Abstract
Ceftriaxone (cfx), a third-generation cephalosporin antibiotic, leads to transient cholelithiasis in some children, also known as pseudolithiasis. However, the underlying pathogenetic mechanism of this adverse effect has not yet been elucidated. We describe 3 children with ceftriaxone-induced pseudolithiasis, who were also carriers of the A(TA)7TAA polymorphism of the UGT1A1 gene, implying that a cause and effect relation may exist.
1. Introduction
Cholelithiasis in childhood is usually considered a secondary effect of various predisposing factors such as hemolytic anemia, hepatobiliary diseases, total parenteral nutrition, sepsis, bowel resection, or a side effect of certain medications [1, 2]. Among the latter, ceftriaxone (cfx), originally described by Schaad et al. [3], has been recognized as a lithogenic substance in a proportion of treated patients. In these cases, fine precipitations or sludge are formed temporarily, resembling gallstones. They resolve shortly after stopping cfx, and various terms, such as “pseudolithiasis” or “reversible lithiasis”, have been used [4].
As the pathogenesis of cfx-induced pseudolithiasis is currently unclear, and main predisposing factors, that is, the drug and the infection are common in all patients, the answer might be hidden in the patient's genomic data. We observed 3 children with cfx-induced pseudolithiasis who were also carriers of the A(TA)7TAA polymorphism of the UGT1A1 gene. UGT1A1 encodes UDP-glucuronosyltransferase (UDPG), an enzyme engaged in the glucuronidation pathway that transforms small lipophilic molecules, (i.e., steroids, bilirubin, hormones, and drugs) into water-soluble, excretable metabolites.
2. Case Presentation
Our 3 patients, consisting of one girl and two boys, aged 5,5 months, 18 months, and 4 years old, respectively, were treated for urinary tract infection and received cfx (100 mg/kg/day) IV for 10–14 days. One patient also received cefotaxime prior to cfx treatment. None had a history of predisposing factors, a family history of gallstones, or was treated with drugs associated with gallbladder lithiasis, such as octreotide or furosemide.
All three patients developed gallbladder pseudolithiasis with typical sonographic appearance: intense, mobile, echogenic material with acoustic shadow (Figure 1). They were repeatedly screened with gallbladder ultrasound (US) while on treatment, and follow-up examination was scheduled weekly until resolution of pseudolithiasis was seen. Extensive laboratory investigation revealed normal blood cell count without overt hemolysis, liver and renal function tests within normal limits, as well as serum lipids and electrolytes. Extensive details of the patients' data can be found in Table 1.
Figure 1.
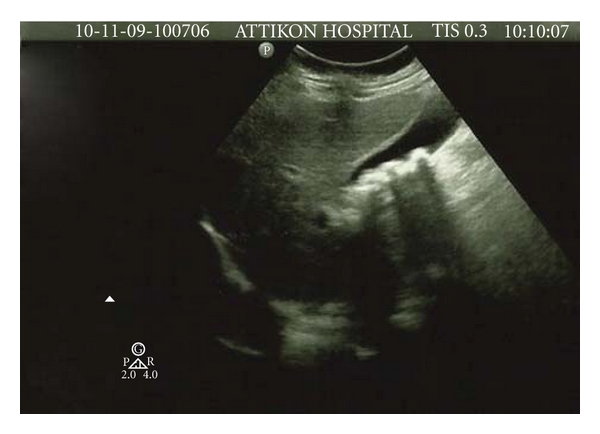
Sonogram in one of our patients showing multiple echogenic material with acoustic shadowing.
Table 1.
Data about the clinical and family history and lab results (inflammation markers and liver function) of the patients.
| Patient #1 | Patient #2 | Patient #3 | |
|---|---|---|---|
| Clinical complications | |||
| Pain | Not referred | Intense abdominal pain | Not referred |
| Others | Not referred | Not referred | Not referred |
| History of prolonged jaundice after birth | Not referred | Not referred | Not referred |
| Family history of gallstones and Gilbert (Meulengracht disease) | Not referred | Not referred | Not referred |
| Other genetic variations | Not referred | G6PD deficiency | Not referred |
|
| |||
| Lab results | |||
| Inflammation markers | |||
| WBC (/μL) | 16.170 | 18.240 | 14.690 |
| NEUT-LYMPH-MONO (%) | 94-4-2 | 65-29-6 | 48-43-3 |
| CRP (mg/L) | 15.5 | 19.9 | 45.90 |
|
| |||
| Liver function | |||
| SGOT (U/L) | 28 | 30 | 24 |
| SGPT (U/L) | 12 | 13 | 17 |
| ALP | 124 | 135 | 172 |
| γGT (U/L) | 9 | 14 | 10 |
| TBIL (mg/dL) | 0.41 | 0.3 | 0.2 |
| DBIL (mg/dL) | 0.13 | 0.11 | 0.1 |
WBC: white blood cell count, NEUT: neutrophils, LYMPH: lymphocytes, MONO: monocytes, CRP: C- reactive protein, SGOT: serum glutamic-oxaloacetic transaminase, SGPT: serum glutamic-pyruvic transaminase, ALP: alkaline phosphatase, γGT: gamma-glutamyl transpeptidase, TBIL: total bilirubin, DBIL: deconjucated bilirubin.
Parents gave informed consent prior to the inclusion of their children in the study. Genomic DNA for TA insertion on the TATAA box of the UGT1A1 gene was analyzed, as previously described [5]. Molecular analysis of the TATAA-box-like sequence of the UGT1A1 promoter revealed that two of the patients were heterozygous (TA7/TA6) and one homozygous (TA7/TA7) for the polymorphism in the TATAA box in the promoter region of the UGT1A1 gene. The study was approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards.
3. Discussion
Although several prospective studies have evaluated the incidence of transient gallbladder pseudolithiasis in patients treated with cfx, the underlying pathogenetic mechanism has not been elucidated as yet [3, 4, 6]. Of interest, our children with cfx-induced pseudolithiasis share a common denominator that is hetero-/homozygosity for the A(TA)7TAA polymorphism of the UGT1A1 gene, implying that a cause and effect relation may exist. In fact, the UDPG encoded by the UGT1A1 gene plays an important role in the handling of anionic substances in general, and in bilirubin elimination in particular. Furthermore, UDPG acts on glucuronidation and formation of bile salts [7].
It has long been recognised that the clinical sequence of reduced UGT1A1 expression and activity of the relevant enzyme is Gilbert syndrome with diverse percentages in different populations. Indeed, a previous study has shown that DNA polymorphisms of extra TA nucleotides in the repetitive TATAA box of the promoter region of UGT1A1 gene in Greek population are prevalent [8].
Numerous studies have tried to clarify the underlying pathogenesis of cfx-induced pseudolithiasis [6, 9]. Beginning with the chemical composition of fine lithogenic precipitations, it should be stated that they are mainly consisted of cholesterol monohydrate and calcium bilirubinate. It is also known that cholesterol precipitates when the cholesterol-solubilizing power of bile acid mixed micelles and phospholipid vesicles is overwhelmed. In addition, bilirubin is excreted as a soluble diglucuronide and deconjugation, either by nonenzymatic hydrolysis or by P-glucuronidase, results in free bilirubin. When the solubility product of calcium and unconjugated bilirubin is exceeded, calcium bilirubinate precipitates [10].
Nevertheless, how cfx has been involved in these mechanisms remains an unanswered question. This drug is a small, anionic, negatively charged organic molecule, mainly eliminated in the urine, and to a lesser extent (40%) excreted into bile [11]. Physicochemically, it is expected that when the solubility product of calcium and cfx is exceeded, precipitation should follow. Precipitation of a drug or a xenobiotic in the biliary system is rarely seen; however, as cfx is an organic anion, it behaves like other calcium-sensitive anions in bile (carbonate, bilirubinate, phosphate, palmitate) that are implicated in the pathogenesis of gallstones [10]. It has been found that cfx and bile acids share a common mechanism for hepatic transport and biliary excretion. It has also been proved that high doses of cfx are more likely to cause pseudolithiasis [9]. However, only a part of patients treated with the maximum dose develop pseudolithiasis, while pseudolithiasis has also been observed in lower doses [10]. On the other hand, considering that the drug's handling in all patients follows a defined process, and what is more, the infectious agent itself does not seem to deter the physicochemical consistency and properties of the drug, but only affects the volume distribution and tissue penetration, we assume that the factor determining the biliary sludge formation is not the infection. As a consequence, the fact that only a number of cfx-treated patients is subject to gallbladder precipitations indicates that predisposed individuals do exist. In addition, as there are no statistically important differences in the characteristics and phenotypes of the affected group (age, gender, etc.), the differentiating factor in this clinical expression must be hidden in patients' genes. For this reason, we have already begun a prospective study in which we follow-up children hospitalised in our department and treated with cfx, studying with molecular analysis the genomic regions with high incidence of the UGT1A1 gene polymorphisms.
In conclusion, we presented three patients with cfx-induced pseudolithiasis and provided suggestions for this occurrence among which the reduced function of UDPG due to UGT1A1 gene polymorphisms seems to be the most compatible.
Conflict of Interests
The authors declare that there is no applicable conflict of interests or source of funding.
Abbreviations
- Cfx:
Ceftriaxone
- UGT1A1:
UDP-glucuronosyltransferase 1 family polypeptide A1
- UDPG:
UDP-glucuronosyltransferase
- US:
Ultrasound.
References
- 1.Ko CW, Sekijima JH, Lee SP. Biliary sludge. Annals of Internal Medicine. 1999;130(4):301–311. doi: 10.7326/0003-4819-130-4-199902160-00016. [DOI] [PubMed] [Google Scholar]
- 2.Trotman BW, Bernstein SE, Bove KE, Wirth GD. Studies on the pathogenesis of pigment gallstones in hemolytic anemia. Journal of Clinical Investigation. 1980;65(6):1301–1308. doi: 10.1172/JCI109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaad UB, Tschäppeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatric Infectious Disease. 1986;5(6):708–710. doi: 10.1097/00006454-198611000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. The Lancet. 1988;2(8625):1411–1413. doi: 10.1016/s0140-6736(88)90596-x. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaidou P, Kostaridou S, Mavri A, Galla A, Kitsiou S, Stamoulakatou A. Glucose-6-phosphate dehydrogenase deficiency and Gilbert syndrome: a gene interaction underlies severe jaundice without severe hemolysis. Pediatric Hematology and Oncology. 2005;22(7):561–566. doi: 10.1080/08880010500198533. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet JP, Abid L, Dabhar A, Lévy A, Soulier Y, Blangy S. Early biliary pseudolithiasis during ceftriaxone therapy for acute pyelonephritis in children: a prospective study in 34 children. European Journal of Pediatric Surgery. 2000;10(6):368–371. doi: 10.1055/s-2008-1072393. [DOI] [PubMed] [Google Scholar]
- 7.Fevery J, Blanckaert N, Heirwegh KP, Preaux AM, Berthelot P. Unconjugated bilirubin and an increased proportion of bilirubin monoconjugates in the bile of patients with Gilbert’s syndrome and Crigler-Najjar disease. Journal of Clinical Investigation. 1977;60:970–979. doi: 10.1172/JCI108877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavazarakis E, Tsezou A, Tzetis M, et al. Gilbert syndrome: analysis of the promoter region of the uridine diphosphate-glucuronosyltransferase 1 gene in the Greek population. European Journal of Pediatrics. 2000;159(11):873–874. doi: 10.1007/s004310000603. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology. 1990;99(6):1772–1778. doi: 10.1016/0016-5085(90)90486-k. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Kestell MF, Lee SP. Gall-bladder sludge: lessons from ceftriaxone. Journal of Gastroenterology and Hepatology. 1992;7(6):618–621. doi: 10.1111/j.1440-1746.1992.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 11.Richards DM, Heel RC, Brogden RN. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984;27(6):429–527. doi: 10.2165/00003495-198427060-00001. [DOI] [PubMed] [Google Scholar]


