Abstract
S. aureus represents a critical cofactor in atopic dermatitis (AD). In this paper, the prevalence of S. aureus infection/colonization was evaluated in 117 children as well as in their cohabitants, in order to assess the value of S. aureus characterization in predicting disease onset and severity and in providing indications for prophylaxis. Results showed that children with AD as well as their cohabitants had a significantly greater incidence of S. aureus infection/colonization as compared to controls. The genetic characterization showed a virtual identity of the bacteria strains collected at different sites of the patients with those found in the cohabitants, suggesting both a direct transmission between the nasal reservoir and the lesions in the same atopic subject and a risk for reinfection within family cohabitants. These data stress the need of preliminary laboratory assessment and posttherapy control in both AD patients and their close contacts for effective S. aureus eradication.
1. Introduction
Atopic dermatitis (AD) is an inflammatory disease affecting the skin and characterized by impaired epidermal barrier function and cutaneous inflammation [1, 2]. It is clinically characterized by an early onset, mostly occurring before 5 years of age [3]. The prevalence of AD has increased in industrialized countries during the past three decades, and among Italian schoolchildren the estimated prevalence is presently of about 5.8% [4]. The eczematous skin of the patients is highly susceptible to bacterial colonization, and Staphylococcus aureus (S. aureus) represents the most frequent isolate [5–8]. S. aureus is a Gram-positive opportunistic bacterium that, although part of the normal flora of the skin, can give rise, as a consequence of clonal evolution, to virulent strains characterized by the expression of virulence factors and the acquisition of resistance to a number of different drugs including β-lactams [9]. S. aureus infection appears to play an important role in the pathogenesis of AD by either causing or exacerbating skin inflammation [10–13]. In fact, S. aureus toxins have potent superantigenic properties (e.g., staphylococcal enterotoxin SEA, SEB, SEC, SED, and toxic shock syndrome toxin (TSST-1)) and may induce monocytes and lymphocytes activation, which in turn are induced to produce several inflammatory cytokines [14, 15]. In a healthy skin, this process causes an inflammatory reaction with a clinical outcome similar to that seen in AD [10].
Although the impact of S. aureus infection in the clinical manifestation of AD is still debated, antibiotic-corticosteroid combination therapy against S. aureus represents an important component of the therapeutic approach to AD [16] and appears effective against both the occurrence of secondary infections and the severity of the disease [17]. However, it should be also considered that the clinical benefits appear often transient and that the onset of an antibiotic resistance represents a serious emerging problem in long-term therapy [18, 19] suggesting the need of an accurate microbiological analysis for a most adequate and effective therapy.
In this study, we investigated the prevalence and relationship between S. aureus infection/colonization, in both skin lesions and anterior nares, and the clinical severity of the disease in children with AD as compared to a control group of asymptomatic children and examined the influence of S. aureus colonization in the family members as a potential source for reinfection. The results indicated that children with AD had a significantly greater prevalence of S. aureus infection/colonization as compared to controls. The genetic characterization confirmed the identity of bacteria strains collected from the patients with those found in the cohabitants, suggesting both a direct transmission between the lesions in the same atopic subject and a risk for reinfection within family cohabitants. These results emphasize the importance of a preliminary laboratory assessment and posttherapy control in both AD patients and their close contacts for effective S. aureus eradication.
2. Patients and Methods
2.1. Patients and Controls
Patients incoming the Pediatric Outpatient Clinic at the San Gallicano Dermatology Institute were examined during a two-year period (2007-2008). One hundred and seventeen children aged between 3 months and 12 years (42 patients were under 12 months of age) of both sexes, suffering from mild to severe AD, were included in the study. Diagnosis of AD was based on the criteria described by Hanifin and Rajka [20]. The severity of AD was measured by the scoring atopic dermatitis index (SCORAD) of the European Task Force on atopic dermatitis. Briefly, a numeric score (1–10) was assigned to (a) extent and (b) intensity of skin lesions and (c) to subjective symptoms like pruritus and sleep loss. The final SCORAD was calculated by the following equation: a/5 + 7b/2 + c. In the present study, the clinical expression of disease was classified as low, medium, or high according to SCORAD values ranging between 0 and 15, 15, and 40 or >40, respectively. Patients did not receive any steroid or antibiotic therapy in the last two months before the initiation of the present study. Parents and cohabitant family members of AD children were asked to voluntarily participate to the study by allowing skin and nose sampling and examination aimed at S. aureus isolation and characterization. One hundred and ten subjects of 37 families accepted to enter the study. S. aureus strains were also examined in asymptomatic carriers consisting of 90 healthy children, of both sexes (39/51 M/F), aged between 3 months and 12 years and 80 adults, of both sexes, aged between 25 and 50, attending the Outpatient Dermatology Clinic to control pigmented lesions. All samples were obtained by sterile swabs, from skin and nose. All patients parents and healthy volunteers gave their written informed consent before study initiation.
2.2. Microbiological Analysis
Swabs were collected from lesions, normal skin areas as well as from nares of atopic children. Nasal swabs were performed in their cohabitants or control subjects. Samples were plated on enriched (blood agar), selective (Mannitol Salt Agar) and differential (MRSA ChromID-BioMérieux, France) media and incubated for 24–48 h at 37°C. Identification and antimicrobial susceptibility of S. aureus were performed by an automated diagnostic system (Card AST P580, Vitek 2, BioMérieux, France) and included the most frequently used drugs for therapeutic use against S. aureus. Bacteria isolates were classified as Methicillin-resistant Staphylococcus aureus (MRSA) on the basis of resistance to oxacillin (≥4 μg/mL) and positivity of the Penicillin-Binding Protein latex agglutination test (PBP2', Oxoid, UK).
2.3. Molecular Characterization
Molecular analysis was performed through Multiplex-PCR as previously described [21] in order to assess the expression of the following genes: capsular antigen (cap 5-cap 8); agr-group (agr); adhesins (hls-spa-ica A-atl-cna-sdr E-sdr C-fnb A-clf A/B); toxins (eta-sea-sej-sec-sed-sek-seq-tst-splB-lukE); Panton-Valentine leukocydin-PVL (lukS/F). Biofilm production was assessed as previously described [22–24].
2.4. Immunoassay
To detect the production of staphylococcal enterotoxins AD and toxic shock syndrome toxin-1 (TSST-1) isolates were incubated in tryptone soya broth and brain heart infusion, respectively, for 24 h at 37°C. Supernatants were tested for the presence of exotoxins by two specific reverse passive latex agglutination commercial kits (SET-RPLA and TST-RPLA) according to the instructions of the manufacturer (Oxoid, UK). The sensitivity of these agglutination assays is 0.5 ng/mL (SET-RPLA) and 2 ng/mL (TST-RPLA), respectively.
2.5. PFGE Analysis
Genotyping of isolated S. aureus strains was performed by pulsed-field gel electrophoresis (PFGE) after DNA digestion with SmaI (Fermentas ER0661), following the CDC (Center of Disease Control and Prevention, USA) protocol [25]. Briefly, electrophoresis was performed with pulses ranging from 5 to 45 seconds at a voltage of 6V/cm at 14°C for 23 hours using the PFGE instrument CHEF DR II (Bio-Rad, USA). The gels were stained for 20 minutes with ethidium bromide solution and observed under UV illumination.
3. Statistical Analysis
The statistical treatment of the results gathered from the different groups of subjects as well as the different clinical outcomes was performed by applying contingency tables and Fisher's t test (between two groups) or χ 2 test (among three groups). The analysis has been performed by the GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, Calif, USA).
4. Results
Of the 117 patients analyzed, 66 (57%) were found to harbor S. aureus (Table 1). In particular, we had positive isolation from skin lesions and nasal swabs in 47 patients (40.1%), while 19 patients (16.2%) had only nasal S. aureus colonization (Table 1). A prevalence of 36.4% of positive isolation were obtained from 31 family members of 20 patients, significantly higher (P = 0.0036) than the nonatopic adults examined as controls (Table 1). In healthy children, S. aureus was isolated at a significantly lower frequency (18 positive out of 90, 20%, P < 0.0001) from nasal swabs. Conversely, the frequency of detection from specimens collected from normal skin areas in patients with AD was low (4/117) and did not significantly differ from that of healthy children (0/90). Considering the age, the rate of positive isolation (including either skin or nares) was significantly higher in the group of children aged more than six (P = 0.0062) (Table 2). Regarding the impact on disease severity, the presence of S. aureus colonization appeared significantly related to a more severe clinical expression of pediatric AD (P = 0.0001) (Table 3).
Table 1.
Prevalence of S. aureus colonization in patients, family members, and controls. Contingency analysis and Fisher's exact test: *P < 0.0001; **P = 0.0036.
| Subjects | Pos/all | Lesional skin and nares | Nares | Uninvolved skin |
|---|---|---|---|---|
| Atopic children | 66/117* (57%) | 47/117 (40.2%) | 19/117 (16.2%) | 4/117 (3.4%) |
| Healthy children | 18/90 (20%) | — | 18/90 | 0/90 |
| Parents/relatives | 31/85** (36.4%) | — | 31/85 | 0/85 |
| Healthy adults | 16/80 (20%) | — | 16/80 | 0/80 |
Table 2.
Prevalence of S. aureus isolates in children with AD according to age.
| Age (yrs) | Pos/neg (n) | Positive (%) |
|---|---|---|
| <3 | 27/22 | 54.1 |
| 3–6 | 13/21 | 38.2 |
| 6–12 | 26/8 | 75.7 |
| *Chi-square test P = 0.0062. |
Table 3.
Prevalence of S. aureus isolates in children with AD according to disease severity.
| Disease score | Pos/neg | Positive (%) |
|---|---|---|
| Low | 3/17 | 15.0 |
| Medium | 25/23 | 52.0 |
| High | 38/11 | 77.5 |
| *Chi-square test P = 0.0001 |
Taking into consideration the expression of virulence factors by S. aureus isolates, the prevalence of MRSA strains among the total number of individual isolates in atopic patients was 7.9%, being present in the nose (4.5%) and, more frequently, in the skin lesion (12.8%). According to the molecular analysis, S. aureus strains isolated from atopic children (a total of 119, of which 96 were from skin lesion and nares of the same patients, 19 from nares of skin negative patients and 4 from healthy skin areas of 4 skin and nare positive patients), 94 (79%) had one or more genes coding for virulence factors such as adhesins, enterotoxins, TSST-1, CAP 5/8, the PV leukocydin, or the capacity to form biofilm (Table 4). Significant associations were found between the severity of the disease and high biofilm producing strains or TSST-1 positivity (P = 0.0003 and P = 0.002, resp.) (Table 5).
Table 4.
Prevalence of S. aureus toxigenic strains in atopic and healthy children.
| Toxigenic strains Pos/all (%) | MRSA Positive (%) | |
|---|---|---|
| AD lesional skin (47) | 37 (79%) | 6 (12.8) |
| AD uninvolved skin (4) | 3 (75%) | |
| AD nares (66) | 51 (77%) | 3 (4.5) |
| CTRLs nares (18) | 5 (28%)* | 0/18 |
| *Chi-square test *P < 0.0001 |
Table 5.
Molecular characterization of bacterial virulence factors of S. aureus strains isolated in children with AD and disease severity.
| Disease Score | Biofilm | Adhesins | Enterotoxins | PVL | TSST-1 | CAP 5/8 |
|---|---|---|---|---|---|---|
| Low/High | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | |
| Low/Medium | 19/15 | 32/0 | 26/6 | 1/31 | 12/20 | 34/0 |
| High | 8/43 | 58/0 | 45/13 | 1/57 | 28/31 | 51/0 |
| Fisher's t test | P = 0.0003 | P = 0.63 | P = 0.18 | P = 0.002 |
To evaluate the possible transmission between nares and skin of the same patient or between patients and their cohabitants, the genetic characterization of strains was assessed by DNA restriction fragmentation by PFGE. Similar PFGE patterns were exhibited by strains collected from skin or nares in the same patient in all 47 positive cases, as reported in Figure 1. The PFGE analysis also showed a close similarity among patients and their cohabitants in 75% of cases. Interestingly, a striking correspondence was found between strains isolated from mothers and atopic children (72%), while fathers and brothers showed a lower extent of identity (53% and 58%, resp.). The data from DNA analysis were confirmed by bacterial phenotyping according to the profile of toxins released in the supernatants of S. aureus cultures as detected by the immunoassay (Table 6). The data showed a correlation between the individual patient and at least one of the family members. Considering the expression of drug resistance, the results indicated that strains isolated from parents had a more complex profile of drug resistance than the similar strains isolated from their children (Table 6).
Figure 1.
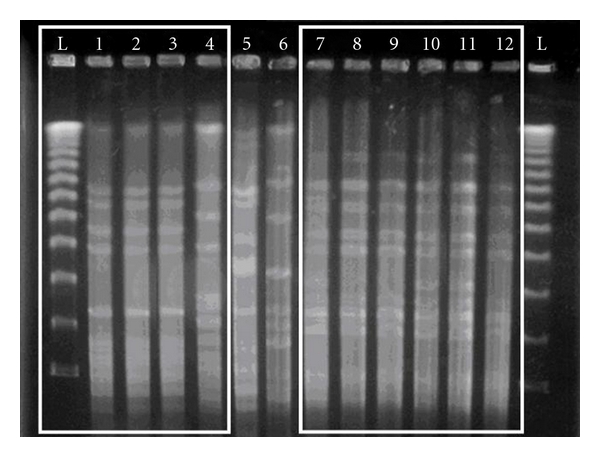
Pulse field gel electrophoresis (PFGE) of different isolates of S. aureus; the first and last lanes correspond to the DNA ladder; lanes 1–4 correspond to strains collected from lesional skin (1), nose (2), healthy skin (3) of an atopic patient (patient 16), and his father (4); lanes 7–12 show similar strains isolated from lesional skin, nose, healthy skin of an atopic patient (patient 26) and related mother, father, and brother.
Table 6.
Immunological detection of toxin array and drug resistance of S. aureus isolates from patients and their family members.
| Source of isolate | Localization | Enterotoxins TSST-1 | Antibiotic resistance |
|---|---|---|---|
| Patient 6 | Lesional skin | SEC | GM, P |
| Nose | SEC | P | |
| Patient 6, father | Nose | SEC | P |
| Patient 6, mother | Nose | SEC | P |
|
| |||
| Patient 10 | Lesional skin | SEC | P |
| Patient 10, father | Nose | SEC | P |
|
| |||
| Patient 15 | Lesional skin | SEB | OX, P, E |
| Nose | SEB | P | |
| Healthy skin | SEB | OX, CM, MUP, RF | |
| Patient 15, mother | Nose | SEB | P, CM, |
|
| |||
| Patient 16 | Lesional skin | SEA, TSST-1 | P, E, MUP |
| Nose | SEA, TSST-1 | P, E, MUP | |
| Healthy skin | SEA, TSST-1 | P, E, MUP | |
| Patient 16, father | Nose | SEA, TSST-1 | OX, P, E, MUP, MXF, |
| CM, TEC, TE, FA | |||
|
| |||
| Patient 19 | Nose | P | |
| Patient 19, mother | Nose | P | |
|
| |||
| Patient 21 | Lesional skin | FF | |
| Nose | Te | ||
| Patient 21, father | Nose | SEA, SED | P |
| Patient 21, mother | Nose | SEA, SED | P |
|
| |||
| Patient 23 | Lesional skin | P | |
| Nose | P | ||
| Patient 23, father | Nose | P, CM, E | |
| Patient 23, mother | Nose | P | |
|
| |||
| Patient 25 | Lesional skin | ||
| Patient 25, mother | Nose | ||
|
| |||
| Patient 26 | Lesional skin | P, FF | |
| Nose | TSST-1 | P | |
| Healthy skin | P, FF | ||
| Patient 26, father | Nose | P, FF | |
| Patient 26, mother | Nose | TSST-1 | P, E, CM |
| Patient 26, brother | Nose | TSST-1 | P, E, CM |
|
| |||
| Patient 27 | Lesional skin | SEB | P, E, CM |
| Nose | SEB | P, E, CM | |
| Patient 27, mother | Nose | SEB | P, E, CM |
| Patient 27, brother | Nose | SEB | P, E, CM |
|
| |||
| Patient 28 | Lesional skin | ||
| Patient 28, father | Nose | P, MUP | |
| Patient 28, mother | Nose | TSST-1 | |
|
| |||
| Patient 29 | Lesional skin | TSST-1 | P |
| Patient 29, mother | Nose | TSST-1 | P |
|
| |||
| Patient 30 | Lesional skin | TSST-1 | P |
| Nose | P | ||
| Patient 30, mother | Nose | TSST-1 | P |
|
| |||
| Patient 31 | Nose | SEC | P, E, CM |
| Patient 31, mother | Nose | SEC | P, E, CM |
|
| |||
| Patient 32 | Nose | ||
| Patient 32, father | Nose | TSST-1 | P |
| Patient 32, mother | Nose | TSST-1 | P |
| Patient 33 | Nose | TSST-1 | P |
| Patient 33, mother | Nose | TSST-1 | P |
|
| |||
| Patient 38 | Lesional skin | P | |
| Nose | P | ||
| Patient 38, father | Nose | P | |
| Patient 38, sister | Nose | P | |
|
| |||
| Patient 44 | Lesional skin | SEC | P, E, CM, FF |
| Nose | SEC | P, E, CM | |
| Healthy skin | SEC | P, E, CM | |
| Patient 44, father | Nose | SEC | |
| Patient 44, mother | Nose | SEC | P, E, CM |
|
| |||
| Patient 45 | Nose | TSST-1 | P |
| Patient 45, mother | Nose | TSST-1 | P |
|
| |||
| Patient 58 | Lesional skin | P | |
| Nose | P | ||
| Patient 58, father | Nose | P | |
| Patient 58, sister | Nose | P | |
Abbreviations: P: Benzylpenicillin; OX: Oxacillin; CM: Clindamycin; E: Erythromycin; FF: Fosfomycin and nitrofurantoin; FA: Fusidic Acid; GM: Gentamicin; MXF: Moxifloxacin; MUP: Mupirocin; RF: Rifampicin; TEC: Teicoplanin; TE: Tetracycline.
5. Discussion
Although the etiology of atopic eczema remains unknown, evidence suggests that both genetic and environmental factors play a role in determining both the susceptibility and the severity of the disease [5, 26]. Recent evidence suggests that S. aureus may play a key role in the pathogenesis of AD [8]. In fact, while S. aureus is rarely found on the skin of healthy subjects, as confirmed by our data on 80 control children, is very frequently found in patients suffering of atopic eczema. Several possible pathogenic mechanisms have been suggested for the role of S. aureus infection/colonization in AD pathogenesis pointing at either a direct chemical irritation or a nonspecific reaction of the S. aureus protein A with immune cells [27] as well as possible mechanisms involving superantigens production [28], which, in turn, exacerbates or maintain skin inflammation in atopic eczema. Indeed, S. aureus produces a group of toxins, which are capable of stimulating large populations of T-lymphocytes, even at distance from the eczematous sites, thus sustaining the activation of the immune system and the persistence of existing lesions. Conversely, the use of oral antibiotics as well as of topical antibiotics/antiseptics in combination with a topical steroid, antifungal agents or antiseptic bath additives, are often effective for the clinical management of AD and help reduce the severity of eczema and at improving the quality of life.
In the present study, we characterized the strains of S. aureus isolated from skin lesion and anterior nares of children with atopic dermatitis and from their cohabitants. The same analysis was performed in healthy subjects either children or adults. Our findings showed that the incidence of S. aureus infection/colonization is higher in children with AD, as compared to matched healthy controls (P < 0.0001) (Table 1). These data are in agreement with the prevalence previously found by others (50%−64.2%) [29, 30]. Interestingly, the prevalence of S. aureus colonization was higher in the patient's relatives (36%) (P = 0.0036) than that found in adult healthy subjects (Table 1). In fact, S. aureus isolates obtained from different sites of the same patients had a close biologic and molecular identity with those collected from patients' cohabitants (P < 0.0001), indicating that they harbored the same S. aureus strain. A reason for such a high rate of S. aureus colonization among the cohabitants of pediatric patients should be probably due to the close physical contact occurring between children and parents/relatives, especially required for care giving. This may concur in establishing a source for reinfection among family members as well as other cohabitants and may sustain intrafamiliar spreading with persistence or reactivation of the disease [30–32]. Considering the expression of drug resistance, results showed in Table 6 indicated that strains isolated from parents had a more complex profile of drug resistance than the similar strains isolated from their children. This could be probably due to an increased exposure to antibiotics given both systemically and locally in adults during their lives. On the other hand, we also found that the presence of S. aureus was associated with an age under three yrs or more than six yrs (P = 0.0062) (Table 2), and with a more marked severity of the disease (Table 3). It could be hypothesized that the exposure during the neonatal period is due to the close contact with their caregivers, while older children more frequently interact with the external environment. The assessment of the bacterial genotypic and phenotypic profiles, through immunologic and molecular methods, showed a significant higher frequency of S. aureus strains expressing molecules associated with virulence, such as enterotoxins, adhesins, PVL, biofilm production, or MRSA in children with AD as compared to asymptomatic carriers. Among these biologic properties, the ability to generate biofilm or TSST-1 were significantly more frequent (P = 0.0003 and P = 0.002, resp.) in patients with a more severe form of the disease as compared to those with a mild disease expression according to the criteria described by Hanifin and Rajka [20]. These factors may thus have an important impact on the clinical outcome and disease severity.
6. Conclusions
Our data suggest that the nasal cavity represent a key source for S. aureus skin colonization and that the familiar environment may play an important role in S. aureus colonization. Therefore, the eradication of these reservoirs might have a key impact on both the frequency and outcome of AD [33]. These results may indicate that clinical management of AD should include a routine testing for S. aureus not only on skin lesion but also in other important body reservoirs such as the nasal cavity [31] and emphasizes the need of performing susceptibility tests for antimicrobial drugs before initiating the therapy. In addition, routine testing should include also the family members of AD patients in order to break the chain of transmission through the establishment of an appropriate antibacterial therapy.
References
- 1.Akdis CA, Akdis M, Bieber T, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European academy of allergology and clinical immunology/American academy of allergy, asthma and immunology/PRACTALL consensus report. Journal of Allergy and Clinical Immunology. 2006;118(1):152–169. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 3.Illi S, Von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy and Clinical Immunology. 2004;113(5):925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 4.Girolomoni G, Abeni D, Masini C, et al. The epidemiology of atopic dermatitis in Italian schoolchildren. Allergy. 2003;58(5):420–425. doi: 10.1034/j.1398-9995.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 5.Bieber T. Atopic dermatitis. The New England Journal of Medicine. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 6.Guzik TJ, Bzowska M, Kasprowicz A, et al. Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: relationship to clinical and immunological parameters. Clinical and Experimental Allergy. 2005;35(4):448–455. doi: 10.1111/j.1365-2222.2005.02210.x. [DOI] [PubMed] [Google Scholar]
- 7.Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. British Journal of Dermatology. 2006;155(4):680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams JV, Vowels BR, Honig PJ, Leyden JJ. S. aureus isolation from the lesions, the hands, and the anterior nares of patients with atopic dermatitis. Pediatric Dermatology. 1998;15(3):194–198. doi: 10.1046/j.1525-1470.1998.1998015194.x. [DOI] [PubMed] [Google Scholar]
- 9.Ring J, Abeck D, Neuber K. Atopic eczema: role of microorganisms on the skin surface. Allergy. 1992;47(4 I):265–269. doi: 10.1111/j.1398-9995.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 10.Arslanagic N, Arslanagic R. Atopic dermatitis and Staphylococcus aureus. Medicinski Arhiv. 2004;58(6):363–365. [PubMed] [Google Scholar]
- 11.Goh CL, Wong JS, Giam YC. Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the national skin centre, Singapore. International Journal of Dermatology. 1997;36(9):653–657. doi: 10.1046/j.1365-4362.1997.00290.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams REA, Gibson AG, Aitchison TC, Lever R, Mackie RM. Assessment of a contact-plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. British Journal of Dermatology. 1990;123(4):493–501. doi: 10.1111/j.1365-2133.1990.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 13.Higaki S, Morohashi M, Yamagishi T, Hasegawa Y. Comparative study of staphylococci from the skin of atopic dermatitis patients and from healthy subjects. International Journal of Dermatology. 1999;38(4):265–269. doi: 10.1046/j.1365-4362.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharp MJ, Rowe J, Kusel M, Sly PD, Holt PG. Specific patterns of responsiveness to microbial antigens staphylococcal enterotoxin B and purified protein derivative by cord blood mononuclear cells are predictive of risk for development of atopic dermatitis. Clinical and Experimental Allergy. 2003;33(4):435–441. doi: 10.1046/j.1365-2222.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- 15.Nomura I, Tanaka K, Tomita H, et al. Evaluation of the staphylococcal exotoxins and their specific IgE in childhood atopic dermatitis. Journal of Allergy and Clinical Immunology. 1999;104(2):441–446. doi: 10.1016/s0091-6749(99)70390-8. [DOI] [PubMed] [Google Scholar]
- 16.Leyden JJ, Marples RR, Kligman AM. Treatment of atopic dermatitis. Allergy. 2004;59:86–92. [Google Scholar]
- 17.Shah M, Mohanraj M. High levels of fusidic acid-resistant Staphylococcus aureus in dermatology patients. British Journal of Dermatology. 2003;148(5):1018–1020. doi: 10.1046/j.1365-2133.2003.05291.x. [DOI] [PubMed] [Google Scholar]
- 18.Suh LM, Honig PJ, Yan AC. Methicillin-resistant Staphylococcus aureus skin abscesses in a pediatric patient with atopic dermatitis: a case report. Cutis. 2006;78(2):113–116. [PubMed] [Google Scholar]
- 19.Hoeger PH. Antimicrobial susceptibility of skin-colonizing S. aureus strains in children with atopic dermatitis. Pediatric Allergy and Immunology. 2004;15(5):474–477. doi: 10.1111/j.1399-3038.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 20.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 21.Campbell SJ, Deshmukh HS, Nelson CL, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. Journal of Clinical Microbiology. 2008;46(2):678–684. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvey MR, MacDougall L, Cholin B, et al. Community-associated methicillin-resistant Staphylococcus aureus, Canada. Emerging Infectious Diseases. 2005;11(6):844–850. doi: 10.3201/eid1106.041146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cafiso V, Bertuccio T, Spina D, Purrello S, Stefani S. Tigecycline inhibition of a mature biofilm in clinical isolates of Staphylococcus aureus: comparison with other drugs. FEMS Immunology and Medical Microbiology. 2010;59(3):466–469. doi: 10.1111/j.1574-695X.2010.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Christensen BB, Sternberg C, Andersen JB, et al. Molecular tools for study of biofilm physiology. Methods in Enzymology. 1999;310:20–42. doi: 10.1016/s0076-6879(99)10004-1. [DOI] [PubMed] [Google Scholar]
- 25.Center of Disease Control and Prevention. Short protocol for Staphylococcus aureus PFGE. pp. 23-24, 2001.
- 26.Bieber T, Novak N. Pathogenesis of atopic dermatitis: new developments. Current Allergy and Asthma Reports. 2009;9(4):291–294. doi: 10.1007/s11882-009-0041-2. [DOI] [PubMed] [Google Scholar]
- 27.White MI, Noble WC. The cutaneous reaction to staphylococcal protein A in normal subjects and patients with atopic dermatitis or psoriasis. British Journal of Dermatology. 1985;113(2):179–183. doi: 10.1111/j.1365-2133.1985.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 28.McFadden JP, Noble WC, Camp RD. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. British Journal of Dermatology. 1993;128(6):631–632. doi: 10.1111/j.1365-2133.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 29.Patel GK, Wyatt H, Kubiak EM, Clark SM, Mills CM. Staphylococcus aureus colonization of children with atopic eczema and their parents. Acta Dermato-Venereologica. 2001;81(5):366–367. doi: 10.1080/000155501317140124. [DOI] [PubMed] [Google Scholar]
- 30.Ricci G, Patrizi A, Neri I, Bendandi B, Masi M. Frequency and clinical role of Staphylococcus aureus overinfection in atopic dermatitis in children. Pediatric Dermatology. 2003;20(5):389–392. doi: 10.1046/j.1525-1470.2003.20503.x. [DOI] [PubMed] [Google Scholar]
- 31.Chiu LS, Chow VC, Ling JM, Hon KL. Staphylococcus aureus carriage in the anterior nares of close contacts of patients with atopic dermatitis. Archives of Dermatology. 2010;146(7):748–752. doi: 10.1001/archdermatol.2010.129. [DOI] [PubMed] [Google Scholar]
- 32.Gilani SJ, Gonzalez M, Hussain I, Finlay AY, Patel GK. Staphylococcus aureus re-colonization in atopic dermatitis: beyond the skin. Clinical and Experimental Dermatology. 2005;30(1):10–13. doi: 10.1111/j.1365-2230.2004.01679.x. [DOI] [PubMed] [Google Scholar]
- 33.Zollner TM, Wichelhaus TA, Hartung A, et al. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clinical and Experimental Allergy. 2000;30(7):994–1000. doi: 10.1046/j.1365-2222.2000.00848.x. [DOI] [PubMed] [Google Scholar]


