Abstract
Pulmonary carcinoids are histologically classified into typical and atypical. It is important to identify these preoperatively for treatment planning and prognosis. Structural imaging cannot conclusively differentiate between them. The aim of this study was to assess the possibility of differentiating the 2 variants using [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) and [68Ga]1,4,7,10-tetraazacyclododecane-NI–IIII-tetraacetic acid-(d)-Phe1-Thy3-octreotide (DOTATOC)-PET/CT. The imaging results of 20 patients with pulmonary carcinoids (13 typical, 7 atypical) on [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT were assessed retrospectively. Six typical carcinoids failed to reveal significant uptake on [18F]FDG-PET/CT. All the atypical carcinoids revealed significant uptake on the [18F]FDG-PET/CT that was higher than that in typical carcinoids (standardized uptake value (SUV)max, 2.9–8.4, P = 0.001). The SUVmax in typical carcinoids on [68Ga]DOTATOC-PET/CT was significantly higher (SUVmax, 8.8–66) compared with atypical carcinoids (SUVmax, 1.1–18.5, P = 0.002). Ratios of SUVmax on [68Ga]DOTATOC-PET/CT to that on [18F]FDG-PET/CT were significantly higher (P < 0.001) in typical carcinoids compared with atypical carcinoids. The different uptake patterns on [18F]FDG and [68Ga]DOTATOC-PET/CT. and the ratio of SUVmax may be helpful in differentiating between typical and atypical carcinoids.
Keywords: Atypical carcinoids; [18F]FDG-PET/CT; [18F] fluorodeoxyglucose-positron emission tomography/computed tomography; [68Ga]DOTATOC-PET/CT; 1,4,7,10-tetraazacyclododecane-N I–IIII-tetraacetic acid-(d)-Phe1-Thy3-octreotide-positron emission tomography/computed tomography; typical carcinoids
Introduction
Carcinoids represent 1–2% of all lung tumours. Histopathologically, they are subclassified into typical and atypical carcinoids. Typical pulmonary carcinoids, in general, have good prognosis in contrast to atypical carcinoids[1–3]. It is important if this differentiation can be made in the preoperative stage as it has therapeutic as well as prognostic implications. To date, histopathological examination is the only means available to make this differentiation. Structural imaging findings are similar in both and differentiation between typical and atypical carcinoids is not possible. [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) has been found to be of limited use in the carcinoid tumours, especially the typical ones, due to their indolent growth and low glucose turnover[4,5]. Carcinoids express somatostatin receptors on their surface. These somatostatin receptors allow functional imaging of these tumours with radiolabelled somatostatin analogues like [68Ga]1,4,7,10-tetraazacyclododecane-NI–IIII-tetraacetic acid-(d)-Phe1-Thy3-octreotide (DOTATOC)[6,7]. The density of these receptors is related to the degree of the tumour differentiation, the most well-differentiated ones (i.e. typical carcinoids) expressing the highest density. This report analyses our experience with a combination of [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT in 20 patients with pulmonary carcinoids to assess the possibility of differentiating the 2 histopathologic variants based on the uptake patterns.
Materials and methods
Ethical clearance was obtained from the institutional ethics committee for this retrospective evaluation of the patients’ records. A total of 20 patients with primary pulmonary carcinoids were assessed retrospectively. All the patients had a thorough clinical examination, haemogram, routine biochemistry, structural imaging by contrast-enhanced CT of the chest, [18F]FDG-PET/CT, [68Ga]DOTATOC-PET/CT, bronchoscopy (with biopsy), followed by surgery (except case 15). The results were analysed against the histopathologic classification (typical or atypical), which was done as per the WHO criteria by a pathologist who had more than 20 years experience in this field. The tissue diagnosis was obtained either by biopsy or histopathologic examination of the operative specimen (in the rest of the cases).
The statistical analysis was done using SPSS Statistical data editor (version 17). Due to the non-parametric nature of the data (as shown by independent sample t-test), median values of standardized uptake value (SUV)max was calculated. The Mann–Whitney test (non-parametric test) was used to calculate the statistical differences in SUVmax values of the carcinoids on both [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT. A P value of less than 0.05 was taken to be significant.
[18F]FDG-PET/CT protocol
PET images were taken on a dedicated PET/CT scanner (Siemens Biograph 64, USA)with lutetium oxyorthosilicate (Lu2SiO5):Ce detectors with attenuation coefficient of 0.89 cm−1, photofraction of 30%, decay constant of 40 ns, and the energy resolution at 511 keV with spatial resolution of 6 mm. After fasting for at least 4 h, verifying the serum glucose level (which should be <140 mg/dl) and with patients in a resting state, in a quiet room, a dose of 370 MBq (10 mCi) of [18F]FDG was injected intravenously. After a 45–60 min uptake period, CT acquisition was performed on spiral dual slice CT with a slice thickness of 4 mm and a pitch of 1. Images were acquired using a matrix of 512 × 512 pixels and pixel size of about 1 mm. After transmission imaging, three-dimensional PET acquisition was taken for 3–5 min per bed position for one or two bed positions. PET data were acquired using a matrix of 128 × 128 pixels. CT-based attenuation correction of the emission images was used.
PET images were reconstructed using an iterative method with ordered subset expectation maximization (2 iterations and 8 subsets) with a 5-mm filter. After completion of PET acquisition, the reconstructed attenuation corrected PET images, CT images and fused images of matching pairs of PET and CT images were available for review.
[68Ga]DOTATOC-PET/CT protocol
[68Ga]DOTATOC-PET/CT was obtained after intravenous injection of 74–111 MBq (2–3 mCi) of radiotracer. During the PET/CT acquisition all PET/CT parameters were kept the same as during [18F]FDG-PET/CT, except for the timing of the three-dimensional PET acquisition, which was 58 min per bed position. After the DOTATOC-PET acquisition, the reconstructed attenuation corrected PET images, CT images and fused images of matching pairs of PET and CT images were available.
A gap of at least 24 h was given between the [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT scans to ensure clearance of the radiotracer and to avoid interference between the 2 radiotracers. Two experienced nuclear medicine physicians evaluated the findings independently and they were blinded to the clinical history, and the clinical and structural imaging findings.
PET images were assessed for areas of increased radiotracer uptake (visual positivity). The corresponding areas in the CT images and fused PET-CT images were corroborated. The SUVmax values of the tumour tissue were calculated. For semi-quantitative analysis, a region of interest (ROI) was drawn around the site of the abnormal uptake in the consequent 4–6 PET/CT slices. The slice with maximal uptake in the ROI was chosen for measurement of metabolic activity of the tracer (SUV). From these ROIs, the SUVmax was calculated.
Results
The patient characteristics, results of structural and functional imaging and the final histopathology are described in Table 1. The mean age of the patients was 32.7 years (range 16–53 years).
Table 1.
Patient characteristics, structural and functional imaging, and final histopathology
| Case no. | Age (years) | CT |
SUVmax on [18F]FDG-PET/CT | SUVmax on [68Ga]DOTATOC- PET/CT | Ratio of SUVmax of DOTATOC/SUVmax of FDG | HPE | |
|---|---|---|---|---|---|---|---|
| Tumour size (mm) | Tumour site | ||||||
| 1 | 40 | 3.2 × 1.8 | R int. Br | 1.3 | 23.2 | 17.8 | TC |
| 2 | 16 | 1.5 × 0.9 | L main Br | 3.1 | 58 | 18.7 | TC |
| 3 | 35 | 3.8 × 3.7 | R main Br | 3.2 | 43 | 13.4 | TC |
| 4 | 44 | 2.5 × 5 | R LL Br | 1.4 | 11 | 7.8 | TC |
| 5 | 40 | 1 × 0.7 | Posterior basal segment of L LL | 1.0 | 8.8 | 8.8 | TC |
| 6 | 23 | 6.8 × 5 | R hilar area | 2.8 | 33 | 11.7 | TC |
| 7 | 40 | 3.6 × 3.4 | R LL Br | 2.6 | 60 | 23.1 | TC |
| 8 | 45 | 4 × 6 | L main Br | 1.7 | 33 | 19.4 | TC |
| 9 | 25 | 1.8 × 1.5 | R main Br | 0.8 | 20 | 25 | TC |
| 10 | 22 | 3 × 2.5 | R int. Br | 2.7 | 43 | 15.9 | TC |
| 11 | 45 | 3.4 × 4.7 | R int. Br | 1.1 | 33 | 30 | TC |
| 12 | 19 | 3.3 × 2.2 | R int. Br | 2.6 | 45 | 17.3 | TC |
| 13 | 22 | 3.2 × 2.8 | R main Br | 3.0 | 66 | 22 | TC |
| 14 | 53 | 8 × 7 | R main Br | 2.9 | 6.3 | 2.1 | AC |
| 15 | 40 | 5 × 7.1 | R main Br + calcification | 6.4 | 18.5 | 2.8 | AC |
| 16 | 34 | 4 × 4.8 | R LL | 3.1 | 12.3 | 3.9 | AC |
| 17 | 16 | 1.5 × 1.5 | L upper lobe bronchus | 3.9 | 13.9 | 3.6 | AC |
| 18 | 31 | 3.4 × 4.7 | R hilar mass, LL bronchial division | 5.9 | 1.1 | 0.2 | AC |
| 19 | 29 | 5.5 × 1.5 | L main Br | 6.8 | 10.3 | 1.5 | AC |
| 20 | 36 | 4.4 × 3.8 | R main Br | 8.4 | 4.7 | 0.5 | AC |
AC, atypical carcinoid; Br, bronchus; HPE, histopathological examination; Int., intermediate; L, left; LL, lower lobe; PE, pleural effusion; R, right, SUV, standardized uptake value; TC, typical carcinoid.
Out of the 20 patients, 13 were fund to have typical carcinoid and 7 had atypical carcinoid. The mean age of the patients with typical carcinoids was 32 years while that of patients with atypical carcinoids was 34.1 years. The difference in age was not statistically significant.
Chest radiographs revealed collapse in 13 patients and mass/opacity in 6 patients. It was normal in one patient (case 5). Contrast-enhanced CT of the chest revealed the primary lesion in all cases. There was no evidence of any additional lesions. All the lesions revealed contrast enhancement. One tumour had evidence of calcification (case 15).
The SUVmax of all carcinoids on [18F]FDG-PET/CT ranged from 0.8 to 8.40 (median SUVmax, 2.8). The SUVmax of typical carcinoids on [18F]FDG-PET/CT ranged from 0.8 to 3.20 (median SUVmax, 2.6). The [18F]FDG-PET/CT images of a typical carcinoid are shown in Fig. 1a,b. Six cases of typical carcinoids failed to reveal any significant radiotracer uptake on [18F]FDG-PET/CT. The other 7 carcinoids had low SUVmax (2.6–3.2). Thus, the overall detection rate of [18F]FDG-PET/CT for typical carcinoid tumours was 53.85%. All the atypical carcinoids revealed uptake on [18F]FDG-PET/CT (detection rate, 100%). The SUVmax ranged from 2.9 to 8.4 (median SUVmax, 5.9) (Table 2). Due to the 6 false-negative tumours, the overall detection rate of [18F]FDG-PET/CT in our series was 70%. Atypical carcinoids revealed significantly higher uptake values on [18F]FDG-PET/CT compared with the typical carcinoids (P = 0.001).
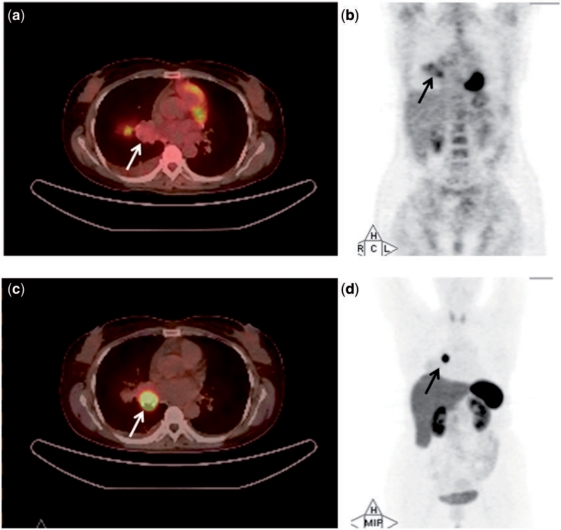
Figure 1.
PET/CT (a) and whole-body projection images (b) of a 40-year-old woman (case 1) with typical carcinoid (arrow) revealing low tumour uptake on [18F]FDG-PET/CT (SUVmax, 1.3). PET/CT image (c) and whole-body projection image (d) of the same patient revealing high tumour uptake on [68Ga]DOTATOC-PET/CT (SUVmax, 23.2).
Table 2.
SUVmax values and ratios of carcinoids on [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT
| Tumour histology | SUVmax on [18F]FDG-PET/CT (median and range) | SUVmax on [68Ga]DOTATOC-PET/CT (median and range) | Ratio of SUVmax on [68Ga]DOTATOC-PET/CT to that on F18 FDG-PET/CT |
|---|---|---|---|
| All carcinoids | 2.8 (0.8–8.4) | 21.6 (1.1–66) | 12.5 (0.2–30) |
| Typical carcinoids | 2.6 (0.8–3.2) | 33 (8.8–66) | 17.8 (7.8–30) |
| Atypical carcinoids | 5.9 (2.9–8.4) | 10.3 (1.1–18.5) | 2.1 (0.2–3.9) |
All but one carcinoid revealed significant uptake on [68Ga]DOTATOC-PET/CT, with the SUVmax ranging from 1.1 to 66 (median SUVmax, 21.6). All the typical carcinoids revealed significant uptake on [68Ga]DOTATOC-PET/CT (detection rate, 100%). The [68Ga]DOTATOC-PET/CT images of a typical carcinoid are shown in Fig. 1c,d. The SUVmax of typical carcinoids ranged from 8.80 to 66 (median SUVmax, 33). One atypical carcinoid did not reveal any significant uptake on [68Ga]DOTATOC-PET/CT (case 18, Fig. 2) thus the detection rate of [68Ga]DOTATOC-PET/CT for atypical carcinoids was 85.7%. The SUVmax in atypical carcinoids ranged from 1.1 to 18.5 (median SUVmax, 10.3) (Table 2). Typical carcinoids revealed significantly higher uptake values on [68Ga]DOTATOC-PET/CT compared with the atypical carcinoids (P = 0.002).
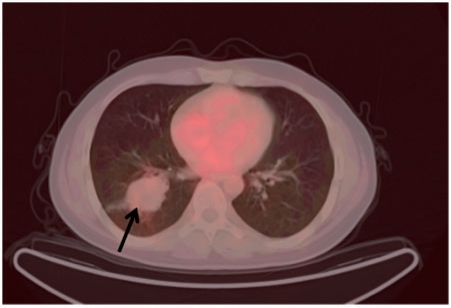
Figure 2.
[68Ga]DOTATOC-PET/CT image of case 18 (atypical carcinoid) revealing no significant uptake in the tumour (arrow) (SUVmax, 1.1).
The ratios of SUVmax on [68Ga]DOTATOC-PET/CT to that on [18F]FDG-PET/CT were calculated for all the patients. The typical carcinoids revealed significantly higher ratios of SUVmax compared with atypical carcinoids. The ratio ranged from 7.8 to 30 in typical carcinoids (median SUVmax ratio, 17.8), and from 0.20 to 3.9 in atypical carcinoids (median SUVmax of 2.1) (Table 2). This difference in the ratios between typical and atypical carcinoids was statistically significant (P < 0.001).
Discussion
Carcinoids are rare pulmonary tumours. The World Health Organization’s histological classification defines them as malignant tumours of epithelial origin with growth patterns that suggest neuroendocrine differentiation. They are subclassified as typical and atypical carcinoids.
The distinction between typical carcinoids and atypical carcinoids is clinically important, because it has therapeutic as well as prognostic implications. Histopathologic features favouring the diagnosis of atypical carcinoids include increased mitotic activity, pleomorphism, irregular nuclei with hyperchromatism and prominent nucleoli, areas of increased cellularity with loss of architecture and areas of necrosis within the tumour. Typical carcinoid tumours have been found to have a much better prognosis with a reported 5-year survival of 87–100% in contrast to the atypical carcinoids, which have a reported 5-year survival rate of 25–69%.
Pulmonary carcinoids are usually detected on structural imaging modalities like CT, which demonstrate a space-occupying lesion but do not have any specific characteristics to differentiate between the typical and atypical carcinoids.
Functional imaging has been used in the workup of carcinoids. The uptake patterns of pulmonary carcinoids on [18F]FDG-PET/CT are variable. Rege et al.[8] reported the [18F]FDG-PET/CT findings in a patient with a pulmonary lesion with history of ectopic adrenocorticotrophic hormone secretion. The lesion was hypometabolic on [18F]FDG-PET and was found be an indolent carcinoid at histology. Erasmus et al.[9] reported a series of 7 cases of pulmonary carcinoids of which 3 were visually negative on [18F]FDG-PET (all typical carcinoids), 3 were hypometabolic (2 typical and 1 atypical) and were erroneously categorized as benign nodules. Only 1 case (typical carcinoid) revealed visual positivity and SUVmax of 6.6. Cheran et al.[10] evaluated 3 patients with pulmonary carcinoids by [18F]FDG-PET and found them to have minimal or no uptake. Most of the tumours evaluated in our study, revealed low uptake on [18F]FDG-PET. Six cases failed to reveal any significant radiotracer uptake on [18F]FDG-PET/CT. The overall detection rate of [18F]FDG-PET/CT in our series was 70%.
Some studies have shown that atypical carcinoids reveal high SUVmax values on [18F]FDG-PET due to their aggressive nature and thus, high metabolism. Wartski et al.[11] evaluated 2 cases of pulmonary carcinoid, one typical and another atypical. The typical carcinoid demonstrated an SUV of 4.87. The atypical carcinoid revealed an SUV of 10.6 on [18F]FDG-PET. Chong et al.[4] evaluated 7 patients with bronchial carcinoid. The typical carcinoids were 3.2 cm and 4.2 cm in size and revealed SUV of 3.2 and 3.4, respectively, on [18F]FDG. The atypical carcinoids revealed SUVs ranging from 3.3 to 7.1 except one, which had an SUV of 1.7. Daniels et al.[5] reviewed 16 patients with bronchial carcinoid who underwent [18F]FDG-PET imaging. They demonstrated a trend toward higher [18F]FDG-PET sensitivity for atypical carcinoid tumours (80%) compared with typical carcinoid tumours (72.7%). Kayani et al.[12] reported that typical pulmonary carcinoids revealed lower uptake values on [18F]FDG-PET/CT (SUVmax range 1.7–16, median SUVmax 4.9) compared with atypical and other less differentiated neuroendocrine tumours (SUVmax range 11.7–20, median SUVmax 16).
In our study, 6 typical carcinoids showed no significant uptake on [18F]FDG-PET/CT and the ones that did reveal uptake were mildly positive (SUVmax 2.6–3.2). All the atypical carcinoids revealed statistically significant higher uptake on [18F]FDG-PET/CT compared with typical carcinoids (Table 2). Thus, the findings correlated well with the literature.
Carcinoid tumours are rich in somatostatin receptors (SSR), so they typically show high uptake on [68Ga]DOTATOC-PET/CT. Various studies in the literature have demonstrated the utility of [68Ga]DOTATOC-PET/CT in the evaluation of carcinoids but the literature on its use in pulmonary carcinoids is scanty. Hofmann et al.[6] evaluated 8 patients with histologically proven metastatic carcinoid tumours by [111In]octreotide scintigraphy and [68Ga]DOTATOC-PET/CT (6 abdominal and 2 bronchial). [68Ga]DOTATOC-PET could identify all lesions in 8 patients whereas [111In]octreotide identified 85% of lesions identified primarily by the morphological imaging. Koukouraki et al.[13] evaluated 15 cases of carcinoids, including 2 cases of pulmonary carcinoids using [18F]FDG-PET and [68Ga]DOTATOC-PET/CT and reported an overall sensitivity of 68.2% and 92%, respectively. Gabriel et al.[7] evaluated 84 cases of neuroendocrine tumours comprising 5 bronchial carcinoids using [68Ga]DOTATOC-PET. They reported a higher sensitivity for tumour detection by [68Ga]DOTATOC compared with single photon emission-computed tomography (SPECT) or CT with a sensitivity of 97% and a specificity of 92%.
The typical carcinoids have been reported to express the somatostatin receptors more abundantly compared with atypical carcinoids. Thus, it seems logical that the typical carcinoids show higher uptake of radiotracer on somatostatin receptor-based imaging studies. This was confirmed in a study by Kayani et al.[12] who found that typical carcinoids revealed higher uptake values on [68Ga]DOTATATE (1,4,7,10-tetraazacyclododecane-NI,NII,NIII, NIV-tetraacetic acid-(d)-Phe1-Thy3-octreotate)-PET/CT[12].
In our study, the typical carcinoids revealed higher SUVmax on [68Ga]DOTATOC-PET/CT compared with atypical carcinoids (statistically significant). The only tumour that did not reveal any significant uptake was an atypical one (Table 2, Fig. 2).
An interesting observation was made when the ratios of SUVmax on [68Ga]DOTATOC-PET/CT and [18F]FDG-PET/CT were calculated. The ratios were significantly and uniformly higher in typical carcinoids (7.8–30, median 17.8) compared with atypical carcinoids (0.20–3.9, median 2.1), P < 0.001.
To the best of our knowledge this is the only study that aims to co-relate the histology of pulmonary carcinoids with the uptake patterns of [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT scans and their SUV ratios. Our study tries to establish the important role that these imaging modalities can play in the management of pulmonary carcinoids. The differential uptake patterns observed in our study can be of immense help for the clinician. Although histopathologic examination by either bronchoscopy or percutaneous biopsy remains the gold standard test for confirmation, nonetheless the information provided by these non-invasive imaging modalities can substantially aid in the prediction of the histopathologic variant of carcinoids and can reliably guide the investigator.
Conclusion
Our study reveals that typical carcinoids had a lower uptake of [18F]FDG compared with the atypical carcinoids. Typical carcinoids showed higher uptake of [68Ga]DOTATOC compared with the atypical carcinoids. The ratio of SUVmax on [68Ga]DOTATOC-PET/CT and [18F]FDG-PET/CT revealed higher values for typical carcinoids. The ratio of SUVmax was a better predictor of the histopathologic variety of the carcinoid tumour compared with the SUVmax on [68Ga]DOTATOC-PET/CT and [18F]FDG-PET/CT scans, individually. This differential uptake has potential use for preoperative assessment, possible prediction of the histology and prognostication of pulmonary carcinoids. A larger study is indicated to validate this observation and to objectively determine a cut-off value for the possible differentiation.
Acknowledgements
No funding of any kind has been received. The authors have no conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001;119:1647–51. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 2.Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumours: surgical management and long-term outcome. J Thorac Cardiovasc Surg. 2002;123:303–9. doi: 10.1067/mtc.2002.119886. [DOI] [PubMed] [Google Scholar]
- 3.Martini N, Zaman MB, Bains MS, et al. Treatment and prognosis in bronchial carcinoid involving regional lymph nodes. J Thorac Cardiovasc Surg. 1994;107:1–7. [PubMed] [Google Scholar]
- 4.Chong S, Lee KS, Kim BT, et al. Integrated PET/CT of pulmonary neuroendocrine tumours: diagnostic and prognostic implications. AJR Am J Roentgenol. 2007;188:1223–31. doi: 10.2214/AJR.06.0503. [DOI] [PubMed] [Google Scholar]
- 5.Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumours presenting as pulmonary nodules. Chest. 2007;131:255–60. doi: 10.1378/chest.06-0711. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann M, Maecke H, Borner R, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–57. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumours: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 8.Rege SD, Hoh CK, Glaspy JA, et al. Imaging of pulmonary mass lesions with whole-body positron emission tomography and fluorodeoxyglucose. Cancer. 1993;72:82–90. doi: 10.1002/1097-0142(19930701)72:1<82::AID-CNCR2820720117>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Erasmus JJ, McAdams HP, Patz EF, Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumours using FDG PET. AJR Am J Roentgenol. 1998;170:1369–73. doi: 10.2214/ajr.170.5.9574618. [DOI] [PubMed] [Google Scholar]
- 10.Cheran SK, Nielsen ND, Patz EF., Jr False-negative findings for primary lung tumours on FDG positron emission tomography: staging and prognostic implications. AJR Am J Roentgenol. 2004;182:1129–32. doi: 10.2214/ajr.182.5.1821129. [DOI] [PubMed] [Google Scholar]
- 11.Wartski M, Alberini JL, Leroy-Ladurie F, et al. Typical and atypical bronchopulmonary carcinoid tumours on FDG PET/CT imaging. Clin Nucl Med. 2004;29:752–53. doi: 10.1097/00003072-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Kayani I, Conry BG, Groves AM, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumours. J Nucl Med. 2009;50:1927–32. doi: 10.2967/jnumed.109.066639. [DOI] [PubMed] [Google Scholar]
- 13.Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:1115–22. doi: 10.1007/s00259-006-0110-x. [DOI] [PubMed] [Google Scholar]