Abstract
The success of cancer vaccines is dependent on the delivery of tumor-associated antigens (TAAs) within lymphoid tissue in the context of costimulatory molecules and immune stimulatory cytokines. Dendritic cells (DCs) are commonly utilized to elicit antitumor immune responses due to their attractive costimulatory molecule and cytokine expression profile. However, the efficacy of DC-based vaccines is limited by the poor viability and lymph-node migration of exogenously generated DCs in vivo. Alternatively, adoptively transferred T cells persist for long periods of time in vivo and readily migrate between the lymphoid and vascular compartments. In addition, T cells may be genetically modified to express both TAA and DC-activating molecules, suggesting that T cells may be ideal candidates to serve as cellular vehicles for antigen delivery to lymph node-resident DCs in vivo. This paper discusses the concept of using T cells to induce tumor-specific immunity for vaccination against cancer.
1. Properties of an Effective Cancer Vaccine
Therapeutic cancer vaccines aim to induce antitumor immune responses through the generation of cytotoxic T cell responses to tumor-associated antigens (TAAs). TAAs are proteins that are either uniquely expressed (e.g., cancer testes antigens, mutated proteins, and viral antigens) or expressed to a higher degree (e.g., overexpressed proteins and differentiation antigens) by tumor cells [1]. The success of a cancer vaccine is contingent on (1) efficient antigen delivery to sites of T cell priming within lymphoid tissue and (2) antigen presentation in the context of costimulatory molecules and immunostimulatory cytokines.
To achieve these goals, a variety of cancer vaccination strategies have been tested clinically, ranging from simple peptide vaccines to more sophisticated approaches using plasmid DNA, viruses, tumor cells, and dendritic cells (DCs) [2]. To date, DC-based vaccine approaches have been most extensively pursued because DCs are considered to be the most potent professional antigen presenting cells (APCs) due to their superior ability to take up, process, and present antigens [3]. Immature DCs reside in peripheral tissues and augment antigen presenting capacity upon activation via the upregulation of MHC class I and II as well as the costimulatory molecules CD80, CD86, and CD83. Concomitantly, activated DCs upregulate CCR7 expression and migrate to T cell zones within lymph nodes, where T cell priming occurs [4]. DCs pulsed with tumor antigens have led to protective immunity and tumor regression in mouse models of cancer [5], and there are a multitude of completed and ongoing vaccine trials in humans that have demonstrated T cell mediated immune responses following DC vaccination.
2. Limitation of Current Strategies
Although much excitement has been generated recently over the FDA approval of sipuleucel-T following prolonged survival in prostate cancer patients [6, 7], the efficacy of DC-based vaccines remain limited in several regards. First, DCs are a terminally differentiated cell type that cannot be expanded ex vivo, resulting in limited numbers of cells with which to vaccinate patients. Furthermore, upon administration of DCs to patients, the vast majority of cells are sequestered at the injection site and fail to migrate to draining lymph nodes [8, 9]. DC viability as well as peptide-MHC complex integrity is lost after 24–48 hours [10], and sequestration allows ample time for DC dedifferentiation, which may result in immune tolerance rather than activation [11]. Attempts to bypass cellular sequestration such as intranodal DC injection are technically difficult as evidenced by an injection accuracy of only 50% despite ultrasound guidance at the hands of an experienced radiologist [12]. The net effect of these limitations is poor antigen delivery to lymphoid tissue and antigen presentation in the absence of immune-stimulatory signals. These limitations have most likely contributed to the low objective clinical responses observed in DC-based vaccine trials. In the case of melanoma, an immunogenic tumor where TAAs have been clearly identified and infiltrating lymphocytes are often observed, clinical response rates may be as high as 10% [13]. However, the overall efficacy of DC-based vaccines has been disappointing when tumor types other than melanoma are taken into account, producing clinical response rates of only 4% [2, 14]. The limited success of DC vaccines can to some extent be attributed to suboptimal vaccination strategies performed in phase I clinical trials, such as the use of immature DCs, lack of vaccine adjuvants, and targeting of single MHC class I-restricted epitopes. Despite an increased understanding of necessary vaccine components, the therapeutic efficacy of DC vaccines is yet to improve significantly. Thus, while DC vaccination remains an attractive strategy, its therapeutic efficacy may be limited, and alternative vaccine approaches should continue to be pursued.
3. Targeting DC In Vivo
Much research has focused on various methods of DC maturation ex vivo to maximize the expression of costimulatory molecules, proinflammatory cytokines, and lymphotropic chemokine receptors to optimize their function in vivo. However, recent evidence has suggested that ex vivo-derived DC vaccines may play a limited role in the priming of T cells in vivo [15]. Antigen delivered by short-lived migratory DCs can be processed by endogenous DCs within the lymph node [16]. The immune effects of exogenous DC vaccination have been demonstrated to be contingent on the transfer of antigen to endogenous DCs but not B cells, and antigen transfer is not due to antigen diffusion, but rather DC-DC molecular transfer [17]. Importantly, vaccination with apoptotic or necrotic DCs abrogated vaccine effects, indicating that viable DCs are needed to migrate to lymphoid tissue and actively deliver antigen. The selective ablation of endogenous lymphoid-resident DCs abrogated T cell responses following DC vaccination, demonstrating the pivotal role this subset of DCs plays in this phenomenon [18].
Murine lymphoid-resident DCs are characterized by the expression of both CD11c and CD8α, and the human equivalent to murine CD8α+ DCs has been recently identified and is characterized by the expression of Clec9A (DNGR-1), BDCA3, and XCR1 [19–22]. The CD8α+ DC subset has been demonstrated to play an important role in the priming of CD8+ T cells due to a unique capacity to cross-present antigens via MHC class I [23–26] and produce high levels of IL-12 following Toll-like receptor activation [27, 28]. CD8α+ DCs are strategically poised to engulf antigen entering the lymph node from the blood and lymphatics as well as from DCs migrating from the periphery into the lymph nodes. Indeed, migratory DCs have been demonstrated to transfer antigen to lymphoid-resident DCs and have led to CD8+ T cell priming following herpes simplex virus [29] and influenza infection [30].
Appreciation for the important role CD8α+ DCs play in CD8+T cell priming has spawned new targeted vaccine strategies that aim to direct antigen specifically to DCs in vivo, and thereby circumvent the various limitations of exogenous DC vaccination [31]. One attractive approach is to administer antigen conjugated to antibodies specific to surface receptors shared by DCs and other cell types, such as the mannose receptor or Fcγ receptors. The specificity of DC-targeting can be narrowed by targeting more DC-restricted receptors. Many of these receptors belong to the C-type lectin receptor (CLR) family, such as DEC-205 and DC-SIGN. Several CLRs have been identified to be expressed uniquely on the surface of CD8α+ DCs, which allows selective targeting of this particular DC subpopulation. Both DEC-205 [32] and Clec9A (DNGR-1) [33, 34] have successfully served as targets for antibody-mediated antigen delivery in vivo.
4. T Cells for Targeting DCs In Vivo
Although DC-targeted strategies using antibody-conjugated antigens are attractive for large-scale clinical application, this method of vaccination often requires the coadministration of immune adjuvants that lack clinical approval, such as agonistic anti-CD40 antibodies. Cellular vehicles like DCs are attractive options for vaccination because in addition to the expression of antigen these cells express the necessary costimulatory molecules and proinflammatory cytokines necessary for the generation of effective cytotoxic T lymphocyte (CTL) responses. However, as stated previously, exogenously generated DCs fail to efficiently deliver antigen to lymphoid tissue. Therefore, a cell type that can efficiently migrate to lymphoid tissue following infusion would be an attractive vehicle for antigen delivery to lymphoid-resident DC in vivo.
One potential cell type with lymphotropic properties is the T cell. Subsets of T cells efficiently migrate from the vasculature to the vicinity of CD8α+ DCs in the lymphatic compartment [35]. Naïve or in vitro-expanded nonpolarized T cells efficiently migrate to lymphoid tissues although T cell polarization toward a type-1 or type-2 phenotype appears to inhibit lymph node migration [35]. To deliver tumor antigens, T cells can be surface-loaded with tumor peptides or be genetically modified to express whole TAAs. In contrast to DCs, T cells can be expanded to large numbers ex vivo to provide an abundance of autologous antigen delivery vehicles to allow for the administration of large vaccinating cell doses and increased frequency of vaccination.
The potential use of T cells as antigen delivery vehicles for vaccination was made apparent following the adoptive transfer of herpes simplex virus thymidine kinase (HSV-TK) gene-modified T cells to human subjects [36, 37]. Infusion of T cells genetically modified with the foreign protein HSV-TK induced robust CD4+ and CD8+ anti-HSV-TK T cell responses which led to the destruction of transferred cells [37]. In addition, HSV-TK gene modified T cells generated memory T cells which led to a boosted T cell response upon additional administrations of HSV-TK T cells. The diversity and stability of the T cell response to HSV-TK generated by gene-modified T cells suggested that antigen delivery by T cells could function as a potential vaccination approach for targeting viral or tumor antigens.
T cells genetically modified to express viral proteins, such as influenza A matrix protein, have been demonstrated to enhance the in vivo persistence of adoptively transferred virus-specific T cells [38]. Although this finding suggests a role for T cell-based vaccine approaches to boost adoptively transferred T cells, much broader vaccine applications could be attained following the demonstration that infusion of antigen-loaded T cells could lead to the priming of T cell responses to TAAs, which are most commonly weakly immunogenic self-antigens. Russo et al. demonstrated that T cells modified to express the melanoma TAA tyrosinase-related protein 2 (TRP-2) could lead to the priming of TRP-2-specific T cell responses following infusion [39]. Vaccination using TRP-2-modified T cells led to the establishment of protective immunity and long-term memory responses in B16F10 melanoma tumor-bearing mice. The authors were able to demonstrate that CD8α+ DCs underwent maturation following the phagocytosis of genetically modified T cells, which could subsequently cross-present TRP-2 antigen and prime TRP-2-specific T cell responses. Importantly, the authors demonstrated that selective ablation of DCs prior to vaccination with T cells modified to express ovalbumin could not induce the expansion of adoptively transferred CFSE-labeled OT-I T cells in vivo, validating that the observed vaccine effects were not mediated by direct antigen presentation by T cells but rather antigen uptake and subsequent presentation by endogenous DCs. These promising results subsequently led to a clinical trial in which 10 melanoma patients were administered MAGE-A3 modified lymphocytes [40]. MAGE-A3-specific T cell responses were detected in 3/10 patients following vaccination. Although the clinical outcomes following this vaccination strategy were underwhelming, this study demonstrated the feasibility of this approach for human application.
5. Genetic Modifications to Enhance the Immune Response Following T Cell Vaccination
Although T cells may efficiently deliver antigen to lymphoid tissue in vivo, delivery of antigen in the absence of concurrent DC maturation would likely lead to inefficient T cell priming or could even induce tolerance. The generation of effector T cell responses requires concurrent activation by costimulatory molecules and proinflammatory cytokines at the time of antigen presentation. Although T cells are not themselves considered professional APCs, upon activation T cells upregulate MHC class I and II molecules [41], the costimulatory molecules CD80, CD83, and CD86 [42, 43] as well as secrete proinflammatory cytokines such as IL-2, IFN-γ, and TNF-α [44]. T cells can induce the proliferation of resting T cells in mixed lymphocyte reactions [45] and preferentially induce cytotoxic T cell responses [46]. T cells are capable of presenting both pulsed and transduced viral or tumor peptide antigens and can process full-length antigens expressed from vectors [47, 48]. Taken together, these observations suggest T cells may function independently of DCs as APCs. However, the antigen presenting role T cells play in vivo is likely insignificant compared to that of professional APCs, due to the relatively lower expression of costimulatory molecules and the complete lack of type-1 polarizing cytokines, such as IL-12. Hence, concurrent DC activation at the time of vaccination is likely necessary to induce effective CTL responses to antigen delivered by T cells.
In addition to antigen expression, T cells may be further modified to express molecules that induce DC maturation (Figure 1). Maturation of DCs is most often mediated through the activation of Toll-like receptors (TLRs). TLR-ligands are well-conserved features of bacteria and viruses known as pathogen-associated molecular patterns (PAMPs). DCs express many different TLRs that can recognize a variety of PAMPs, such as lipopolysaccharide, double-stranded RNA, and unmethylated CpG dinucleotides.
Figure 1.
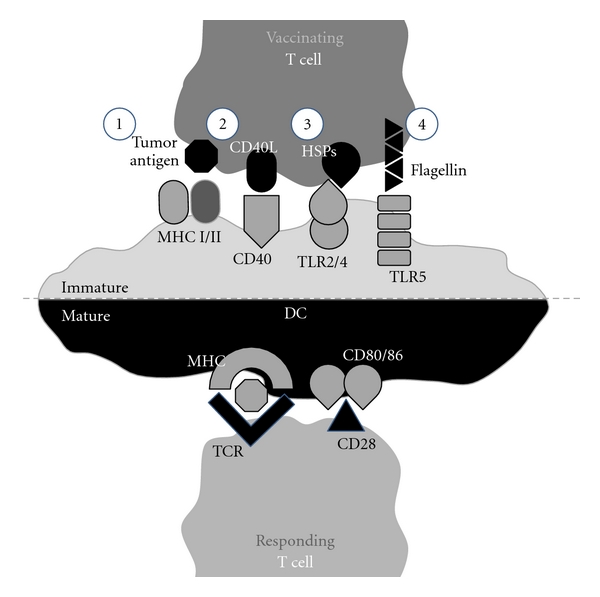
Targeting dendritic cells (DCs) in vivo using T cells for cancer vaccination. Upon infusion, T cells efficiently home to lymphoid tissue where they encounter lymph node-resident DCs. T cells may be genetically modified to express tumor-associated antigens as well as molecules that can induce DC activation, such as CD40L, heat shock proteins (HSPs), and flagellin. Interacting DCs engulf and present antigen delivered by T cells on MHC class I and II molecules. T cell-mediated DC maturation results in the upregulation of costimulatory molecules, such as CD80 and CD86, which are necessary for the generation of potent helper CD4+ and effector CD8+ T cell responses.
Flagellin is one TLR ligand of interest, because it is one of the few TLR ligands that is a protein, allowing for transgenic expression by T cells. Flagellin is the major protein constituent of bacterial flagellum and is recognized by TLR5. TLR5 is expressed on the surface of DCs isolated from lymph nodes [49]. Flagellin has been demonstrated to enhance the priming of antigen-specific CD4+ T cells [50] which results in a strong humoral response to produce protective antibodies [51], and flagellin fusion proteins have been shown to augment the generation of antigen-specific CD8+ cytotoxic T cell responses [51]. Flagellin, is a foreign protein, thus antiflagellin immune responses could potentially lead to the elimination of flagellin-expressing T cells and limit the effectiveness of vaccine boosting. However, preexisting immunity to flagellin does not appear to limit its effectiveness as a vaccine adjuvant [52, 53].
Various endogenous proteins, such as heat shock proteins (HSPs), have been found to bind TLRs and lead to DC maturation. HSPs play a central role in intracellular protein folding and transport. They are an abundant intracellular protein not expressed on the cell surface under normal physiologic conditions. The presence of HSPs in the extracellular compartment has been demonstrated to both facilitate antigen uptake and presentation as well as act as a danger signal to indicate cellular destruction due to bacterial or viral infections or mechanical damage [54]. HSP-peptide complexes can be internalized by professional APCs via receptor-mediated endocytosis [55–57] leading to the induction of not only helper CD4+ T cell responses via MHC class II presentation, but also CD8+ CTL responses via antigen cross-presentation on MHC class I molecules. Furthermore, several HSPs, such HSP60, HSP70, HSP90, gp96, and calreticulin, have been shown to induce DC maturation via recognition by TLR2 and TLR4 [58]. Surface expression of HSP70 and gp96 by tumor cells has been found to induce DC maturation and lead to antitumor immune responses in vivo [59, 60], and transgenic expression of gp96 in mice leads to systemic autoimmunity [61]. Such findings suggest that HSP expression could augment responses to TAAs delivered by T cells.
TLR-independent DC maturation can be achieved via the CD40 receptor. CD40 is a cell-surface receptor belonging to the tumor necrosis factor-receptor family and is expressed by a variety of cell types including B cells, monocytes, and DCs as well as endothelial and epithelial cells [62]. The natural ligand for CD40, CD154 (CD40L), is transiently expressed by CD4+ T cells and serves as a positive feedback signal for DC activation following T cell activation. CD40 cross-linking induces DCs to upregulate MHC class II and costimulatory molecule expression [63] as well as produce high levels of proinflammatory cytokines [64]. CD40 signaling results in CD8+ T cell priming independent of helper CD4+ T cells [65], and antibodies to CD40 can evoke strong antitumor CD8+ T cell responses in vivo [66–68]. CD154 expression on the surface of activated CD4+ T cells is tightly regulated, being expressed only transiently for <24 hours [69]. Therefore, stable CD154 expression may be attained by transgenic modification of T lymphocytes. Using retroviral-mediated gene modification, Higham et al. modified tumor-specific CD8+ T cells to express CD154 [70]. Despite stable gene integration, transgenic CD154 expression remained tightly regulated with decreasing expression 72 hours after transduction. The authors were able to increase transgenic CD154 expression by deletion of the intracellular domain of CD154 and reactivation of T cells. CD154-expressing T cells were subsequently demonstrated to mature DCs in vitro as well as activate tolerogenic DCs within tumor draining lymph nodes in vivo. Such an approach could be envisioned to facilitate DC activation for the purpose of enhancing immune responses to antigens delivered by T cells.
6. Summary
For cancer vaccines, delivery of antigen in the context of immune stimulatory signals that activate lymph-node resident DCs is a critical step in generating a robust antitumor immune response. Because of their natural lymph-node tropism and that they can be easily expanded ex vivo and genetically modified to alter biologic function, T cells represent a novel and flexible platform for cancer vaccine design. In addition to transgenic expression of tumor antigens and DC activating molecules, T cells may be further modified to secrete cytokines (e.g., IL-2, IL-7, IL-12, IL-15, and IL-21), improve tissue-specific migration via expression of chemokine receptors (e.g., CCR7, CXCR4, and CCR2), and express molecules that suppress immune regulatory cells, including CD4+CD25+FoxP3+ regulatory T cells. In contrast to more conventional DC vaccine strategies, we propose that using T cells to deliver tumor antigens into lymphoid organs, while providing essential immune activating signals required for the induction of antitumor immune responses, may ultimately improve the efficacy of cell-based cancer vaccines.
References
- 1.Stevanovic S. Identification of tumour-associated T-cell epitopes for vaccine development. Nature Reviews Cancer. 2002;2(7):514–520. doi: 10.1038/nrc841. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunological Reviews. 2011;239(1):27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. European Journal of Immunology. 1998;28(9):2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nature Medicine. 1995;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 6.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. Journal of Clinical Oncology. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 7.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 8.De Vries IJM, Krooshoop DJEB, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Research. 2003;63(1):12–17. [PubMed] [Google Scholar]
- 9.Okada N, Mori N, Koretomo R, et al. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Therapy. 2005;12(2):129–139. doi: 10.1038/sj.gt.3302358. [DOI] [PubMed] [Google Scholar]
- 10.Kukutsch NA, Roßner S, Austyn JM, Schuler G, Lutz MB. Formation and kinetics of MHC class I-ovalbumin peptide complexes on immature and mature murine dendritic cells. Journal of Investigative Dermatology. 2000;115(3):449–453. doi: 10.1046/j.1523-1747.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. Journal of Experimental Medicine. 2000;192(9):1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarntzen EHJG, Figdor CG, Adema GJ, Punt CJA, de Vries IJM. Dendritic cell vaccination and immune monitoring. Cancer Immunology, Immunotherapy. 2008;57(10):1559–1568. doi: 10.1007/s00262-008-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature Reviews Immunology. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE. 2010;5(6, article e11144) doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaba K, Turley S, Yamaide F, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. Journal of Experimental Medicine. 1998;188(11):2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. Journal of Immunology. 2003;170(6):2817–2823. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 18.Petersen TR, Sika-Paotonu D, Knight DA, Simkins HMA, Hermans IF. Exploiting the role of endogenous lymphoid-resident dendritic cells in the priming of NKT cells and CD8+ T cells to dendritic cell-based vaccines. PLoS ONE. 2011;6(3, article e17657) doi: 10.1371/journal.pone.0017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachem A, Güttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. Journal of Experimental Medicine. 2010;207(6):1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. Journal of Experimental Medicine. 2010;207(6):1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. Journal of Experimental Medicine. 2010;207(6):1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulin LF, Salio M, Griessinger E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. Journal of Experimental Medicine. 2010;207(6):1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Den Haan JMM, Lehar SM, Bevan MJ. CD8+ but not CD8- dendritic cells cross-prime cytotoxic T cells in vivo. Journal of Experimental Medicine. 2000;192(12):1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. Journal of Experimental Medicine. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. Journal of Immunology. 2001;166(9):5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 26.Schnorrer P, Behrens GMN, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(28):10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. Journal of Immunology. 2001;166(9):5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 28.Edwards AD, Manickasingham SP, Spörri R, et al. Microbial recognition via toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. Journal of Immunology. 2002;169(7):3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 29.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Belz GT, Smith CM, Kleinert L, et al. Distinct migrating and nonmigrating dendritic cell population are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(23):8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacken PJ, De Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nature Reviews Immunology. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 32.Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. Journal of Experimental Medicine. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caminschi I, Proietto AI, Ahmet F, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112(8):3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancho D, Mourão-Sá D, Joffre OP, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. Journal of Clinical Investigation. 2008;118(6):2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. Journal of Experimental Medicine. 2001;193(8):987–993. doi: 10.1084/jem.193.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 37.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107(6):2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper LJN, Al-Kadhimi Z, Serrano LM, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105(4):1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 39.Russo V, Cipponi A, Raccosta L, et al. Lymphocytes genetically modified to express tumor antigens target DCs in vivo and induce antitumor immunity. Journal of Clinical Investigation. 2007;117(10):3087–3096. doi: 10.1172/JCI30605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontana R, Bregni M, Cipponi A, et al. Peripheral blood lymphocytes genetically modified to express the self/tumor antigen MAGE-A3 induce antitumor immune responses in cancer patients. Blood. 2009;113(8):1651–1660. doi: 10.1182/blood-2008-07-168666. [DOI] [PubMed] [Google Scholar]
- 41.Triebel F, De Roquefeuil S, Blanc C. Expression of MHC class II and Tac antigens on IL2-activated human T cell clones that can stimulate in MLR, AMLR, PLT and can present antigen. Human Immunology. 1986;15(3):302–315. doi: 10.1016/0198-8859(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 42.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. Journal of Experimental Medicine. 1993;177(3):845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyss-Coray T, Mauri-Hellweg D, Baumann K, Bettens F, Grunow R, Pichler WJ. The B7 adhesion molecule is expressed on activated human T cells: functional involvement in T-T cell interactions. European Journal of Immunology. 1993;23(9):2175–2180. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- 44.Foster AE, Leen AM, Lee T, et al. Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12. Journal of Immunotherapy. 2007;30(5):506–516. doi: 10.1097/CJI.0b013e318046f3b1. [DOI] [PubMed] [Google Scholar]
- 45.De Haan A, Van Der Gun I, Van Dijk E, Hepkema BG, Prop J, De Leij LFMH. Activation of alloreactive T cells by allogeneic nonprofessional antigenpresenting cells and interleukin-12 from bystander autologous professional antigenpresenting cells. Transplantation. 2000;69(8):1637–1644. doi: 10.1097/00007890-200004270-00020. [DOI] [PubMed] [Google Scholar]
- 46.Mauri D, Wyss-Coray T, Gallati H, Pichler WJ. Antigen-presenting T cells induce the development of cytotoxic CD4+ T cells: I. Involvement of the CD80-CD28 adhesion molecules. Journal of Immunology. 1995;155(1):118–127. [PubMed] [Google Scholar]
- 47.Melenhorst JJ, Solomon SR, Shenoy A, et al. Robust expansion of viral antigen-specific CD4+ and CD8 + T cells for adoptive T cell therapy using gene-modified activated T cells as antigen presenting cells. Journal of Immunotherapy. 2006;29(4):436–443. doi: 10.1097/01.cji.0000211302.52503.93. [DOI] [PubMed] [Google Scholar]
- 48.Atanackovic D, Matsuo M, Ritter E, et al. Monitoring CD4+ T cell responses against viral and tumor antigens using T cells as novel target APC. Journal of Immunological Methods. 2003;278(1-2):57–66. doi: 10.1016/s0022-1759(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 49.Song XT, Turnis ME, Zhou X, et al. A Th1-inducing adenoviral vaccine for boosting adoptively transferred T cells. Molecular Therapy. 2011;19(1):211–217. doi: 10.1038/mt.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. Journal of Immunology. 2002;169(7):3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 51.Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. Journal of Immunology. 2009;182(12):7539–7547. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ben-Yedidia T, Arnon R. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunology Letters. 1998;64(1):9–15. doi: 10.1016/s0165-2478(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 53.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infection and Immunity. 2006;74(2):1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nature Reviews Immunology. 2002;2(3):185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 55.Blachere NE, Li Z, Chandawarkar RY, et al. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. Journal of Experimental Medicine. 1997;186(8):1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnold-Schild D, Hanau D, Spehner D, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. Journal of Immunology. 1999;162(7):3757–3760. [PubMed] [Google Scholar]
- 57.Singh-Jasuja H, Toes REM, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens: on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. Journal of Experimental Medicine. 2000;191(11):1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Medical Microbiology and Immunology. 2008;197(1):1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- 59.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. Journal of Immunology. 1999;163(3):1398–1408. [PubMed] [Google Scholar]
- 60.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. Journal of Immunology. 2001;167(12):6731–6735. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z. Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15824–15829. doi: 10.1073/pnas.2635458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Seminars in Immunology. 2009;21(5):265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. Journal of Experimental Medicine. 1994;180(4):1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. Journal of Experimental Medicine. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoenberger SP, Toes REM, Van Dervoort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD4OL interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 66.French RR, Chan HTC, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nature Medicine. 1999;5(5):548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 67.Diehl L, Den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nature Medicine. 1999;5(7):774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 68.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of cd40. Nature Medicine. 1999;5(7):780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 69.Van Kooten G, Banchereau J. CD40-CD40 ligand. Journal of Leukocyte Biology. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 70.Higham EM, Dane Wittrup K, Chen J. Activation of tolerogenic dendritic cells in the tumor draining lymph nodes by CD8+ T cells engineered to express CD40 ligand. Journal of Immunology. 2010;184(7):3394–3400. doi: 10.4049/jimmunol.0903111. [DOI] [PMC free article] [PubMed] [Google Scholar]


