Abstract
Leishmaniases are a group of vector-borne diseases with different clinical manifestations caused by parasites transmitted by sand fly vectors. In Mexico, the sand fly Lutzomyia olmeca olmeca is the only vector proven to transmit the parasite Leishmania mexicana to humans, which causes leishmaniasis. Other vector species with potential medical importance have been obtained, but their geographic distributions and relation to transmission areas have never been assessed. We modeled the ecological niches of nine sand fly species and projected niches to estimate potential distributions by using known occurrences, environmental coverages, and the algorithms GARP and Maxent. All vector species were distributed in areas with known recurrent transmission, except for Lu. diabolica, which appeared to be related only to areas of occasional transmission in northern Mexico. The distribution of Lu. o. olmeca does not overlap with all reported cutaneous leishmaniasis cases, suggesting that Lu. cruciata and Lu. shannoni are likely also involved as primary vectors in those areas. Our study provides useful information of potential risk areas of leishmaniasis transmission in Mexico.
Introduction
Studies of ecological and geographic patterns of occurrence of vector-borne diseases are relevant for understanding modes of parasite transmission.1 Recent research in ecological niche modeling, integrating point occurrence data with digital environmental maps, provides a useful and powerful framework for understanding the eco-epidemiologic geography of zoonotic diseases because their transmission cycles involve different sylvatic (enzootic) and domestic reservoir and vector species, each responding to environmental variation, according to its own ecological niche.2–6 The ecological niche is defined as the set of environmental conditions in which species can maintain populations without immigrational subsidy, and provides a framework for testing hypotheses regarding roles of environmental variables in shaping distributional patterns of species and evaluating how different species' ecological niches relate to one another.3,7
An interesting and complex disease system from the point of view of the variety of species involved in the transmission cycle and public health relevance are the leishmaniases, a group of diseases with different clinical manifestations, caused by parasites transmitted by sand fly vectors (Diptera: Psychodidae: Phlebotominae) among mammal reservoir hosts. Vectors and reservoir hosts may differ depending on the geographic region.8–11
In the New World, insect vectors known to be responsible for Leishmania transmission to humans belong to the genus Lutzomyia. Although the taxonomy of sand flies has seen major changes in nomenclature after the proposal of Galati,12 throughout this report we use the name Lutzomyia (sensu) in a more conservative sense.12,13 Most sand fly species are only classified as suspected leishmaniasis vectors because ultimate proof of involvement is rather difficult to achieve.9,14,15
In Mexico, at least for cutaneous leishmaniasis (CL), only one taxon, Lutzomyia olmeca olmeca, has been incriminated as a vector of this disease. Other species, such as Lu. cruciata, Lu. shannoni, Lu. panamensis, and Lu. ylephiletor, have recently been found infected with Leishmania mexicana in Campeche.16,17 Previous studies in Campeche documented Lu. o. olmeca as infected with Leishmania; Lu. cruciata was also found infected with similar parasites, although the particular Leishmania species could not be identified.18 Lutzomyia cruciata is a suspected leishmaniasis vector in Nicaragua and Belize, and its abundances at some collecting sites in the Yucatan Peninsula exceed those of the known vector species.19–23 Other species that feed on humans and that have been found infected with Leishmania in Central America include Lu. panamensis, Lu. shannoni, and Lu. ylephiletor.10,24 Lutzomyia gomezi has been found infected with Leishmania panamensis in Panama.25,26 In northern Mexico and the southern United States, sand fly species suspected of being involved in parasite transmission to humans are Lu. diabolica and Lu. anthophora.27,28 Lutzomyia diabiolica is suspected of being a vector of L. mexicana and has been infected experimentally with L. infantum, and Lu. anthophora was able to transmit L. mexicana experimentally to mice.29–31
Visceral leishmaniasis (VL) is caused by L. infantum (L. chagasi) and is transmitted in the New World to humans by Lu. longipalpis and Lu. evansi, which alternate their relevance as vectors depending on ecological characteristics of transmission focus.32 Lutzomyia longipalpis is generally considered the principal vector of L. infantum and is known to comprise a species complex distributed in the Neotropics from Mexico to Argentina.33–36 Lutzomyia evansi is distributed from Mexico to Colombia and Venezuela.26,34,37–40 In some localities in Colombia, this species acts as the main vector,37 and in Costa Rica it is dominant when Lu. longipalpis shows low abundance.38
In Mexico, the first clinically documented records of CL were from forested parts of the Yucatan Peninsula.41,42 Currently, although cases are not reported by public health authorities, they are nonetheless a significant public health problem in the region. Cases of VL have been reported from the states of Chiapas, Puebla, Guerrero, Oaxaca, Morelos, and Tabasco,41,42 and several foci in the state of Chiapas and the Balsas River Basin are found in tropical dry forest areas (Becker I, Laboratorio de Inmunoparasitología, Universidad Nacional Autónoma de Mexico and Ramsey J, Centro Regional de Investigación en Salud Pública, unpublished data).
Thus, in Mexico, knowing the ecology and geography of vector species involved in Leishmania transmission is of great relevance to public health because entomologic surveillance is one of the most important intervention strategies of vector-borne diseases. The official policy of the Mexican government public health agency regarding entomologic surveillance is Norma Oficial Mexicana sobre la Vigilancia Epidemiológica, Prevención y Control de las Enfermedades Transmitidas por Vector (PROY-NOM 032-SSA2-2000).
The aim of this study was to estimate likely distributions of all sand fly species with potential medical importance for leishmaniasis transmission in Mexico and to relate these distributions to known transmission areas. This comparison will enable assessment of whether current knowledge of vector diversity is likely to be complete. By this means, we identified vector species likely to be involved in Leishmania transmission cycles. Because leishmaniasis cases in Mexico have not been followed-up with research on local transmission, information regarding leishmaniasis etiology is scarce. Distributional maps on a national scale will facilitate understanding of the relative roles of different sand fly vector species. The ultimate aim is to identify high-risk areas and use this information for designing control and prevention strategies.
Methods
Eleven sand fly species (Lutzomyia) of at least potential medical importance are found in Mexico. These species were selected based primarily on their vector status and public health relevance in nearby countries, and also on the findings obtained by different authors in recent years in Mexico. For CL, we included nine species: the proven vector Lu. o. olmeca and eight suspected vectors: Lu. cruciata, Lu. shannoni, Lu. ylephiletor, Lu. gomezi, Lu. diabolica, Lu. ovallesi, Lu. panamensis, and Lu. anthophora.10,16,20,22,24,26,28,30,43–48 For VL, we included the two known vectors, Lu. longipalpis and Lu. evansi.33,40
A database summarizing known occurrences for these species in Mexico was assembled from a variety of sources, such as scientific publications,49–53 records of the entomologic collections of the Instituto de Diagnóstico y Referencia Epidemiológicos54 and the Universidad Autónoma de Yucatán, and personal communications with Dr. Oscar Velasco-Castrejón (Departamento de Medicina Experimental, Universidad Nacional Autónoma de Mexico). Geographic coordinates were added to each record on the basis of the Instituto Nacional de Estadística y Geografía 2000 locality database (www.inegi.gob.mx), Biogeomancer (www.biogeomancer.org), and Falling Rain Genomics, Inc. (Palo Alto, CA) (www.fallingrain.com).55
Ecological niche modeling.
We modeled ecological niches and estimated potential geographic distributions for each species by using the computer algorithms Genetic Algorithm for Rule-Set Prediction (GARP) and Maxent.56,57 We decided to use both algorithms because the two methods have been widely used, although sometimes showing contrasts in performance:58–60 GARP often overpredicts potential distributions of species somewhat, and Maxent may overfit models and underpredict.57,59 Also, obtaining predictions using different methodologic approaches generally produces more reliable results than under a single approach.61
For those species with ≥ 13 available records, we divided occurrence points randomly into two pools: training data (80%) and testing data (20%). Models were built using training data, reserving the testing data for model evaluation; species with < 13 records were modeled using all available points as training data. Using GARP, we developed 100 replicate models for each species; the best subset was chosen considering an extrinsic omission measure for species with a test dataset and intrinsic omission measure for the rest, and soft omission threshold (20% of distribution) for all species. Models generated using Maxent were run choosing logistic output.62
For all model outputs, we then used a threshold for presence that was set such that 90% of all occurrences on which the model was based were included in the predicted area (i.e., E = 10%, in the terminology of Peterson8), and remaining areas were classed as absent. The areal extent of distributional areas was calculated in a geographic information sytem based on the number of pixels predicted as suitable.6 Testing data were overlaid on the binary outputs to evaluate which of the three output models (GARP, Maxent, and coincident areas between them) better fit to known vector distributions. We used a binomial test to evaluate statistical significance of predictions by comparing observed successes and failures with random expectations.63
WorldClim climatic coverages used for modeling were chosen to minimize inter-variable correlations measured on the basis of 5,000 random points in Mexico.64 We generated a correlation matrix in JMP 6.0 among all variables in the WorldClim dataset version 1.4 (approximately 1 km resolution; www.worldclim.org), and retained variables that were relatively uncorrelated (r < 0.75). The nine environmental variables chosen to build the models were mean diurnal range, isothermality, maximum temperature of warmest month, temperature annual range, mean temperature of wettest quarter, mean temperature of driest quarter, precipitation seasonality, precipitation of warmest quarter, and precipitation of coldest quarter from the WorldClim database. We also included slope, aspect, and topographic index from the Hydro-1K dataset.65,66
Areas of public health importance.
To define important areas for leishmaniasis transmission in Mexico, we used the states historically known to report cases of leishmaniasis, clipped with the Mexican ecoregions, to set geographic limits with ecological meaning.67 Two transmission levels were set such that states with recurrent transmission had annual records for at least 15 consecutive years and states with occasional transmission had sporadic records obtained from the Sistema Único de Información para la Vigilancia Epidemiológica, Dirección General de Epidemiología, Secretaría de Salud. Predicted distributions for vector species were overlapped with the map produced depicting the areas of transmission to estimate the percentage of the distribution of each vector in recurrent and occasional transmission areas (Table 1).
Table 1.
Percentage of Lutzomyia species potential distributions related to transmission level and land use coverage, Mexico
| Species | Relation to transmission areas | Relation to land cover | Eco-epidemiologic traits | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Occasional % | Recurrent % | Human settlements % | Crops and pastures % | Forested areas % | Infected | No. infected/no. collected (%), reference | Abundance | Vector ability | |
| Lu. cruciata | 17.35 | 60.41 | 0.58 | 39.45 | 59.96 | Yes | 1/128 (0.8)17 6/6 (100)65 | High | Likely |
| Lu. diabolica | 25.95 | 12.60 | 0.77 | 34.38 | 64.84 | No | Suspected | ||
| Lu. gomezi | 9.98 | 75.91 | 0.64 | 31.26 | 68.1 | No | Suspected | ||
| Lu. longipalpis | 44.40 | 26.65 | 0.66 | 39.02 | 60.32 | No | Suspected | ||
| Lu. olmeca | 8.31 | 81.84 | 0.57 | 37.84 | 61.6 | Yes | 1/453 (0.2) to 11/144 (7.6)17 6/38 (15.7)65 | High | Confirmed |
| Lu. ovallesi | 18.27 | 70.44 | 0.73 | 38.73 | 60.54 | No | Suspected | ||
| Lu. panamensis | 6.57 | 91.08 | 0.38 | 24.18 | 75.44 | Yes | 2/46 (4.3)17 | High | Likely |
| Lu. shannoni | 16.46 | 66.24 | 0.57 | 39.27 | 60.16 | Yes | 3/56 (5.4) to 8/53 (15.1)17 2/7 (28.5)65 | High | Likely |
| Lu. ylephiletor | 14.49 | 62.42 | 1.03 | 32.46 | 66.51 | Yes | 1/8 (12.5)17 | Low | Likely |
Land use coverage.
We used a land use map (www.conabio.gob.mx) to assess the presence of suitable forested areas for sand fly breeding sites and evaluated the percent of species' potential distribution in each of three land coverage types: human settlements, agriculture (including crops and pastures), and forested areas. In their sylvatic cycle, sand flies require humid and shadowed substrates provided by forested areas in which they lay eggs to ensure larval survival and development. This coarse-resolution land use map included only a broad classification of agriculture that did not enable identifying shadowed crops potentially providing similar suitable ecological conditions for sand flies; we considered crops and pasture as transformed areas.
Results
In all, 172 presence records for Leishmania vector species in Mexico were assembled (Figures 1 and 2). Not surprisingly, we found that sampling of sand flies was focused in the southern parts of the country. Among states, sampling was most intense in Campeche (n = 86), Oaxaca (n = 47), and Quintana Roo (n = 40). In contrast, only single sampling localities were available for the states of Coahuila, Chihuahua, and Tamaulipas. The species most commonly obtained were Lu. cruciata (n = 102) and Lu. shannoni (n = 61), and only few records were available for Lu. anthophora (n = 2), Lu. ylephiletor (n = 3), Lu. gomezi (n = 3), and Lu. evansi (n = 1). Lutzomyia o. olmeca, the only demonstrated CL vector in Mexico, has been obtained at 29 localities, all in the southern part of the country. Overall, species richness of potential vectors was highest in southeastern Mexico, including the states of Veracruz, Tabasco, Campeche, and Quintana Roo (Figures 1 and 2).
Figure 1.
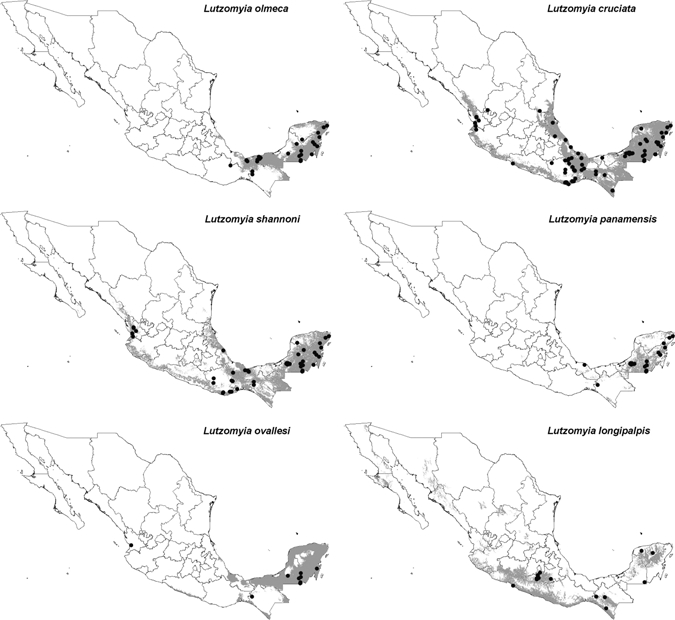
Ecological niche model-based distributional predictions for the species of Lutzomyia with known or potential medical importance in Mexico (gray). Black dots indicate species' known occurrence points. Non-significant models are not shown.
Figure 2.
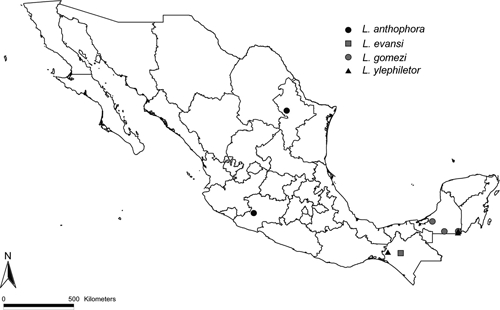
Known occurrence points for non-modeled Lutzomyia species, Mexico.
Although GARP models predicted larger areas of potential distribution for six of the seven species modeled, Maxent models performed slightly better in terms of independent testing (Table 2). In general, GARP models had statistically significant predictions for all species except for Lu. diabolica and Lu. longipalpis, and Maxent was not significant only for Lu. diabolica. The areas of predicted distribution generated by both algorithms were also highly coincident for all species and were generally the same as the area predicted only with Maxent (i.e., the Maxent prediction was a subset of the GARP prediction). On the basis of these results, we decided to use only outputs generated by Maxent as predicted areas of potential distribution.
Table 2.
Area predicted and statistical significance of models from GARP and Maxent, and their coincidence, Mexico*
| Algorithm | Lutzomyia species | Area predicted (km2) | P | N (training data) |
|---|---|---|---|---|
| GARP | Lu. cruciata | 703,980 | 6.04 × 10−6 | 82 |
| Lu. diabolica | 669,718 | 0.331 | 11 | |
| Lu. longipalpis | 300,985 | 0.079 | 14 | |
| Lu. olmeca | 183,971 | 7.27 × 10−5 | 25 | |
| Lu. shannoni | 660,361 | 2.38 × 10−5 | 50 | |
| Lu. panamensis | 203,306 | 0.002 | 26 | |
| Lu. cruciata | 314,851 | 1.31 × 10−13 | 82 | |
| Maxent | Lu. diabolica | 511,085 | 0.025 | 11 |
| Lu. longipalpis | 171,022 | 0.001 | 14 | |
| Lu. olmeca | 123,742 | 1.30 × 10−7 | 25 | |
| Lu. shannoni | 253,205 | 4.10 × 10−8 | 50 | |
| Lu. panamensis | 53,263 | 1.58 × 10−7 | 26 | |
| Lu. cruciata | 290,770 | 9.19 ×10−10 | 82 | |
| Lu. diabolica | 277,042 | 0.406 | 11 | |
| Coincidence | Lu. longipalpis | 83,335 | 0.006 | 14 |
| Lu. olmeca | 98,055 | 3.26 × 10−6 | 25 | |
| Lu. shannoni | 218,944 | 9.79 ×10−9 | 50 | |
| Lu. panamensis | 37,096 | 0.006 | 26 |
Models were performed by using 80% of point data (training data). The remaining 20% of occurrence data were overlapped on the predicted area, and a binomial table of presence/absence was built. Statistical significance of predictions was assessed by comparing observed successes and failures with random expectations and using a binomial test.
The potential distribution of Lu. o. olmeca was restricted to Campeche, Quintana Roo, Tabasco, southern Veracruz, and some areas in Chiapas and Yucatan. Lutzomyia cruciata was the species with the largest predicted area of potential distribution, ranging across the Yucatan Peninsula and the states of Chiapas, Oaxaca, and Veracruz, and along the Pacific Coast north to Nayarit. Interestingly, Lu. shannoni showed a similar, although smaller, area of potential distribution compared with that of Lu. cruciata. The potential distribution of Lu. panamensis was restricted to the Yucatan Peninsula, southern Veracruz, and the Chontalpa region of Tabasco. Lutzomyia ovallesi had a small potential distributional area in Mexico restricted to Campeche and Quintana Roo (Figure 1). In northern Mexico, the most broadly distributed species was Lu. diabolica: its potential distribution reached the southern part of the United States, supporting the idea of its potential importance as a vector in Texas (Figure 1).29
The Yucatan Peninsula has areas of recurrent leishmaniasis transmission (Figure 3). In those areas, the sand flies Lu. olmeca, Lu. panamensis, and Lu. ovallesi had almost 80% of their Mexican distributional areas (Figure 1 and Table 1). Widely-distributed vector species, such as Lu. shannoni and Lu. cruciata, were present in all transmission areas, and were dominant in regions with recurrent transmission. In general, all vector species were distributed in areas with recurrent transmission, except for Lu. diabolica, which appeared related only to areas of occasional transmission, corresponding to the cases reported from northern Mexico (Table 1). Interestingly, areas of recurrent transmission are concentrated in moist forest ecoregions, and occasional transmission areas were mainly distributed in the tropical dry forests and Tamaulipan mezquital ecoregions (Figure 4). We observed that sand fly species occur mainly in forested areas, although at least one-third of their potential distributions overlapped with crops and pastures, probably reflecting the fragmented nature of Mexican forests; only minimal overlap of sand fly species potential distribution with human settlements was observed (Table 1).
Figure 3.
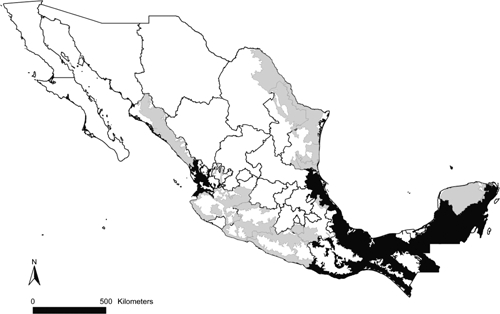
Leishmaniasis transmission areas in Mexico. Areas of recurrent transmission are in black, and areas of occasional transmission are in gray.
Figure 4.
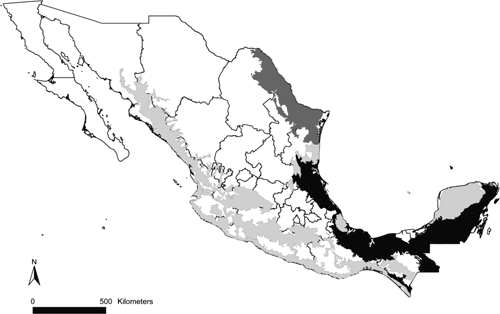
Ecoregions where leishmaniasis transmission is known to occur in Mexico. Moist forests are in black, Tamaulipan mezquital is in dark gray, and tropical deciduous forests are in light gray.
Discussion
Studies of geographic and ecological distributions of vectors and cases of diseases offer powerful tools for identifying risk zones and exploring potential interactions between hosts, vectors, and parasites.2,3 Our results provide a baseline against which to improve research on Leishmania and their vectors in Mexico. Because one of the criteria set by the World Health Organization for linking species to disease transmission is that vector and reservoir geographic distributions must coincide with human case distributions,68 our comparisons of modeled potential distributions enabled us to assess disease transmission in places where entomologic surveillance has scarcely been conducted.4
The distribution of the only proven CL vector in Mexico, Lu. olmeca, does not overlap all of the known CL cases in the country, suggesting that other sand fly species must be involved as vectors in those areas. On the basis of numbers of records and broad geographic distributions, we suggest that Lu. cruciata and Lu. shannoni would be the most suitable candidates: both are distributed broadly in southeastern Mexico and in the coastal lowlands. Lutzomyia cruciata, Lu. shannoni, Lu. panamensis, and Lu. ylephiletor have been found infected with Leishmania, although no studies on vector capacity of these species for disease transmission have been conducted in Mexico. The proportion of infected sand flies varied between species, places, and seasons (Table 1).17,69 Because Lu. shannoni occurs well north into the United States, incorporation of occurrences in the United States into next-generation models would be useful.70 In this regard, Leishamnia mexicana has also been found in the United States.71–74
Leishmaniasis transmission in the states of Campeche and Quintana Roo is related to areas of moist forest, suggesting that infection is occurring by human proximity to areas with original vegetation coverage.10,75 A curious variation occurs in the state of Yucatan, where case incidence is low compared with the rest of the Peninsula, although the known reservoirs are present. The proven vector Lu. olmeca has been reported in this state, although its abundance is low compared with other sand flies in the area (e.g., Lu. cruciata). It is possible that the number of human cases caused by Lu. o. olmeca is minimal.75
In Tabasco, most of the original vegetation coverage has been replaced, especially with cacao crops. In this region, Lu. o. olmeca has been identified as the primary vector, and clinical cases with a high prevalence of patients with diffuse CL have been recorded.76 Areas with homogenous land coverage, such as crops, offer a less complex habitat where only disturbance-tolerant species can survive. Vector species with medical importance have shown to have the ability to establish populations in transformed areas after human interventions.77 Our results showed a high proportion of potential distributions of species overlapping with transformed habitats, suggesting ample potential for domiciliation of the sand flies by shifting from a sylvatic to a peridomestic cycle. Clearly, landscape analyses along ecological gradients should be conducted to establish population dynamics of vectors and their impacts on parasite incidence and persistence.
In Nayarit, cases have been related to coffee plantations, and recent records of infected patients with Lu. nia mexicana in the adjacent states of Durango and Sinaloa suggest a wider geographic range of the disease or a recent spread of the disease northwest.78 Given their geographic distribution, vector species responsible for transmission in this region could include Lu. shannoni, Lu. diabolica, and Lu. cruciata.
Lutzomyia anthophora has been obtained only in northeastern Mexico in the states of Nuevo Leon and Tamaulipas. Given the low number of records for Lu. anthophora in Mexico, ecological niche models could not be developed in this study. However, models based on collection points in the United States predict potential distributional areas for this vector in the states of Sinaloa, Sonora, and Baja California Sur in Mexico.79 Lutzomyia anthophora is a non-anthropophilic feeder. Thus, although no human cases have been recorded in these states, sylvatic transmission may be occurring.72 The absence of an anthropophilic vector species provides a barrier for parasite transmission to humans. However, cases occurring along the Mexico–United States border area must be monitored carefully because other vector species can be incriminated in transmission of parasites to humans. For example, the spread of Lu. diabolica from the east would favor appearance of zoonotic transmission foci in the western states. Clearly, intensive monitoring of sand fly species along the Mexico–United States border is crucial for identifying potential risk areas of infection in the region, particularly in light of changing climates and shifting vector species distributions.79
Little is known about VL in Mexico. The known vector Lu. longipalpis is distributed in tropical deciduous forests corresponding to areas of VL transmission. No parasite isolations from either vectors or reservoirs have been reported, and no surveillance has been undertaken by government health authorities. However, cases are known to occur and are likely underestimated because of limited access of rural communities to health services. Spread of VL has been documented in South America as a result of socio-environmental factors such as deforestation and human and domestic dog migration.80,81 The appearance of new foci in South America involve Lu. longipalpis as the principal VL vector and domestic dogs as reservoirs, as was assessed in the 2006 VL outbreaks in Brazil, Paraguay, and Argentina.81 These new transmission trends are leading increasingly to VL urbanization. Thus, we consider initiation of research on VL in Mexico to be of paramount importance.
Although leishmaniasis distribution and incidence in Mexico is likely underestimated, the general picture is one of focal regions with recurrent transmission. In Quintana Roo and Tabasco, where most cases have been historically reported, cases are usually related to areas with changing land use, suggesting the possibility of a domiciliation process. It is critical to evaluate the course of disease transmission under dynamic scenarios related to land use or climatic change.79 More generally for leishmaniasis, given specific transmission areas in northern Mexico, the Balsas River Basin, the southeastern region, and the state of Nayarit, where particular species interactions seem to be occurring, we believe that different Leishmania strains are involved in different foci.
ACKNOWLEDGMENTS
We thank the Entomology Laboratory at the Instituto de Diagnóstico y Referencia Epidemiológica (H. Huerta, and O. Velasco) for providing sand fly collection data; the Geographic Positioning Sustem Unit at Centro Internacional de Agricultura Tropical, especially A. Jarvis and S. Castaño, for providing support with the geographic analyses; and two anonymous reviewers for helping to improve the clarity of the manuscript.
Footnotes
Financial support: This study was partially supported by Consejo Nacional de Ciencia y Tecnología Programa de (Project 80156) and the Universidad Nacional Autónoma de México (Investigación e Innovación Tecnológica project IN225408 to Víctor Sánchez-Cordero). Camila González was a recipient of a Dirección General de Estudios de Posgrado fellowship for the Posgrado en Ciencias Biomédicas, Facultad de Medicina, Universidad Nacional Autónoma de México.
Authors' addresses: Camila González, Centro de Ciencias de la Complejidad (C3), Universidad Nacional Autónoma de México, Torre de Ingeniería, Ciudad Universitaria, Mexico City 04510 DF, Mexico and Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Circuito Exterior, Edificio Nuevo, Módulo C, Apartdo, Postal 70-153, Mexico City DF 04510, Mexico, E-mail: c.gonzalez2592@uniades.edu.co. Eduardo A. Rebollar-Téllez, Laboratorio de Entomología Médica, Departamento de Zoología de Invertebrados, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Ave. Universidad s/n, Ciudad Universitaria, Apartado, Postal 109-F, San Nicolás de los Garza, Nuevo León C.P. 66451, Mexico, E-mail: eduardo.rebollart1@uanl.edu.mx. Sergio Ibáñez-Bernal, Instituto de Ecología, AC Red Ambiente y Sustentabilidad, Carretera Antigua a Coatepec No. 351, El Haya, Xalapa, 91070, Veracruz, México, E-mail: sergio.ibanez@inecol.edu.mx. Ingeborg Becker-Fauser, Laboratorio de Inmunoparasitología, Facultad de Medicina, Universidad Nacional Autónoma de México, Hospital General de México, Dr. Balmis 148, Mexico City 06726 DF, Mexico, E-mail: becker@servidor.unam.mx. Enrique Martínez-Meyer and Víctor Sánchez-Cordero, Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Circuito Exterior, Edificio Nuevo, Módulo C, Apartado, Postal 70-153, México City DF 04510, Mexico, E-mails: emm@ibiologia.unam.mx and victor@ibiologia.unam.mx. A. Townsend Peterson, Biodiversity Institute, The University of Kansas, Lawrence, KS, E-mail: town@ku.edu.
References
- 1.Mullner RM, Chung K, Croke KG, Mensah EK. Geographic information systems in public health and medicine. J Med Syst. 2004;28:215–221. doi: 10.1023/b:joms.0000032972.29060.dd. [DOI] [PubMed] [Google Scholar]
- 2.Peterson AT, Sánchez-Cordero V, Beard CB, Ramsey JM. Ecologic niche modeling and potential reservoirs for Chagas disease, México. Emerg Infect Dis. 2002;8:662–667. doi: 10.3201/eid0807.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson AT, Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. Int J Parasitol. 2003;33:919–931. doi: 10.1016/s0020-7519(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 4.Peterson AT, Scachetti R, Fonseca de Camargo V. Using epidemiological survey data to infer geographic distributions of leishmaniasis vector species. Rev Soc Bras Med Trop. 2004;37:10–14. doi: 10.1590/s0037-86822004000100003. [DOI] [PubMed] [Google Scholar]
- 5.Peterson AT, Martínez-Campos C, Nakazawa Y, Martínez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans R Soc Trop Med Hyg. 2005;99:647–655. doi: 10.1016/j.trstmh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Peterson AT. Biogeography of diseases: a framework for analysis. Naturwissenschaften. 2008;95:483–491. doi: 10.1007/s00114-008-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinnell J. Field tests of theories concerning distribution control. Am Nat. 1917;51:115–128. [Google Scholar]
- 8.Velasco-Castrejón O. Leishmaniasis in Mexico. Rev Latinoam Microbiol. 1987;29:119–126. [PubMed] [Google Scholar]
- 9.Travi B, Montoya J. CIDEIM. Manual de Entomología Médica para Investigadores de América Latina. Cali, Colombia: Fundación Centro Internacional de Entrenamiento e Investigaciones Médicas Fundación CIDEIM; 1994. [Google Scholar]
- 10.Rebollar-Téllez E, Reyes-Villanueva F, Fernández-Salas I, Andrade-Narváez FJ. Population dynamics and biting rhythm of the anthropophilic sandfly Lutzomyia cruciata in southeast México. Rev Inst Med Trop Sao Paulo. 1996;38:29–33. doi: 10.1590/s0036-46651996000100006. [DOI] [PubMed] [Google Scholar]
- 11.Monroy-Ostria A, Hernandez–Montes O, Barker DC. Aetiology of visceral leishmaniasis in Mexico. Acta Trop. 2000;75:155–161. doi: 10.1016/s0001-706x(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 12.Galati BE. Phylogenetic systematics of phlebotominae (Diptera: Psychodidae) with emphasis on American groups. Bol Dir Malariol San Amb. 1995;35:133–142. [Google Scholar]
- 13.Lewis DJ, Young DG, Fairchild GB, Minter DM. Proposals for a stable classification of the phlebotomine sandflies (Diptera: Psychodidae) Syst Entomol. 1977;2:319–332. [Google Scholar]
- 14.Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 15.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 16.Biagi FF, De Biagi AM, Beltrán F. Phlebotomus flaviscutellatus, transmisor natural de Leishmania mexicana. Prensa Med Mex. 1965;30:276–272. [PubMed] [Google Scholar]
- 17.Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, Rebollar-Téllez EA. Incrimination of four sandfly species previously unrecognized as vectors of Leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150–161. doi: 10.1111/j.1365-2915.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- 18.Rebollar-Téllez E, Ramírez-Fraire A, Andrade-Narvaez FJ. A two-year study on vectors of cutaneous leishmaniasis: evidence for sylvatic transmission in the state of Campeche, Mexico. Mem Inst Oswaldo Cruz. 1996;91:555–560. doi: 10.1590/s0074-02761996000500004. [DOI] [PubMed] [Google Scholar]
- 19.Disney RH. Observations on a zoonosis: leishmaniasis in British Honduras. J Appl Ecol. 1968;5:1–59. [Google Scholar]
- 20.Williams P. The biting rhythms of some anthropophilic phlebotomine sandflies in British Honduras. Ann Trop Med Parasitol. 1966;60:357–364. doi: 10.1080/00034983.1966.11686425. [DOI] [PubMed] [Google Scholar]
- 21.Williams P. Phlebotomine sandflies and leishmaniasis in British Honduras (Belize) Trans R Soc Trop Med Hyg. 1970;64:317–364. doi: 10.1016/0035-9203(70)90171-9. [DOI] [PubMed] [Google Scholar]
- 22.Rebollar-Téllez E, Reyes-Villanueva F, Fernández-Salas I, Andrade-Narváez FJ. Abundance and parity rate of Lutzomyia cruciata in an endemic focus of localized cutaneous leishmaniasis in southern Mexico. J Med Entomol. 1996;33:683–685. doi: 10.1093/jmedent/33.4.683. [DOI] [PubMed] [Google Scholar]
- 23.Rebollar-Téllez E, Manrique-Saide P. New distributional record of Lutzomyia cruciata in the state of Yucatan, Mexico. Entomol News. 2001;112:337–339. [Google Scholar]
- 24.Porter CH, Steurer FJ, Kreutzer RD. Isolation of Leishmania mexicana mexicana from Lutzomyia ylephiletor in Guatemala. Trans R Soc Trop Med Hyg. 1987;81:929–930. doi: 10.1016/0035-9203(87)90355-5. [DOI] [PubMed] [Google Scholar]
- 25.Miranda A, Carrasco R, Paz H, Pascale JM, Samudio F, Saldaña A, Santamaría G, Mendoza Y, Calzada JE. Molecular epidemiology of American tegumentary leishmaniasis in Panama. Am J Trop Med Hyg. 2009;81:565–571. doi: 10.4269/ajtmh.2009.08-0265. [DOI] [PubMed] [Google Scholar]
- 26.Young DG, Duncan MA. Guide to the Identification and Geographic Distribution of Lutzomyia Sand Flies in Mexico, The West Indies, Central and South America (Diptera: Psychodidae) Memoirs of the Entomological Institute, No. 54, Gainesville, FL: Associated Publishers; 1994. [Google Scholar]
- 27.McHugh CP, Grogl M, Kreutzer RD. Isolation of Leishmania mexicana (Kinetoplastida: Trypanosomatidae) from Lutzomyia anthophora (Diptera: Psychodidae) collected in Texas. J Med Entomol. 1993;30:631–633. doi: 10.1093/jmedent/30.3.631. [DOI] [PubMed] [Google Scholar]
- 28.McHugh CP, Melby PC, LaFon S. Leishmaniasis in Texas: epidemiological and clinical aspects of human cases. Am J Trop Med Hyg. 1996;55:547–555. doi: 10.4269/ajtmh.1996.55.547. [DOI] [PubMed] [Google Scholar]
- 29.Lawyer PG, Young DG, Butler JF, Akin DE. Development of Leishmania mexicana in Lutzomyia diabolica and Lutzomyia shannoni (Diptera: Psychodidae) J Med Entomol. 1987;24:347–355. doi: 10.1093/jmedent/24.3.347. [DOI] [PubMed] [Google Scholar]
- 30.Endris RG, Young DG, Perkins PV. Experimental transmission of Leishmania mexicana by a North American sand fly, Lutzomyia anthophora (Diptera: Psychodidae) J Med Entomol. 1987;24:243–247. doi: 10.1093/jmedent/24.2.243. [DOI] [PubMed] [Google Scholar]
- 31.Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera: Psychodidae) Mosq News. 1984;44:263–304. [Google Scholar]
- 32.Travi BL, Montoya J, Gallego J, Jaramillo C, Llano R, Velez ID. Bionomics of Lutzomyia evansi (Diptera: Psychodidae) vector of visceral leishmaniasis in northern Colombia. J Med Entomol. 1996;33:278–285. doi: 10.1093/jmedent/33.3.278. [DOI] [PubMed] [Google Scholar]
- 33.Lainson R, Ward RD, Shaw JJ. Experimental transmission of Leishmania chagasi, causative agent of neotropical visceral leishmaniasis, by the sandfly Lutzomyia longipalpis. Nature. 1977;266:628–630. doi: 10.1038/266628a0. [DOI] [PubMed] [Google Scholar]
- 34.Young DG. A Review of the Bloodsucking Psychodid Flies of Colombia (Diptera: Phlebotominae and Sycoracinae) Gainesville, FL: University of Florida; 1979. Institute of Food and Agricultural Sciences Technical Bulletin 806. [Google Scholar]
- 35.Montoya-Lerma J, Ferro C. In: Insectos de Colombia. Amat-G G, Andrade-C G, Fernández F, editors. Volume II. Santa Fe de Bogotá, Mexico: Editora Guadalupe; 1999. pp. 210–245. (Flebótomos (Diptera: Psychodidae) de Colombia). Academia Colombiana de Ciencias Exactas, Fisicas y Naturales. Colección Jorge Alvarez Lleras No 13. [Google Scholar]
- 36.Maingon RD, Ward RD, Hamilton JG, Bauzer LG, Peixoto AA. The Lutzomyia longipalpis species complex: does population sub-structure matter to Leishmania transmission? Trends Parasitol. 2007;24:12–17. doi: 10.1016/j.pt.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Travi BL, Vélez ID, Brutus L, Segura I, Jaramillo C, Montoya J. Lutzomyia evansi, an alternate vector of Leishmania chagasi in a Colombian focus of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84:676–677. doi: 10.1016/0035-9203(90)90142-2. [DOI] [PubMed] [Google Scholar]
- 38.Zeledón R, Murillo J, Gutiérrez H. Observaciones sobre la ecología de Lutzomyia longipalpis (Lutz & Neiva, 1912) y posibilidades de existencia de leishmaniasis visceral en Costa Rica. Mem Inst Oswaldo Cruz. 1984;79:455–459. doi: 10.1590/s0074-02761984000400010. [DOI] [PubMed] [Google Scholar]
- 39.Montoya-Lerma J, Cadena H, Oviedo M, Ready PD, Barazarte R, Travi BL, Lane RP. Comparative vectorial efficiency of Lutzomyia evansi and Lu. longipalpis for transmitting Leishmania chagasi. Acta Trop. 2003;85:19–29. doi: 10.1016/s0001-706x(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 40.Ibáñez-Bernal S, Rodríguez-Domínguez G, Gómez-Dantes CH, Ricardez-Esquinca JR. First record of Lutzomyia evansi (Nuñez-Tovar 1924) in Mexico (Diptera: Psychodidae, Phlebotominae) Mem Inst Oswaldo Cruz. 2004;99:127–129. doi: 10.1590/s0074-02762004000200002. [DOI] [PubMed] [Google Scholar]
- 41.Seidelin H. Leishmaniasis and babesiasis in Yucatán. Ann Trop Med Parasitol. 1912;6:295–299. [Google Scholar]
- 42.Beltrán E, Bustamante ME. Datos epidemiológicos acerca de la úlcera de los chicleros (Leishmania americana) en México. Rev Inst Salubr Enferm Trop. 1942;3:1–28. [Google Scholar]
- 43.Beltrán E. Cutaneous leishmaniasis in Mexico. Sci Mon. 1944;59:108–119. [Google Scholar]
- 44.Rebollar-Téllez EA, Tun-Ku E, Manrique-Saide PC, Andrade-Narvaez FJ. Relative abundances of sand fly species (Diptera: Phlebotominae) in two villages in the same area of Campeche, in southern Mexico. Ann Trop Med Parasitol. 2005;99:193–201. doi: 10.1179/136485905X16390. [DOI] [PubMed] [Google Scholar]
- 45.Rowton E, de Mata M, Rizzo N, Navin T, Porter C. Vectors of Leishmania braziliensis in the Petén, Guatemala. Parasitologia. 1991;33:501–504. [PubMed] [Google Scholar]
- 46.Feliciangeli MD, Reyes RM, Limongi JE. Natural infection of Lutzomyia ovallesi (Diptera: Psychodidae) with parasites of the Leishmania braziliensis complex in a restricted focus of cutaneous leishmaniasis in northern Venezuela. Mem Inst Oswaldo Cruz. 1988;83:393–394. doi: 10.1590/s0074-02761988000300019. [DOI] [PubMed] [Google Scholar]
- 47.Bonfante-Garrido R, Spinetti H, Cupillo E, Momen H, Grimaldi G. Lutzomyia ovallesi (Diptera: Psychodidae) as a vector of cutaneous leishmaniasis in Venezuela. Parasitologia. 1991;33:99–104. [PubMed] [Google Scholar]
- 48.Christensen HA, Fairchild GB, Herrer A, Johnson CM, Young DG, de Vázquez AM. The ecology of cutaneous leishmaniasis in the Republic of Panama. J Med Entomol. 1983;20:463–484. doi: 10.1093/jmedent/20.5.463. [DOI] [PubMed] [Google Scholar]
- 49.Ibáñez-Bernal S. Phlebotominae (Diptera: Psychodidae) de México. I. Brumptomyia França y Parrot; Lutzomyia França, las especies de L. (Lutzomyia) Fança y del grupo Verrucarum. Folia Entomol Mex. 1999;107:61–118. [Google Scholar]
- 50.Ibáñez-Bernal S. Phlebotominae (Diptera: Psychodidae) de México. II. Las especies de Lutzomyia (Coromyia) Barretto, del grupo Delpozoi y de Lutzomyia (Dampfomyia) Addis. Folia Entomol Mex. 2001;40:17–46. [Google Scholar]
- 51.Ibáñez-Bernal S. Phlebotominae (Diptera: Psychodidae) de México. III. Las especies de Lutzomyia (Psathyromyia) Barretto, del grupo Aragoi, de L. (Trichopygomyia) Barreto, del grupo Dreisbachi y de L. (Nyssomyia) Barretto. Folia Entomol Mex. 2002;41:149–183. [Google Scholar]
- 52.Ibáñez-Bernal S. Phlebotominae (Diptera: Psychodidae) de México. IV. Las especies de Lutzomyia (Psychodopygus) Mangabeira, L. (Micropygomyia) Barretto, Lutzomyia del grupo Oswaldoi, L. (Helcocyrthomyia) Barretto, y especies del género sin agrupar. Folia Entomol Mex. 2003;42:109–152. [Google Scholar]
- 53.Van Wynsberghe NR, Canto-Lara SB, Sosa-Bibiano EI, Rivero-Cárdenas NA, Andrade-Narváez FJ. Comparison of small mammal prevalence of Leishmania (Leishmania) mexicana in five foci of cutaneous leishmaniasis in the state of Campeche, Mexico. Rev Inst Med Trop Sao Paulo. 2009;51:87–94. doi: 10.1590/s0036-46652009000200006. [DOI] [PubMed] [Google Scholar]
- 54.Godinez-Alvarez A, Ibáñez-Bernal S. Catálogo de Psychodidae (Diptera) de la colección de artrópodos con importancia médica del InDRE, Secretaria de Salud, Mexico. Acta Zool Mex. 2010;26:99–121. [Google Scholar]
- 55.Comisión Nacional para el Conocimiento y Uso de la Biodiversidad . Cabeceras Municipales, 2000. Extraído de Principales Resultados por Localidad. XII Censo de Población y Vivienda 2000 del Instituto Nacional de Estadística Geografía e Informática (INEGI) Mexico City: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; 2004. [Google Scholar]
- 56.Stockwell DR, Peters D. The GARP modeling system: problems and solutions to automated spatial prediction. Int J Geogr Inf Sci. 1999;13:143–158. [Google Scholar]
- 57.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190:231–259. [Google Scholar]
- 58.Elith J, Graham CH, Anderson RP, Dudik M, Ferrier S, Guisan A, Hijmans RJ, Huettman F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Overton JM, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, Williams SE, Wisz MS, Zimmermann NE. Novel methods to improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 59.Peterson AT, Papes M, Eaton M. Transferability and model evaluation in ecological niche modeling: comparison of GARP and Maxent. Ecography. 2007;30:550–560. [Google Scholar]
- 60.Graham CH, Elith J, Hijmans R, Guisan A, Peterson AT, Loiselle BA. The influence of spatial errors in species occurrence data used in distribution models. J Appl Ecol. 2007;45:239–247. NCEAS Species Distribution Modeling Group. [Google Scholar]
- 61.Araujo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecol Modell. 2003;162:211–232. [Google Scholar]
- 63.Raxworthy CJ, Martinez-Meyer E, Horning N, Nussbaum RA, Schneider GE, Ortega-Huerta MA, Peterson AT. Predicting distributions of known and unknown reptile species in Madagascar. Nature. 2003;426:837–841. doi: 10.1038/nature02205. [DOI] [PubMed] [Google Scholar]
- 64.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 65.Hijmans RJ, Cameron S, Parra J. WorldClim, Versión 1.3. 2005. http://biogeo.berkeley.edu/worldclim/worldclim.htm Available at.
- 66.U.S. Geological Survey . HYDRO 1k Elevation Derivative Database. Washington, DC: U.S. Geological Survey; 2001. http://edcdaac.usgs.gov/gtopo30/hydro/ Available at. [Google Scholar]
- 67.Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood EC, D'Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR. Terrestrial ecoregions of the world: a new map of life on earth. Biocience. 2001;51:933–938. [Google Scholar]
- 68.World Health Organization Control of the leishmaniases. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1990;793:1–158. [PubMed] [Google Scholar]
- 69.Sánchez-García L, Berzunza-Cruz M, Becker-Fauser I, Rebollar-Téllez EA. Sand flies naturally infected by Leishmania mexicana in the peri-urban area of Chetumal city, Quintana Roo, México. Trans R Soc Trop Med Hyg. 2010;104:406–411. doi: 10.1016/j.trstmh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Minter L, Kovacic B, Claborn DM, Lawyer P, Florin D, Brown GC. New state records for Lutzomyia shannoni and Lutzomyia vexator. J Med Entomol. 2009;46:965–968. doi: 10.1603/033.046.0432. [DOI] [PubMed] [Google Scholar]
- 71.McHugh CP, Grogl M, Kerr SF. Isolation of Leishmania mexicana from Neotoma micropus collected in Texas. J Parasitol. 1990;76:741–742. [PubMed] [Google Scholar]
- 72.Kerr SF, McHugh CP, Merkelz R. A focus of Leishmania mexicana near Tucson, Arizona. Am J Trop Med Hyg. 1999;61:378–379. doi: 10.4269/ajtmh.1999.61.378. [DOI] [PubMed] [Google Scholar]
- 73.Chablé-Santos JB, Van Wynsberghe NR, Canto-Lara SB, Andrade-Narvaez FJ. Isolation of Leishmania (L.) mexicana from wild rodents and their possible role in the transmission of localized cutaneous leishmaniasis in the state of Campeche, Mexico. Am J Trop Med Hyg. 1995;53:141–145. doi: 10.4269/ajtmh.1995.53.141. [DOI] [PubMed] [Google Scholar]
- 74.Van Wynsberghe NR, Canto-Lara SB, Damián-Centeno AG, Itzá-Ortiz MF, Andrade-Narváez FJ. Retention of Leishmania (Leishmania) mexicana in naturally infected rodents from the state of Campeche, Mexico. Mem Inst Oswaldo Cruz. 2000;95:595–600. doi: 10.1590/s0074-02762000000500001. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez-Tejeda G, Rodríguez N, Parra CI, Hernández-Montes O, Barker DC, Monroy-Ostria A. Cutaneous leishmaniasis caused by members of Leishmania braziliensis complex in the state of Nayarit, Mexico. Mem Inst Oswaldo Cruz. 2001;96:15–19. doi: 10.1590/s0074-02762001000100002. [DOI] [PubMed] [Google Scholar]
- 76.Rebollar-Téllez EA, Orilla-Moguel H, Dzul-Manzanilla F, Che-Mendoza A, Manrique-Saide P, Zapata-Peniche A. An update on the phlebotomid sandfly fauna from Yucatan, Mexico. Entomol News. 2006;117:21–24. [Google Scholar]
- 77.Berzunza-Cruz M, Bricaire G, Zuloaga Romero S, Pérez-Becker R, Saavedra-Lira E, Pérez-Montfort R, Crippa-Rossi M, Velasco-Castrejón O, Becker I. Leishmania mexicana mexicana: genetic heterogeneity of Mexican isolates revealed by restriction length polymorphism analysis of kinetoplast DNA. Exp Parasitol. 2000;95:277–284. doi: 10.1006/expr.2000.4541. [DOI] [PubMed] [Google Scholar]
- 78.Pérez-Vega J, López-Moreno C, López-Valenzuela J, Rendón-Maldonado J, López-Moreno H. Cutaneous leishmaniasis caused by Leishmania mexicana in Durango, Mexico: first clinical case report. Gac Med Mex. 2009;145:433–435. [PubMed] [Google Scholar]
- 79.González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrade Barata R, França-Silva JC, Mayrink W, Costa da Silva J, Prata A, Seixas Lorosa E, Araújo Fiúza J, Macedo Gonçalves C, de Paula MK, Santos Dias E. Aspectos da ecologia e do comportamento de flebotomíneos em área endêmica de leishmaniose visceral, Minas Gerais. Rev Soc Bras Med Trop. 2005;38:421–425. doi: 10.1590/s0037-86822005000500012. [DOI] [PubMed] [Google Scholar]
- 81.Salomón OD, Quintana MG, Bruno MR, Quiriconi RV, Cabral V. Visceral leishmaniasis in border areas: clustered distribution of phlebotomine sand flies in Clorinda, Argentina. Mem Inst Oswaldo Cruz. 2009;104:801–804. doi: 10.1590/s0074-02762009000500024. [DOI] [PubMed] [Google Scholar]


