100,000. On the other hand, 90% of leishmaniasis cases in HIV-positive patients occurred in the 25–49 years of age group. None of the cases of leishmaniasis found in patients younger than 16 years f age were co-infected with HIV, although the overall hospitalization rate associated with HIV in that age group was 5.52/100.000 inhabitants. This pattern was independent of the diagnostic position occupied by leishmaniasis to define hospitalizations (results not shown).
Figure 1.
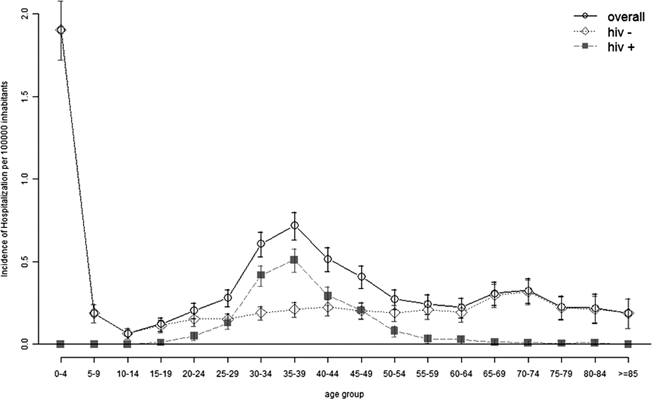
Incidence of hospitalizations related to leishmaniasis by age and human immunodeficiency virus (HIV) status in Spain (1997–2008).
The geographical distribution of hospitalizations of patients with leishmaniasis in the first diagnostic position during the 12-year study period is represented in Figure 2, showing that the highest hospitalization rates occurred in Madrid, Valencia, and the Balearic Islands. An increase in the number of hospitalizations can be observed when comparing the north-west to the south-east part of the country. When gathering the HIV infection status, the distribution for HIV-negative hospitalizations with leishmaniasis remains similar. When only HIV-positive patients are considered, the geographical distribution is more homogeneous.
Figure 2.
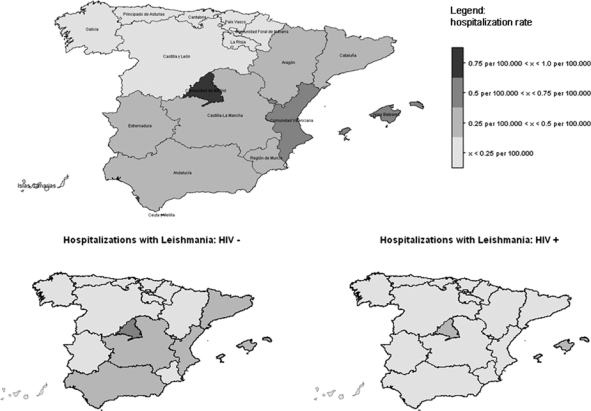
Incidence of hospitalizations related to leishmaniasis by autonomous region and human immunodeficiency virus (HIV) status in Spain (1997–2008).
First incident leishmaniasis was responsible for 34,990 days of hospitalization in public hospitals during 1997–2008. During the 12-year period, the average cost/inpatient hospital care was $9,601.01. Estimated total cost of these hospitalizations was $19,469,829, which corresponds to an annual cost of leishmaniasis to the national health system of more than € 1.1 million.
Discussion
The first hospitalization rate for leishmaniasis was 0.41 cases/100,000 of the total population of Spain. The case fatality rate was 3.3%, with no significant trend during the 12-year period. These figures are in line with previously published data.3,15 The HIV infection has changed leishmaniasis epidemiology.3,4,16 Meanwhile, in HIV-negative patients the highest incidence of new cases requiring hospitalization was found in children < 5 years of age, no cases in HIV-positive patients occurred in children up to 16 years of age. The HIV infection was associated with an increase in the number of cases in the young adult group, being all the new cases in the 17–65 years of age group, with the majority in the 25–49 years of age group.
The prevalence of HIV-positive patients in the general hospitalized population in Spain during this study period was 0.58%.17 The prevalence of HIV-positive patients among the 2,028 patients hospitalized with leishmaniasis indicated as principal diagnostic code was 37%. The figures in our study are a clear indication of the higher susceptibility of HIV-positive patients to get infected with and develop leishmaniasis. The high association of Leishmania with HIV has been explained with two non-exclusive approaches, immunosupression as a factor activating latent infections,18 and transmission of Leishmania parasites among parenteral drugs users.19 Interestingly, in our analysis we did not find evidence for different mortality among HIV-positive and HIV-negative patients during the prime attack. This result can probably be explained by the restoration of the CD4 count thanks to HAART, which permits a long survival of the co-infected patients.
Clinical leishmaniasis is frequently reported in the whole country, excluding the northwest. Traditionally, prevalence was higher in the Madrid region, the Balearic Islands, and the Mediterranean coast.15 As shown in Figure 2, our results among immunocompetent hospitalized leishmaniasis patients are consistent with this previously reported distribution. The co-infected group largely contributed to the overall burden of hospitalizations. The higher incidence of hospitalizations found in Madrid can be explained by the high endemicity of Leishmania combined with the higher prevalence of HIV compared with rural areas. Unfortunately, the CMBD does not contain information on the geographical origin of the patients. The codes 085.1 to 085.5 have a higher chance to reflect imported cases, but because their combined prevalence was lower than 3%, these cases did not affect the pattern of the geographical distribution of leishmaniasis in Spain in the study period. Our results differ substantially from the 209 cases reported by the Spanish decentralized surveillance system (EDO) in the weekly epidemiological reports for the study period (http://www.isciii.es/htdocs/centros/epidemiologia/boletin_semanal/). Considering that 1,574 cases were reported between 1982 and 1995 before the national surveillance system was decentralized, the existent surveillance system is not informed about the real burden of leishmaniasis in Spain. We postulate that the CMBD, including only hospitalized cases of leishmaniasis, gives a more realistic estimate of the burden of the more severe cases.
As mentioned, HIV/Leishmania co-infected patients previously showed lower cure trend, higher relapse rates, and higher mortality than those with leishmaniasis but immunocompetent.4
The homogenous distribution of co-infected cases in our study is an expression of the opportunistic behavior of the parasite that is circulating quite frequently in the Mediterranean region and not causing symptomatic leishmaniasis as shown in several epidemiological studies using delayed immune skin test, with prevalences of 15% in Tuscany, Italy,20 36% in the French Alps Maritimes,21 or 46% in the Alpujarras, south of Granada in Spain.22 The high proportion of asymptomatic carriers in endemic zones in Spain should be taken into account in blood donation for potential parasite transmission, and in immunocompromised people, whose asymptomatic carriage can evolve to clinical visceral leishmaniasis. Concerning the HIV seropositive population, it generally occurs in the advanced stages of the HIV disease. Immunocompromised asymptomatic carriers are at high risk of developing clinical visceral leishmaniasis. Screening of this population for parasite presence should be highly recommended.
Interestingly, the average length of hospitalization between HIV-positive and -negative patients, although significant, differed only by 2.3 days. This could be explained by the relatively good health condition of HIV patients because of HAART favoring a good response to leishmaniasis treatment but also because of an increased prescription of Amphotericin B treatment with less side effects instead of toxic antimonials.
No specific public health program for leishmaniasis control is implemented in Spain. The most immediate prevention method is protection against sand fly bites by using repellents, appropriate clothes, and using mosquito nets in endemic areas. Furthermore, in areas where canine reservoirs present a high prevalence of infection, dogs should be controlled, treated when infected, and in some cases avoided as pets.23
The economical impact of leishmaniasis to the health system is almost unknown worldwide. In French Guyana, cutaneous leishmaniasis represented 0.13% of the general budget and 0.43% of the global budget of the French Guiana Social Security in 1997.24 The low endemicity of leishmaniasis in the Mediterranean countries may have led to underestimate the cost to the health system. According to our study, it represents a cost in Spain € 1.1 million/year for the inpatient setting alone. In addition, canine leishmaniasis is highly prevalent in Spain with more than 10% seroprevalence in many areas, implying a high infection risk for humans, animal suffering, and significant costs to the owners.23
There are some important aspects that need to be considered when interpreting the findings from this study. Our study only included cases of leishmaniasis requiring hospitalization, which in all cases corresponded to subjects with visceral leishmaniasis and is not equivalent to the true incidence in the population. Subjects with cutaneous leishmaniasis caused by Leishmania infantum becoming ulcered are usually treated intralesionally with antimonials in dermatological clinics and rarely require hospitalization. Therefore, it can reasonably be assumed that unspecified leishmaniasis is referring to visceral leishmaniasis, which accounts in our study for more than 82% of the hospitalizations and 14% of unspecified leishmaniasis.
Another limitation concerns the economic analyses. Our economic analysis only covers the costs of the initial incident hospitalization using DRG. As such the real burden of leishmaniasis is higher because of recurring hospital visits, on average 1.76/individual, outpatient treatment of cutaneous leishmaniasis, and other indirect costs could not be considered.
Nonetheless, hospital discharge databases provide a complete record of all hospitalizations and are a sensitive measure of the impact and costly end of a disease. In general, these data are not subject to underdiagnosis and deficiencies in reporting that can limit outpatient surveillance systems. The reliability of the CMBD depends on the quality of the discharge report and the clinical history, and the variable codification process.25 Unfortunately, it was impossible to compare our results with those previous to HAART introduction in Spain, because no data were available for the time period before 1997.
Although the epidemiology of leishmaniasis in Spain was relatively well known before and after the apparition of acquired immunodeficiency syndrome (AIDS) in the eighties, thanks to numerous patched studies, this is one of the few studies in which the prevalence estimates have been done based on reliable information comprising the whole country for more than a decade. Moreover, it has allowed estimating the economical impact of the disease to the health system. A holistic consideration of the burden of leishmaniasis is required by the Spanish health authorities not only taking into account the dog as the reservoir host involved in the transmission to humans, but also as a matter of social reputation and international esteem.
ACKNOWLEDGMENTS
We thank the Subdirección General del Instituto de Información Sanitaria for providing the information used as the basis for this study.
Footnotes
Authors' addresses: Ruth Gil-Prieto and Angel Gil de Miguel, Department of Preventive Medicine and Public Health and Medical Immunology and Microbiology, Offices 217 and 202, Rey Juan Carlos University, Madrid, Spain, E-mails: ruth.gil@urjc.es and angel.gil@urjc.es. Stefan Walter, Department of Public Health, Erasmus MC, Office 2187, Rotterdam, The Netherlands and Department of Epidemiology, Erasmus MC, Rotterdam, The Netherlands, E-mail: s.walter@erasmusmc.nl. Jorge Alvar, Leishmaniasis Control Program, Department of Neglected Tropical Diseases, Office L344, World Health Organization, Geneva 27, Switzerland, E-mail: alvarj@who.int.
References
- 1.WHO Leishmaniasis. 2010. http://www.who.int/topics/leishmaniasis/en/ Available at. Accessed July 25, 2010.
- 2.Alvar J. Leishmaniasis: from boilogy to control. Second edition. Salamanca: Laboratorios Intervet S.A; 2001. [Google Scholar]
- 3.Desjeux P, Alvar J. Leishmania/HIV co-infections: epidemiology in Europe. Ann Trop Med Parasitol. 2003;97((Suppl 1)):3–15. doi: 10.1179/000349803225002499. [DOI] [PubMed] [Google Scholar]
- 4.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortajada C, Pérez-Cuevas B, Moreno A, Martínez E, Mallolas J, García F, Valls E, Miró JM, De Lazzari E, Gatell JM. Highly active antiretroviral therapy (HAART) modifies the incidence and outcome of visceral leishmaniasis in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:364–366. doi: 10.1097/00126334-200207010-00015. [DOI] [PubMed] [Google Scholar]
- 6.López-Vélez R, Casado JL, Pintado V. Decline of a visceral leishmaniasis epidemic in HIV-infected patients after the introduction of highly active antiretroviral therapy (HAART) Clin Microbiol Infect. 2001;7:394–395. doi: 10.1046/j.1198-743x.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 7.Mira JA, Corzo JE, Rivero A, Macias J, De Leon FL, Torre-Cisneros J, Gomez-Mateos J, Jurado R, Pineda JA. Frequency of visceral leishmaniasis relapses in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. Am J Trop Med Hyg. 2004;70:298–301. [PubMed] [Google Scholar]
- 8.World Health Assembly Control of leishmaniasis. 60th World Health Assembly, 9th plenary meeting, 21 May 2007. 2007. http://apps.who.int/gb/ebwha/pdf_files/WHA60/A60_R13-en.pdf Available at. Accessed April 11, 2011.
- 9.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2:e313. doi: 10.1371/journal.pntd.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministerio de Sanidad y Consumo International Classification of Diseases 9th revision Clinical Modification. 2010. http://www.msc.es/estadEstudios/estadisticas/normalizacion/clasifEnferm/instrucNorma.htm Available at. Accessed March 22, 2011.
- 11.National Health Insurance . Deputy Directors Office of Administrative Coordination. National Information System for Hospital Data (CMBD) INSALUD hospitals 2001. Madrid, Spain: 2002. www.ingesa.msc.es/ Available at. Accessed March 22, 2011. [Google Scholar]
- 12.Rivero Cuadrado A. (The minimum data set in the NHS: early and ongoing developments).Rev Fuentes Estadísticas. 2000;49:18–19. http://www.webcitation.org/query.php?url=http://www.fuentesestadisticas.com/Numero49/Paginas/18-19.htm&refdoi=10.1186/1471-2458-8-109 Available at. Accessed January 20, 2010. [Google Scholar]
- 13.Gil Prieto R, San Román Montero J, Gómez Alejandre C, Alvaro Meca LA, Rivero A, Gil de Miguel A. Epidemiology of pneumococcal meningitis hospitalizations in pediatric population in Spain (1998–2006) Vaccine. 2009;27:2669–2673. doi: 10.1016/j.vaccine.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 14.Schreyögg J, Stargardt T, Tiemann O, Busse R. Methods to determine reimbursement rates for diagnosis related groups (DRG): a comparison of nine European countries. Health Care Manage Sci. 2006;9:215–223. doi: 10.1007/s10729-006-9040-1. [DOI] [PubMed] [Google Scholar]
- 15.Valcárcel Y, Bastero R, Anegón M, González S, Gil A. The epidemiology of hospital admissions due to leishmaniasis in Spain (1999–2003) Enferm Infecc Microbiol Clin. 2008;26:278–281. doi: 10.1157/13120414. [DOI] [PubMed] [Google Scholar]
- 16.Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CMBD CMBD date. 2010. http://www.msc.es/en/estadEstudios/estadisticas/cmbdhome.htm Available at. Accessed May 30, 2010.
- 18.Kubar J, Marty P, Lelièvre A, Quaranta JF, Staccini P, Caroli-Bosc C, Le Fichoux Y. Visceral leishmaniasis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS. 1998;12:2147–2153. doi: 10.1097/00002030-199816000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Cruz I, Morales MA, Noguer I, Rodríguez A, Alvar J. Leishmania in discarded syringes from intravenous drug users. Lancet. 2002;359:1124–1125. doi: 10.1016/s0140-6736(02)08160-6. [DOI] [PubMed] [Google Scholar]
- 20.Bettini S, Gramiccia M, Gradoni L, Pozio E, Mugnai S, Maroli M. Leishmaniasis in Tuscany (Italy). VIII. Human population response to leishmanin in the focus of Monte Argentario (Grosseto) and epidemiological evaluation. Ann Parasitol Hum Comp. 1983;58:539–547. doi: 10.1051/parasite/1983586539. [DOI] [PubMed] [Google Scholar]
- 21.Marty P, Le Fichoux Y, Giordana D, Brugnetti A. Leishmanin reaction in the human population of a highly endemic focus of canine leishmaniasis in Alpes-Maritimes, France. Trans R Soc Trop Med Hyg. 1992;86:249–259. doi: 10.1016/0035-9203(92)90295-n. [DOI] [PubMed] [Google Scholar]
- 22.Acedo Sánchez C, Martín Sánchez J, Vélez Bernal ID, Sanchís Marín MC, Louassini M, Maldonado JA, Morillas Márquez F. Leishmaniasis eco-epidemiology in the Alpujarra region (Granada Province, southern Spain) Int J Parasitol. 1996;26:303–310. doi: 10.1016/0020-7519(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 23.Alvar J, Cañavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Adv Parasitol. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- 24.Dedet JP, Pillot B, Gentilini M. Evaluation of the socioeconomic costs of cutaneous leishmaniasis in French Guiana. Rev Epidemiol Sante Publique. 1991;39:129–133. [PubMed] [Google Scholar]
- 25.Peiro S, Librero J. The quality assessment from the minimum basic hospital discharge data set. Rev Neurol. 1999;29:651–661. [PubMed] [Google Scholar]


