Abstract
Histoplasma capsulatum var. capsulatum infection is rare outside disease-endemic areas. Clinical presentation and outcome of acquired immunodeficiency syndrome–related histoplasmosis are unknown in non-endemic areas with wide access to highly active anti-retroviral therapy (HAART). Retrospective analysis of cases recorded at the French National Reference Center for Mycoses and Antifungals during two decades: pre-HAART (1985–1994) and HAART (1997–2006). Clinical features and outcome of all adults with proven acquired immunodeficiency syndrome–related histoplasmosis were compared between the two periods. One hundred four patients were included (40 during the pre-HAART era and 64 during the HAART era). Diagnosis was established a mean of 62 days after onset of symptoms. One-year overall mortality rates decreased from 53% (pre-HAART era) to 22% (HAART era). Diagnosis during the pre-HAART era and an older age were the only independent factors associated with death. Histoplasmosis is a rare invasive fungal infection outside disease-endemic areas. Its prognosis improved significantly during the HAART era.
Introduction
Extrapulmonary infection caused by Histoplasma capsulatum var. capsulatum is an acquired immune deficiency syndrome (AIDS)–defining opportunistic infection.1 This systemic fungal infection is endemic in certain areas of North and South America, Asia, and Africa.2 It represented the first manifestation of human immunodeficiency virus (HIV) infection in up to 25% of AIDS patients in highly endemic areas before the availability of highly active anti-retroviral therapy (HAART).3 AIDS-related histoplasmosis is almost always disseminated and mortality is high in the absence of accurate diagnosis and prompt appropriate antifungal treatment.3,4 Liposomal amphotericin B has proved superior to deoxycholate amphotericin B in reducing overall mortality and iatrogenic renal failure in the HIV infection context and is the recommended first-line therapy of severe cases.5,6 Retrospective7,8 and prospective9,10 studies from endemic areas have identified various prognostic factors. The impact of HAART on the epidemiology and outcome of histoplasmosis has not been extensively studied, even in endemic areas, and only a limited number of cases of immune reconstitution inflammatory syndrome (IRIS) cases have been reported in AIDS patients.11
Disseminated histoplasmosis can also be diagnosed in AIDS patients living in non-endemic area after acute exposure or delayed reactivation of a latent infection providing the patient had previously lived or traveled to a disease-endemic area.2 Updated data on AIDS-related histoplasmosis in non-endemic areas are lacking. A recent review of the literature identified only 68 cases in Europe published during 1984–2004,12 and an epidemiologic survey reported 45 cases diagnosed in 10 countries in Europe during 1995–1999.13
We thus performed a 10-year (1997–2006) nationwide retrospective study to describe AIDS-related histoplasmosis during the HAART era in metropolitan France, a non-endemic country. We compared these recent cases to those recorded during the pre-HAART era (1985–1994) to investigate the impact of HAART on the epidemiology, clinical manifestations, and prognosis of AIDS-related imported histoplasmosis.
Methods
Study design.
A retrospective study was implemented at the French National Reference Center for Mycoses and Antifungals (NRCMA). All adult cases of AIDS-related H. capsulatum infections diagnosed in metropolitan France during January 1, 1985–December 31, 1994 (first period, pre-HAART era) and during January 1, 1997–December 31, 2006 (second period, HAART era) and reported to the NRCMA were reviewed. Additional cases were identified by direct contact with members of the French Mycosis Study Group. Because HAART was not routinely available as standard care during 1995 and 1996 in metropolitan France, cases diagnosed during January 1, 1995–December 31, 1996 were not included.
Inclusion criteria were an age > 18 years, serologically confirmed HIV infection, proven histoplasmosis according to updated European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group definition14 (i.e., illness consistent with histoplasmosis and positive culture, direct examination, or histopathologic results), and a diagnosis established in metropolitan France. Patients were not eligible when the diagnosis was established in French overseas departments (i.e., French Guiana and French West Indies, regions in which H. capsulatum var. capsulatum infections are endemic).
A standardized questionnaire was established and data were obtained by reviewing the medical, microbiologic, and pathologic charts in the corresponding centers and by discussion with the clinician/mycologist in charge of the patient whenever needed. The following data were extracted: epidemiologic data (date and place of birth, living area, travel to or residency in disease-endemic areas, time since last travel to these disease-endemic areas), HIV infection parameters (date of diagnosis, CD4 cell count, and viral load at the time of histoplasmosis diagnosis, previous opportunistic infections, anti-retroviral therapy) and histoplasmosis characteristics (clinical presentation, diagnostic modalities, antifungal treatment and follow-up up to April 2008).
Data from the pre-HAART cohort were obtained in 1995 by using the same questionnaire and methods and were reported in part in 1996 (Lortholary O and others, Abstract I174, 36th Interscience Conference on Chemotherapy and Antimicrobial Agents, New Orleans, LA, September 15–18, 1996).
Definitions.
Histoplasmosis was defined by isolation of H. capsulatum from any body site or by visualization of small ovoid (3–4 μm) intracellular yeasts compatible with H. capsulatum upon examination of tissue section or fluid pellet in a context compatible with the diagnosis.
Clinical pulmonary signs included cough, dyspnea, sputum, and hemoptysis. Mucocutaneous symptoms were defined as presence of any of skin ulceration, papule, pseudo-molluscum lesions, and/or pharyngeal ulceration. Neurologic signs were defined as presence of any of seizure, motor deficit, coma, meningitis. Histoplasmosis was considered as disseminated if at least two non-contiguous organs were involved.
Previously established criteria of severity were used7,10 and corresponded to the presence of at least one of the following: hypoalbuminemia (< 35 g/L), disseminated intra-vascular coagulation, lactate dehydrogenase levels > 2 times the upper limit of the reference range, creatinine concentration > 185 μM (2.1 mg/dL), systolic blood pressure < 90 mm of Hg, and hypoxemia (PaO2 < 60 mm of Hg).
Amphotericin B treatment should have lasted at least 14 days to be considered for analysis. In case of successive therapy with deoxycholate amphotericin B and liposomal amphotericin B, only the formulation that had been received longer was considered for analysis. Maintenance therapy was considered as therapy that started three months after initiation of antifungal treatment. Relapse was defined as new onset of clinical signs with new isolation of H. capsulatum on culture or visualization of small ovoid (3–4 μm) intracellular yeasts upon examination of tissue section or fluid pellet. The existence of histoplasmosis-related IRIS was systematically investigated using published criteria (i.e., progression of organ dysfunction or enlargement of pre-existing lesion or exaggerated inflammatory reaction and decrease in plasma HIV viral load by > 1 log copies/mL).11,15 All IRIS and relapse cases were reviewed and validated by two infectious diseases specialists who were experts in the field. The underlying cause of death was considered to be histoplasmosis in the presence of clinical symptoms related to histoplasmosis and with a positive direct examination result or culture of H. capsulatum within one month before death.
Statistical analysis.
The current study based on specific missions of the NRCMA has been approved by the appropriate Institutional Review Board. Information was obtained anonymously and entered coded in a secured website (approved by the French commission on personal data and freedom). Statistical analysis was performed by using StataSE10 software (StataCorp, College Station, TX) and R software (http://cran.r-project.org). Distributions of variables were compared by using the chi-square test or Fisher's exact test for categorical variables and the Student's t-test for continuous variables.
Overall survival was the time interval between the date of diagnosis and the date of death or last follow-up. Kaplan-Meier estimates were used to describe survival distributions. The log-rank test was used to compare survival distributions. Multivariate analyses were performed by using Cox proportional hazards regression model.
Histoplasmosis-related death probabilities were obtained from crude cumulative incidence estimates, which treated death from other causes as competing risk events. Cumulative incidences of death related to histoplasmosis were compared between subgroups by using the method of Fine and Gray.16 Multivariate proportional hazards models accounting for death from other causes as a competing risk were fitted by using the same method.
Results
Baseline demographic and HIV parameters during the pre-HAART and HAART eras.
Among the 104 patients studied (Table 1), 40 were diagnosed during the pre-HAART era (1985–1994) and 64 during the HAART era (1997–2006). In the second period, the proportion of women (P = 0.04) and that of patients born in Africa (P < 0.001) increased. The proportion of females born in Africa increased from 5% (2 of 40) to 31% (20 of 64; P = 0.001). There was a significant change in the mode of HIV transmission with a decrease in the proportion of homosexual or bisexual men and intravenous drug users and an increase in the proportion of heterosexual patients (P < 0.001). Histoplasmosis was significantly more frequently the initial event leading to the diagnosis of HIV infection during the HAART era. Median CD4+ T cell count was similar during the two periods.
Table 1.
Demographic and virologic characteristics of 104 HIV-infected patients with imported histoplasmosis, France*
| Characteristic | Pre-HAART (1985–1994), n = 40 | HAART (1997–2006), n = 64 | P |
|---|---|---|---|
| Mean (SD) age in years | 38 (7) | 40 (11) | 0.22 |
| Male, no. (%) | 31 (78) | 37 (58) | 0.04 |
| Continent of birth, no. (%) | < 10−3 | ||
| Africa | 6 (15) | 36 (58) | |
| United States | 15 (38) | 18 (29) | |
| Europe | 17 (44) | 7 (11) | |
| Asia | 1 (3) | 0 | |
| Oceania | 0 | 1 (2) | |
| HIV characteristics | |||
| Median CD4 cell count (/μL) (IQR, 95% CI) | 21 (10–33) | 12 (5–30) | 0.74 |
| HIV status at the time of histoplasmosis diagnosis, no. (%) | 0.02 | ||
| Histoplasmosis indicating HIV infection | 7 (17) | 27 (42) | |
| HIV infection already known | 15 (38) | 22 (34) | |
| AIDS already known | 19 (45) | 15 (23) | |
| Mode of HIV transmission, no. (%) | 0.001 | ||
| Heterosexual | 17 (42) | 48 (75) | |
| Homosexual | 12 (32) | 3 (5) | |
| Intravenous drug use | 4 (10) | 2 (3) | |
| Transfusion | 3 (8) | 2 (3) | |
| Unknown | 4 (10) | 9 (14) | |
| Geographic areas of suspected Histoplasma capsulatum exposure, no. (%)†‡ | |||
| Africa | 10 (25) | 39 (61) | < 10−3 |
| United States | 15 (38) | 9 (14) | 0.008 |
| French Guiana | 13 (33) | 14 (22) | 0.23 |
| French West Indies | 8 (20) | 6 (9) | 0.15 |
| Asia | 3 (8) | 2 (3) | 0.37 |
| Unknown | 1 (3) | 0 | 0.39 |
HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy; IQR = interquartile range; CI = confidence interval; AIDS = acquired immunodeficiency syndrome.
For whom information was available.
The same patient may have traveled to several disease-endemic areas.
All patients traveled to areas in which histoplasmosis was endemic. Geographic areas of exposure were diverse but mostly in Africa and French Guiana (Table 1). The time interval between the last travel to a disease-endemic area and the diagnosis was known for 93 patients (interval < 1 month [n = 18, 19%], 1 month–1 year [n = 20, 22%], 1–10 years [n = 42, 45%], and > 10 years [n = 13, 14%]). The longest time interval recorded was 15 years.
Characteristics of histoplasmosis at diagnosis.
Histoplasmosis was diagnosed nearly two months after the first symptoms in both periods (Table 2). Clinical presentation did not differ during the two periods for more frequent clinical signs (fever, peripheral adenopathies, liver or spleen enlargement, abdominal pain, diarrhea). However, pulmonary symptoms (cough and sputum) were more frequently recorded during the pre-HAART era but the same proportion of patients had an abnormal chest radiograph. The pattern of dermatologic lesions evolved and showed a significant decrease in papules (37% versus 17%; P = 0.02). Neurologic symptoms remained scarce. Overall, histoplasmosis was disseminated in 73 (70%) of 104 patients. Biological parameters remained similar and showed only a trend towards decreased hemoglobin levels during the HAART era (9.1 ± 2.2 versus 8.3 ± 1.9 g/dL; P = 0.07). Serum alkaline phosphatase levels were increased (> 2× the upper limit of the reference value) in 43% and 39% of patients, respectively (P > 0.05) and aspartate aminotransferase levels were increased (> 2× the upper limit of the reference value) in 32% and 55%, respectively (P = 0.03).
Table 2.
Baseline characteristics of imported histoplasmosis in 104 HIV-infected patients, France*
| Characteristic | Pre-HAART (1985–1994), n = 40 | HAART (1997–2006), n = 64 | P |
|---|---|---|---|
| Clinical presentation | |||
| Time between onset of symptoms and diagnosis (days) | 59 (66) | 65 (65) | 0.66 |
| Fever, no. (%) | 35 (88) | 54 (84) | 0.78 |
| Adenopathy, no. (%) | 21 (49) | 40 (63) | 0.17 |
| Hepatomegaly, no. (%) | 19 (48) | 27 (43) | 0.56 |
| Splenomegaly, no. (%) | 14 (36) | 28 (44) | 0.39 |
| Pulmonary symptoms, no. (%) | 24 (60) | 22 (34) | 0.01 |
| Cough | 23 (58) | 17 (27) | 0.002 |
| Sputum | 12 (30) | 2 (3) | < 10−3 |
| Dyspnea | 11 (28) | 13 (20) | 0.40 |
| Abnormal chest radiograph | 25 (61) | 33 (52) | 0.34 |
| Mucocutaneous symptoms, no. (%) | 23 (56) | 28 (44) | 0.17 |
| Papule | 15 (37) | 11 (17) | 0.02 |
| Oral ulceration | 6 (15) | 4 (6) | 0.18 |
| Skin ulceration | 3 (8) | 2 (3) | 0.37 |
| Abdominal symptoms, no. (%) | 16 (40) | 23 (36) | 0.68 |
| Neurologic symptoms, no. (%) | 6 (15) | 7 (11) | 0.56 |
| Biologic parameters, no. (%) | |||
| Hemoglobin (g/dL) | 9.1 (2.2) | 8.3 (1.9) | 0.07 |
| Leukocytes (109/L) | 2.9 (1.2) | 3.5 (4.4) | 0.37 |
| Platelets (109/L) | 188 (104) | 147 (150) | 0.14 |
| AST (N× upper reference value) | 2.1 (2.1) | 3.9 (4.5) | 0.04 |
| ALP (N× upper reference value) | 3.9 (7.1) | 1.8 (1.2) | 0.03 |
HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy; AST = aspartate aminotransferase; ALP = alkaline phosphatase.
Clinical severity was assessed only during the second period. Most (n = 41, 64%) patients had at least one of the severity criteria, including hypoalbuminemia (48%), a lactate dehydrogenase level > 2× the upper limit of the reference value (36%), hypotension (14%), disseminated intravascular coagulation (13%), renal insufficiency (11%), and hypoxemia (5%).
The diagnostic tools used remained similar during the two periods (Table 3). More than 90% of the patients had a positive histopathologic or direct examination results and approximately 80% had a positive fungal culture. The most frequently contributive clinical specimens were bone marrow (60%), skin or mucosal specimen (39%), blood (36%), and respiratory samples (36%). Positive skin/mucosal samples were slightly more frequent during the pre-HAART era (50% and 31%; P = 0.06). There was also a trend towards an increase in the prevalence of positive blood culture (35% and 54%; P = 0.10).
Table 3.
Tools for diagnosis of histosplasmosis in 104 HIV-infected patients with imported histoplasmosis, France*
| Tools | Pre-HAART (1985–1994), n = 40 | HAART (1997–2006), n = 64 | P |
|---|---|---|---|
| Diagnostic tools, no. (%) | |||
| Histopathology or direct examination | 38 (95) | 60 (94) | 1 |
| Positive Histoplasma capsulatum culture | 31 (78) | 52 (81) | 0.80 |
| Blood | 11 (35) | 27 (54) | 0.11 |
| Respiratory specimen | 12 (30) | 20 (31) | 0.89 |
| Skin or mucosal specimen | 20 (50) | 20 (31) | 0.06 |
| Bone marrow | 22 (55) | 42 (66) | 0.28 |
| Lymph node | 7 (18) | 18 (28) | 0.35 |
| Gastrointestinal tract | 8 (20) | 8 (13) | 0.57 |
| Liver | 5 (13) | 2 (3) | 0.10 |
| Cerebrospinal fluid | 0 | 2 (3) | 0.52 |
| Detection of antibodies against H. capsulatum | 3 (8) | 1 (2) | 0.16 |
HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy.
Histoplasmosis-related IRIS.
Seven (11%) patients diagnosed during the HAART era showed development of IRIS related to histoplasmosis (Table 4). Four of them had been reported elsewhere.11,17 In two cases, a 51-year old woman treated with HAART and chemotherapy for Kaposi's sarcoma and a 33-year old man who had AIDS-defining toxoplasmosis and onset of HAART three weeks before IRIS, IRIS was the first manifestation of histoplasmosis. Histoplasmosis was the first manifestation of HIV infection for the five other patients. IRIS occurred 1–2 months after diagnosis of histoplasmosis in three patients. Two patients had late-onset IRIS but they had unplanned interruption of HAART, resumed HAART without medical advice 2–3 months before IRIS, and consulted a physician only when IRIS occurred. IRIS was severe in two patients: one had uveitis that finally required enucleation, and one had intestinal obstruction that required colectomy.
Table 4.
Clinical, virologic, and outcome data in seven patients with histoplasmosis-related IRIS, France*
| Patient no. | Age, y/sex | Time interval, months† | Baseline‡ | IRIS | ||||
|---|---|---|---|---|---|---|---|---|
| CD4 cells/μL | Viral load (copies/mL) | Manifestation | CD4 cells/μL | Viral load (copies/mL) | Manifestation | |||
| 1 | 51/F | 0 | 25 | 350,000 | None | 144 | < 50 | Peripheral necrotic adenopathies |
| 2§ | 33/M§ | 0 | 13 | 250,000 | None | 59 | 150 | Hemophagocytic syndrome |
| 3§ | 57/M§ | 1 | 55 | > 500,000 | Pulmonary involvement | 436 | < 200 | Intestinal obstruction caused by granulomatous colitis |
| 4§ | 20/F§ | 2 | 4 | 196,000 | Papule and hepatomegaly | 108 | 25,000 | Arthritis, uveitis |
| 5 | 29/F | 43¶ | 92 | 3,328,000 | Meningitis | 113 | 68,000 | Aseptic meningitis |
| 6 | 51/M | 2 | 14 | 1,000,000 | Splenomegaly and peripheral adenopathies | 180 | < 50 | Rash |
| 7§ | 36/M§ | 35¶ | 2 | 290,000 | Hepatosplenomegaly and peripheral adenopathies | 106 | < 50 | Peripheral necrotic adenopathies |
IRIS = immune reconstitution inflammatory syndrome.
Time interval is the period between diagnosis of histoplasmosis and IRIS.
Baseline was diagnosis of acquired immunodeficiency syndrome (AIDS). AIDS was detected by histoplasmosis for all patients except patients 1 and 2 for whom IRIS was the first manifestation of histoplasmosis. Patient 1 was treated with chemotherapy and highly active anti-retroviral therapy (HAART) for Kaposi's sarcoma. Histoplasmosis-related adenopathy appeared three months after onset of HAART, when chemotherapy was stopped. Patient 2 had AIDS-defining toxoplasmosis and onset of HAART three weeks before IRIS.
These two patients had an unplanned interruption of HAART and became compliant again 2 months before IRIS. No immunovirologic data were available during HAART interruption.
Outcome of 104 patients with histoplasmosis.
Ninety-five (91%) patients received antifungal drugs for at least 48 hours. Most (64%) patients received one amphotericin B formulation. Forty-seven (45%) patients received deoxycholate amphotericin B, including 38 patients subsequently switched to itraconazole. Twenty (19%) patients were prescribed liposomal amphotericin B, including 15 patients switched to itraconazole. Itraconazole was the only antifungal drug prescribed to 25 (24%) patients. Voriconazole or fluconazole use was exceptional (one patient each). Five (5%) patients who died less than 48 hours after diagnosis did not receive an antifungal drug. No information related to treatment was available for the remaining four patients.
Median follow-up was 31.5 months (range = 0–120 months) and 41 deaths were observed. Overall mortality rate was 39% in the entire cohort. Mortality rate was higher in the pre-HAART era (hazard ratio [HR] = 0.25, 95% confidence interval [CI] = 0.13–0.48; P < 0.001) (Figure 1). Death was related to histoplasmosis in 21 (51%) patients. All related deaths were observed before one year. The histoplasmosis-related mortality rate was higher during pre-HAART era than during the HAART era (HR = 0.30, 95% CI = 0.12–0.74, P = 0.009). Two (5%) patients relapsed during the pre-HAART era, and 4(6%) relapsed during the HAART era.
Figure 1.
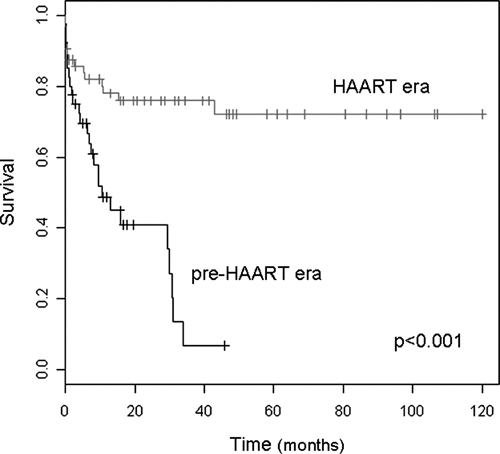
Overall survival curves of acquired immunodeficiency syndrome–associated histoplasmosis patients during pre–highly active anti-retroviral therapy (HAART) and HAART periods, France.
Factors associated with mortality.
Overall mortality and histoplasmosis-related mortality were significantly associated with arterial hypotension (HR = 2.38, 95% CI =1.05–5.38, P = 0.037 and HR = 10.49, 95% CI = 2.59–42.45, P < 0.001, respectively) by univariate analysis (Table 5). Pulmonary involvement, dyspnea, cutaneous involvement, platelet count, and hemoglobin level were not associated with overall or histoplasmosis-related mortality (Table 6). There was a trend towards lower mortality among patients treated with liposomal amphotericin B (HR = 0.63, 95% CI = 0.25–1.58, P = 0.38) or itraconazole alone (HR = 0.54, 95% CI = 0.21–1.34, P = 0.18) than among those treated with deoxycholate amphotericin B.
Table 5.
Comparison of AIDS-related histoplasmosis cases from disease-endemic areas with 104 cases from France, a non-endemic area*
| Area | Period | No. | Age, years | Men, % | CD4 cell count/μL | HAART, % | AIDS defining, % | Clinical features, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Adp | HM, SM | Skin | Pulm | Abdo | Neuro | ||||||||
| Indiana, USA | 1981–1989 | 72 | NA | NA | NA | 0 | 69 | 96 | 17 | 26, 13 | 1 | 53 | 3 | 18 |
| United States | 1996–1999 | 92 | 39 | 89 | 25 | 35 | > 50 | 88 | NA | NA | NA | 55 | 33 | 16 |
| Colombia | 1979–2001 | 30 | 36 | 97 | 45 | 37 | ND | 90 | 57 | 13 | 53 | 80 | 47 | NA |
| Panama | 1997–2003 | 104 | 37 | 85 | 65 | 11 | 68 | 92 | 19 | 42 | 17 | 64 | 50 | NA |
| French Guiana | 1982–2007 | 200 | 40 | 68 | 34 | 8 | 78 | 89 | 46 | 33,19 | 12 | 35 | 47 | 15 |
| Brazil | 1995–2004 | 164 | 34 | 80 | 104 | 18 | ND | 95 | 3 | 34,29 | 10 | 75 | 61 | 17 |
| France† | 1985–1994 | 40 | 38 | 78 | 32 | 0 | 17 | 88 | 49 | 48, 36 | 56 | 60 | 40 | 15 |
| France† | 1997–2006 | 64 | 40 | 58 | 28 | 23 | 42 | 84 | 63 | 43, 44 | 44 | 34 | 36 | 11 |
| Area | Period | No. | AIDS-defining, % | Mortality, % (follow-up, months) | Relapse | Mean for diagnosis, % | ||
|---|---|---|---|---|---|---|---|---|
| Blood culture | Histology | Urinary/blood antigen detection | ||||||
| Indiana, USA | 1981–1989 | 72 | 69 | 22 (NA) | 18 (NA) | 90 (19) | NA | 97, 83 |
| United States | 1996–1999 | 92 | > 50 | 12 (3) | NA | 71 (51) | NA | 96, 85 |
| Colombia | 1979–2001 | 30 | ND | 20 (12) | NA | 96 | 81 | NA |
| Panama | 1997–2003 | 104 | 68 | 10 (1) | 6 (NA) | NA (68) | 34 | NA |
| French Guiana | 1982–2007 | 200 | 78 | 31 (6) | NA | NA (8) | NA | NA |
| Brazil | 1995–2004 | 164 | ND | 32 (NA) | NA | 48 (NA) | 81 | NA |
| France† | 1985–1994 | 40 | 17 | 53 (12) | 5 | 78 (35) | 95 | NA |
| France† | 1997–2006 | 64 | 42 | 22 (12) | 6 | 81 (54) | 94 | NA |
HAART = highly active anti-retroviral therapy; AIDS = acquired immunodeficiency syndrome; Adp = adenopathy; HM = hepatomegaly; SM = splenomegaly; Pulm = pulmonary symptoms; Abdo = abdominal symptoms; Neuro = neurologic symptoms; NA = not available.
This study.
Table 6.
Univariate analysis of factors associated with overall mortality and histoplasmosis-related mortality, France*
| Factor | Overall mortality | Histoplasmosis-related mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Demographic | ||||||
| Male sex | 1.50 | 0.75–2.99 | 0.25 | 1.07 | 0.43–2.63 | 0.89 |
| Age (10-year increase) | 1.36 | 0.97–1.90 | 0.08 | 1.69 | 1.01–2.84 | 0.05 |
| Continent of birth: Africa | 0.40 | 0.20–0.82 | 0.01 | 0.30 | 0.10–0.88 | 0.03 |
| Diagnosis during HAART-era | 0.25 | 0.13–0.48 | < 0.001 | 0.30 | 0.12–0.74 | 0.009 |
| HIV status at the time of histoplasmosis diagnosis | ||||||
| AIDS already known | 1.67 | 0.77–3.62 | 0.20 | 1.16 | 0.42–3.15 | 0.78 |
| HIV infection already known | 1.28 | 0.58–2.83 | 0.55 | 0.74 | 0.25–2.20 | 0.59 |
| Mode of HIV transmission | ||||||
| Heterosexual | 0.55 | 0.30–1.02 | 0.06 | 0.60 | 0.26–1.41 | 0.24 |
| Clinical characteristics | ||||||
| Hypotension | 2.38 | 1.05–5.38 | 0.04 | 10.49 | 2.59–42.44 | 0.001 |
| Pulmonary symptoms | 1.43 | 0.77–2-64 | 0.25 | 1.72 | 0.73–4.05 | 0.21 |
| Dyspnea | 1.27 | 0.64–2.53 | 0.50 | 1.33 | 0.52–3.38 | 0.55 |
| Papule | 1.61 | 0.84–3.08 | 0.15 | 0.87 | 0.33–2.31 | 0.78 |
| Biologic parameters | ||||||
| Platelet count (50 × 109/L increase) | 1.17 | 0.91–1.52 | 0.23 | 1.06 | 0.84–1.34 | 0.61 |
| Hemoglobin (g/dL) | 0.90 | 0.76–1.06 | 0.20 | 0.49 | 0.15–1.58 | 0.23 |
HZ = hazard ratio; CI = confidence interval; HAART = highly active anti-retroviral therapy; AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus.
Age and diagnosis during the HAART era were independently associated with overall mortality (HR = 1.72 per 10-years increase, 95% CI = 1.17–2.54, P = 0.006 and HR = 0.19, 95% CI = 0.01–0.39, P < 0.001, respectively) and histoplasmosis-related mortality (HR = 2.16 per 10-years increase, 95% CI = 1.10–4.22, P = 0.025, and HR = 0.22, 95% CI = 0.08–0.61, P = 0.04, respectively) by multivariate analysis.
Discussion
We analyzed 104 cases of proven AIDS-related histoplasmosis in metropolitan France. This study represents the largest reported cohort from a non-endemic area.12 Histoplasmosis caused by H. capsulatum is a rare, invasive, fungal infection in France, and diagnosis is frequently delayed. All patients had traveled to a disease-endemic area, including 14% for more than 10 years before given a diagnosis of histoplasmosis, and all were strongly immunocompromised (median CD4 cell count = 15 cells/μL).
Comparison of our data concerning patients from a non-endemic area with those related to patients from North, Central, and South America is summarized in Table 5. Our patients were characterized by a high frequency of extra-pulmonary lesions (skin, adenopathy, hepatomegaly, splenomegaly). This finding could reflect a high fungal burden, a hypothesis strengthened by the high mortality rate (60%) recorded in our study during the pre-HAART era, and potentially explained by delayed diagnosis caused in part by lack of awareness of clinicians in France. An alternative hypothesis to explain the differences in clinical patterns between patients from different areas could be the implication of different, area-restricted H. capsulatum isolates.18 This hypothesis could not be further explored in our study because of lack of storage of the clinical isolates.
Histoplasmosis caused by H. capsulatum is more frequent in AIDS patients than histoplasmosis caused by H. capsulatum var. duboisii. Less than 20 cases of AIDS-related H. duboisii histoplasmosis have been reported in the literature.19 The two variants infect patients who have poor immunologic status. Skin lesions and adenopathies are common during both diseases. However, patients infected with H. duboisii have less hepatosplenomegaly and pulmonary involvement than patients infected with H. capsulatum (Table 7). Bone lesions appear to be suggestive of H. duboisii infection. The mortality rate is probably higher in H. capsulatum patients (39% for the H. capsulatum patients in this study and 24% for H. duboisii patients in published studies).
Table 7.
Comparison between AIDS-related imported Histoplasma capsulatum and H. duboisii histoplasmosis, France*
| Characteristic | H. capsulatum, pre-HAART era | H. capsulatum, HAART era | H. duboisii |
|---|---|---|---|
| No. | 40 | 64 | 17 |
| Mean age (years) | 38 | 40 | 34 |
| No. men | 78 | 58 | 71 |
| Clinical features, % | |||
| Fever | 88 | 84 | 58 |
| Adenopathy | 49 | 63 | 53 |
| Hepatosplenomegaly | 48 (HM), 36 (SM) | 43 (HM), 44 (SM) | 12 |
| Pulmonary symptoms | 60 | 34 | 0 |
| Skin | 56 | 44 | 59 |
| Bone | 0 | 0 | 18 |
| Abdominal | 40 | 36 | 12 |
| Diagnosis tools, % | |||
| Direct examination | 95 | 94 | 100 |
| Culture | 78 | 81 | 64 |
| Blood culture | 35 | 54 | 12 |
| Outcome, % | |||
| Death | 53 | 22 | 24 |
| Relapse | 5 | 3 | 12 |
AIDS = acquired immunodeficiency syndrome; HAART = highly active antiretroviral therapy; HM = hepatomegaly; SM = splenomegaly. Data for H. duboisii-infected patients are from Loulergue and others.19
One-year overall mortality significantly decreased from 53% during the pre-HAART era to 22% during the HAART era among AIDS-related histoplasmosis patients from metropolitan France. Although clinical features of histoplasmosis were similar during the two periods, some significant changes occurred and could be in part responsible for improvement in survival: modification of HIV infection epidemiology in France, availability of liposomal amphotericin, and widespread use of HAART.
The typical AIDS-related histoplasmosis patient during the pre-HAART era was a homosexual/bisexual male from Europe with previously defined AIDS and one or several prior opportunistic infections. However, heterosexual women from Africa without a known history of AIDS were more frequently seen during the HAART era. The data support the conclusion that physicians should no longer assume that histoplasmosis is caused by H. duboisii in all cases originating in Africa.
During the HAART era, 31% of patients were treated with liposomal amphotericin, a drug that was not previously available, which explains why only deoxycholate amphotericin B and/or itraconazole were prescribed during the first period. Because liposomal amphotericin was more effective than deoxycholate amphotericin B in reducing overall mortality after two weeks of treatment in a randomized controlled trial,5 it was anticipated that short-term (two weeks) survival would improve during the second period. This prediction was not observed. The design of our study (a cohort study and not a therapeutic trial), and therefore the lack of standardization preclude any definitive conclusions. Finally, all patients in the second period who survived more than two weeks after diagnosis of histoplasmosis received HAART.
Treatment with HAART was started 1–3 months after onset of histoplasmosis. However, the impact of HAART on outcome of AIDS-related histoplasmosis has not been established.20 Our data suggest that HAART had a major impact in decreasing overall mortality in AIDS patients with histoplasmosis.
Such a positive impact of HAART on late mortality has already been established for other opportunistic infections such as those with Pneumocystis jirovecii, cryptococcosis, or tuberculosis in industrialized countries.21–24 A significant decrease in early mortality had not been reported for any other opportunistic infections.21
Patient age and the period of diagnosis were the only independent prognostic factors identified in our study. Hypoalbuminemia, disseminated intravascular coagulation, increased lactate dehydrogenase levels, renal failure, hypotension, and hypoxemia have been reported as prognostic factors7,10 but were not identified in our study because of the small size of the cohort and the reduced number of deaths.
Immune reconstitution inflammatory syndrome occurred in 11% of the patients during the HAART era. This incidence is similar to that identified for cryptococcosis25 and lower than that for tuberculosis (40%) in AIDS patients who never received HAART.26 All histoplasmosis-related IRIS patients had a dramatic increase in CD4 cell count and a decrease in HIV viral load, similar to Mycobacterium tuberculosis-related IRIS patients.26 Severe forms requiring surgery were diagnosed in two patients. Interestingly, IRIS was the first manifestation of histoplasmosis in two patients. Such a finding is consistent with those of a retrospective study that reported that HIV patients living in French Guiana had an increased risk of histoplasmosis during the first two months after beginning HAART.27 The IRIS cases occurred in three of the patients more than two months after the beginning of HAART. However, two patients had an unplanned interruption of HAART and became compliant again two months before IRIS, and IRIS occurred in one patient shortly after the interruption of chemotherapy for Kaposi's sarcoma.
In conclusion, overall survival after AIDS-related histoplasmosis increased in metropolitan France, a non-endemic area, during the HAART era. Because most patients came from Africa, H. capsulatum histoplasmosis should no longer be considered as American histoplasmosis, but must be considered in every febrile severely immunocompromised HIV-infected patient who had already traveled to a disease-endemic area. Immune reconstitution inflammatory syndrome occurred after HAART in up to 11% of patients and may be an indicator of histoplasmosis.
ACKNOWLEDGMENTS
This work was presented in part at the 21st European Congress of Clinical Microbiology and Infectious Diseases, 27th International Congress of Chemotherapy, Milan, Italy, May 2011. Members of the French Mycosis Study Group who participated to the study are (in alphabetical order of the city) Amiens, Centre Hospitalier Universitaire: T. Chouaki (Mycologie), Y. Samad (Maladies Infectieuses et Tropicales); Annecy: J. Gaillat (Maladies Infectieuses et Tropicales); Argenteuil: F. Leturdu (Mycologie), M. Touahri (Hématologie); Aulnay, Hôpital Robert Ballanger: X. Delassus (Maladies Infectieuses et Tropicales); Bobigny, Hôpital Avicenne: O. Bouchaud (Maladies Infectieuses et Tropicales), M. Bentata (Médecine Interne), C. Bouges-Michel (Mycologie); Bondy, Hôpital Jean Verdier: Isabelle Poilane (Mycologie), O. Fain (Médecine Interne); Bordeaux: B Couprie (Mycologie), H. Dutronc (Maladies Infectieuses et Tropicales); Boulogne-Billancourt, Hôpital Ambroise Paré: E. Rouveix (Médecine Interne), J. Dunan (Mycologie); Créteil, Hôpital Henri-Mondor: S. Bretagne (Mycologie), A. Sobel, Y. Lévy (Immunologie Clinique); Hôpital Intercommunal: V. Garrait (Médecine Interne), N. Fauchet (Mycologie); Gonesse: F. Deliu (Médecine Interne); Kremlin-Bicêtre, Hôpital du Kremlin-Bicètre: O. Lambotte, M. De Lavaissiere (Médecine Interne), P. Bourrée (Mycologie), J. Jabot (Réanimation Médicale); Lyon, Hospices Civils de Lyon: M.A. Piens, F. de Montbrison, A.-L. Bienvenu (Mycologie), E. Togent (Réanimation Médicale), B. Ponseau, A. Boibieu (Maladies Infectieuses et Tropicales); Mantes La Jolie: V. Perronne (Maladies Infectieuses et Tropicales), O. Eloy (Mycologie) ; Marseille, Assistance Publique-Hôpitaux de Marseille: S. Genot (Maladies Infectieuses et Tropicales), S. Ranque (Mycologie); Nantes: Y. Lepape (Mycologie); Nice, Centre Hospitalier Universitaire: P. Dellamonica, P.-M. Roger (Maladies Infectieuses et Tropicales), M. Gari-Toussaint (Mycologie); Nimes, Centre Hospitalier Universitaire: C. Lechiche (Maladies Infectieuses et Tropicales), L. Lachaud (Mycologie); Orléans: L. Hocqueloux, T. Prazuk (Maladies Infectieuses et Tropicales), D. Poisson (Mycologie); Paris, Hôpital Bichat: S. Houzet, C. Chochillon (Mycologie), C. Leport, S. Matheron (Maladies Infectieuses et Tropicales), M. Wolff (Réanimation Médicale), L. Choudat, A. Rousseau (Anatomopathologie); Hôpital Cochin: D. Salmon, P. Blanche (Médecine Interne), N. Dupin, N. Franck (Dermatologie), M. T. Baixench (Mycologie); Hôpital Saint-Antoine: JL Poirot (Mycologie), M. C. Meyohas, D. Bollens (Maladies Infectieuses et Tropicales); Hôpital Tenon: S. Jaureguiberry (Maladies Infectieuses et Tropicales), M. Develoux (Mycologie); Hôpital Européen Georges Pompidou: E. Dannaoui (Mycologie), M. Karmochkine (Immunologie Clinique); Hôtel-Dieu: E. Aslangul (Médecine Interne), M. Cornet (Mycologie); Hôpital Lariboisière, V. Delcey (Médecine Interne); Hôpital Necker-Enfants Malades: J. P. Viard, B. Dupont, F. Lanternier, M. E. Bougnoux (Mycologie); Hôpital de la Pitié-Salpêtrière: A. Datry (Mycologie); Hôpital Rothschild: W. Rozenbaum, A Trylezinski (Maladies Infectieuses et Tropicales); Hôpital Saint-Louis: J. M. Molina (Maladies Infectieuses et Tropicales), C. Lacroix (Mycologie), E. Azoulay (Réanimation Médicale) ; Poitiers: C. Kauffman-Lacroix (Mycologie), C. Godet (Maladies Infectieuses et Tropicales); Rennes, Hôpital Pontchaillou: C. Arvieux (Maladies Infectieuses et Tropicales), S. Chevrier (Mycologie); Rouen: L. Favennec (Mycologie), F. Tamion (Réanimation Médicale); Saint-Étienne: P. Lucht (Maladies Infectieuses et Tropicales) ; in Saint-Mandé, Hôpital Bégin: P. Imbert; Toulouse, Hôpital Purpan: L. Porte (Maladies Infectieuses et Tropicales), M.-D. Linas (Mycologie); Troyes: F. Benaoudia (Mycologie).
Footnotes
Authors' addresses: Vincent Peigne, Unité de Mycologie Moléculaire, Centre National de Référence Mycologie et Antifongiques Institut Pasteur, 25–28 Rue du Docteur Roux, 75015 Paris, France (present address: Service de Réanimation Médicale, Hôpital Européen Georges Pompidou, 20 Rue Leblanc, 75015 Paris, France), E-mail: vincent.peigne@egp.aphp.fr. Françoise Dromer, Unité de Mycologie Moléculaire, Centre National de Référence Mycologie et Antifongiques, Centre National de la Recherche Scientifique Unité de Raccordement des Abonnés 3012, Institut Pasteur, 25–28 Rue du Docteur Roux, 75015 Paris, France, E-mail: francoise.dromer@pasteur.fr. Caroline Elie, Département de Biostatistiques, Hôpital Necker-Enfants Malades, 149 Rue de Sèvres, 75015 Paris, France, E-mail: caroline.elie@parisdescartes.fr. Olivier Lidove, Service de Médecine Interne, Centre Hospitalier Universitaire Bichat, 48 Rue Henri Huchard, 75018 Paris, France, E-mail: olivier.lidove@bch.ap-hop-paris.fr. Olivier Lortholary, Unité de Mycologie Moléculaire, Centre National de Référence Mycologie et Antifongiques, Centre Hospitalier Universitaire 3012, Institut Pasteur, 25–28 Rue du Docteur Roux, 75015 Paris, France and Université Paris Descartes, Hopital Necker-Enfants Malades, Centre d'Infectiologie Necker Pasteur, 149 Rue de Sèvres, 75015 Paris, France, E-mail: olortho@pasteur.fr.
References
- 1.Leads from the MMWR. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. JAMA. 1987;258:1143–1145, 1149, 1153–1154. [PubMed] [Google Scholar]
- 2.Wheat LJ. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49:274–282. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 3.Wheat LJ, Connolly-Stringfield PA, Baker RL, Curfman MF, Eads ME, Israel KS, Norris SA, Webb DH, Zeckel ML. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Segura L, Rojas M, Pelaez N, Shor-Posner G, RosaRe D, Moreno J, Klaskala W, Baum MK. Disseminated histoplasmosis and human immunodeficiency virus type 1 infection: risk factors in Guatemala. Clin Infect Dis. 1997;25:343–344. doi: 10.1086/514547. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, Powderly WG, Hafner R, Kauffman CA, Dismukes WE. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137:105–109. doi: 10.7326/0003-4819-137-2-200207160-00008. [DOI] [PubMed] [Google Scholar]
- 6.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 7.Wheat LJ, Chetchotisakd P, Williams B, Connolly P, Shutt K, Hajjeh R. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin Infect Dis. 2000;30:877–881. doi: 10.1086/313824. [DOI] [PubMed] [Google Scholar]
- 8.de Francesco Daher E, de Sousa Barros FA, da Silva Junior GB, Takeda CF, Mota RM, Ferreira MT, Martins JC, Oliveira SA, Gutierrez-Adrianzen OA. Risk factors for death in acquired immunodeficiency syndrome-associated disseminated histoplasmosis. Am J Trop Med Hyg. 2006;74:600–603. [PubMed] [Google Scholar]
- 9.Hajjeh RA, Pappas PG, Henderson H, Lancaster D, Bamberger DM, Skahan KJ, Phelan MA, Cloud G, Holloway M, Kauffman CA, Wheat LJ. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin Infect Dis. 2001;32:1215–1220. doi: 10.1086/319756. [DOI] [PubMed] [Google Scholar]
- 10.Couppie P, Sobesky M, Aznar C, Bichat S, Clyti E, Bissuel F, El Guedj M, Alvarez F, Demar M, Louvel D, Pradinaud R, Carme B. Histoplasmosis and acquired immunodeficiency syndrome: a study of prognostic factors. Clin Infect Dis. 2004;38:134–138. doi: 10.1086/379770. [DOI] [PubMed] [Google Scholar]
- 11.Breton G, Adle-Biassette H, Therby A, Ramanoelina J, Choudat L, Bissuel F, Huerre M, Dromer F, Dupont B, Lortholary O. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. AIDS. 2006;20:119–121. doi: 10.1097/01.aids.0000199014.66139.39. [DOI] [PubMed] [Google Scholar]
- 12.Antinori S, Magni C, Nebuloni M, Parravicini C, Corbellino M, Sollima S, Galimberti L, Ridolfo AL, Wheat LJ. Histoplasmosis among human immunodeficiency virus-infected people in Europe: report of 4 cases and review of the literature. Medicine (Baltimore) 2006;85:22–36. doi: 10.1097/01.md.0000199934.38120.d4. [DOI] [PubMed] [Google Scholar]
- 13.Ashbee HR, Evans EG, Viviani MA, Dupont B, Chryssanthou E, Surmont I, Tomsikova A, Vachkov P, Enero B, Zala J, Tintelnot K. Histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Med Mycol. 2008;46:57–65. doi: 10.1080/13693780701591481. [DOI] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 16.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.De Lavaissiere M, Manceron V, Bouree P, Garcon L, Bisaro F, Delfraissy JF, Lambotte O, Goujard C. Reconstitution inflammatory syndrome related to histoplasmosis, with a hemophagocytic syndrome in HIV infection. J Infect. 2009;58:245–247. doi: 10.1016/j.jinf.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Karimi K, Wheat LJ, Connolly P, Cloud G, Hajjeh R, Wheat E, Alves K, Lacaz Cd Cda S, Keath E. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J Infect Dis. 2002;186:1655–1660. doi: 10.1086/345724. [DOI] [PubMed] [Google Scholar]
- 19.Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard JP, Dromer F, Lortholary O. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13:1647–1652. doi: 10.3201/eid1311.070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ., Jr Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis. 2008;62:151–156. doi: 10.1016/j.diagmicrobio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Lortholary O, Poizat G, Zeller V, Neuville S, Boibieux A, Alvarez M, Dellamonica P, Botterel F, Dromer F, Chene G. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 22.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 23.Morris A, Wachter RM, Luce J, Turner J, Huang L. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS. 2003;17:73–80. doi: 10.1097/00002030-200301030-00010. [DOI] [PubMed] [Google Scholar]
- 24.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, Battegay M, Vernazza P, Bernasconi E, Opravil M, Kaufmann D, Sudre P, Francioli P, Telenti A. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 25.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 26.Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo Mvondo D, Longuet P, Leport C, Vilde JL. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 27.Nacher M, Sarazin F, El Guedj M, Vaz T, Alvarez F, Nasser V, Randrianjohany A, Aznar C, Carme B, Couppie P. Increased incidence of disseminated histoplasmosis following highly active antiretroviral therapy initiation. J Acquir Immune Defic Syndr. 2006;41:468–470. doi: 10.1097/01.qai.0000209927.49656.8d. [DOI] [PubMed] [Google Scholar]


