Abstract
Imaging a new mass lesion in a child requires careful consideration of a variety of issues. The age of the child is an important factor in determining the appropriate test to start with and the age also helps provide an appropriate differential diagnosis, which can then be used to guide further imaging. The long-term outcome for most children with cancer is very good, with over 70% achieving 5-year survival and presumed cure. Consequently their imaging requirements should be regarded as equal to all other children. Minimizing exposure to ionizing radiation, particularly where follow-up imaging is required is an important consideration. This article focuses specifically on general paediatric radiology and neuro-oncology imaging is not addressed. The pitfalls to be aware of in plain radiography, ultrasonography, computed tomography, magnetic resonance imaging and nuclear medicine (positron emission tomography-computed tomography and single photon emission computed tomography) in children with a proven or suspected malignancy are discussed.
Keywords: Paediatric, radiology, oncology, ultrasonography, CT, MRI, PET/CT, SPECT
Introduction
The incidence of malignancy in childhood is rare with less than 1% of cancer cases in children aged 0–14 years[1]. Imaging a new mass lesion in a child requires careful consideration of a variety of issues. What is best for the child? Will the patient be able to cooperate? How much information is sufficient for initial management of the case? What is the age of the child?
The age of the child is an important factor in determining the appropriate test to start with and also helps to provide an appropriate differential diagnosis, which can then be used to guide further imaging. For example, lymphoma is rarely seen before the age of 2 years and increases in incidence with age, where as embryonal tumours such as neuroblastoma, Wilms tumours, retinoblastoma and rhabdomyosarcoma are most common in the first few years of life and are extremely rare after childhood.
The long-term outcome for most children with cancer is very good, with over 70% achieving 5-year survival and presumed cure[2]. Consequently, their imaging requirements should be regarded as equal to all other children[3]. Minimizing exposure to ionizing radiation, particularly where follow-up imaging is required, is an important consideration.
This article focuses on the diagnostic pitfalls in plain radiography, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and nuclear medicine studies including positron emission tomography (PET).CT and single photon emission computed tomography (SPECT) in children with proven or suspected extracranial malignancy.
Plain radiography
Confusing the thymus with a mediastinal mass is not an uncommon pitfall in the interpretation of chest radiographs as it is extremely variable in appearance. The normal thymus is homogeneous, continuous with superior heart border and can occupy both upper lung lobes, demonstrating widening on expiration (Fig. 1). The normal thymus does not displace the trachea or vessels.
Figure 1.
Normal cardiothymic contour on a chest radiograph in a 6-month-old girl. The normal thymus is homogeneous, continuous with the superior heart border and can occupy both upper lung lobes. The normal thymus does not displace the trachea or vessels.
External densities such as nipples, dense pre-pubertal breasts and rib deformities can mimic intrapulmonary masses. Round pneumonia in a child with an infective history should not be misinterpreted as a neoplastic lesion (Fig. 2).
Figure 2.
Round pneumonia. Chest radiograph in a 3-year-old girl with infective symptoms demonstrates a well-defined round opacity in the right mid-zone.
It is uncommon for a malignant mass lesion in the chest to be diagnosed incidentally on a chest radiograph and the differential is much more likely to include a congenital or an inflammatory lesion. The notable exception to this rule is thoracic neuroblastic tumours, which may be incidentally picked up on a radiograph performed for a minor coryzal illness. These tumours are paraspinal and are often associated with posterior rib erosion or separation, which helps increase confidence in that diagnosis (Fig. 3).
Figure 3.
Neuroblastoma. Chest radiograph in a 5-month-old boy shows an incidental right paracardiac mass. Close inspection of the right-sided ribs shows some separation posteriorly between the 6th and 7th ribs, and subtle erosion of the inferior surface of the posterior 6th rib medially (arrow). These findings all point to a diagnosis of neuroblastoma, confirmed on biopsy.
Primary lung tumours are rare and include bronchogenic tumours, bronchial carcinoids, pleuropulmonary blastomas (Fig. 4)[4] and mesenchymal tumours. Metastatic lung disease is still relatively rare but most commonly from osteosarcoma (where the lung lesions may ossify), Ewings sarcoma, rhabdomyosarcoma, hepatoblastoma and Wilms tumours. The chest radiograph is much less sensitive for pulmonary metastases and tumour assessment compared with CT of the chest (Fig. 5).
Figure 4.
Pleuropulmonary blastoma. Axial CT through the lower chest at mediastinal window settings after intravenous contrast enhancement in a 2-year-old girl, shows a left basal mass lesion displacing the heart to the right, with some compression of the left atrium and ventricle.
Figure 5.
Lung nodule. (a) A chest radiograph demonstrates a small opacity located medially in the left upper zone (arrow). (b) Coronal reformatted CT of the chest at lung window settings demonstrates that this opacity is a well-defined parenchymal nodule in the left upper lobe (arrow).
In the context of a new abdominal mass, the abdominal radiograph has largely been replaced by other modalities such as US, CT, or MRI and is seldom worth performing routinely. Abdominal radiographs have an important role to play in the oncology patient in the assessment of possible bowel obstruction, inflammation (suspected typhilitis) or perforation.
Radiographs are crucial as the first-line imaging study in the assessment of a suspected bone tumour. Targeted films may be required when a staging bone scan is abnormal suggesting skeletal metastases but overall are less sensitive than bone scintigraphy or MRI. Primary bone tumours in young children are statistically most likely to be from Ewing sarcoma or primitive neuroectodermal tumour (PNET) families, solely on the basis of age. Osteosarcomas classically manifest as tumours around the knee in adolescents. Not all lytic destructive lesions in childhood are malignant. Langerhans cell histiocytosis, occurring most frequently in the first few years of life, is a relatively common benign entity and a great mimic of more serious pathology (Fig. 6). The use of gonadal shielding is recommended in all follow-up paediatric pelvic radiographs but must not be used if the clinicians are specifically concerned about bony metastatic disease to the pelvis which can potentially be obscured by shielding.
Figure 6.
Langerhans cell histiocytosis. Anterioposteror and lateral films of the right humerus in a 2-year-old girl show an aggressive lytic lesion with prominent periosteal reaction. Biopsy confirmed Langerhans cell histiocytosis.
Complications of treatment may be evident on radiographs during long-term follow-up. Examples include ifosfamide rickets, non-specific osteopenia, avascular necrosis and osteochondromas secondary to irradiation[5].
Ultrasonography
Lymph nodes are routinely encountered during US examination and lack of normal lymph node morphology requires follow-up and/or further investigation. Round, enlarged abnormal lymph nodes are likely involved by locoregional tumour spread, for example. A limitation of all imaging, including US, however, is when non-enlarged nodes harbour malignant cells but, by standard imaging criteria, the nodes are normal in size and appearance.
Most renal tumours have a similar range of appearances in all childhood age groups. Many lesions are largely solid but other tumours, of the same histology, may be very cystic in appearance. In neonates, congenital mesoblastic nephroma is the most common renal mass lesion, whereas Wilms tumour becomes statistically the more likely renal tumour after 6 months of age until adolescence.
Age similarly influences the differential diagnosis for tumours elsewhere. Hepatoblastoma is the most common hepatic malignancy in childhood; its prevalence is increasing, and over 90% occur in children aged 3 years or younger[6,7].
Linear array high-resolution (10–12 MHz) transducers may show abnormalities in the liver or spleen not visible with lower resolution curvilinear probes including liver infiltration in 4S neuroblastoma[8] and diffuse fungal infiltration of the liver or spleen as a complication of the neutropenia induced by chemotherapy. The International Neuroblastoma Staging System (INSS) describes disseminated disease limited to liver, skin and bone in patients less than 1 year of age as 4S, soon to be referred to as MS or special metastatic disease as described in the International Neuroblastoma Risk Group Staging System (INRGSS). Most solid tumours ultimately require a biopsy for a tissue diagnosis and this can often achieved with US guidance[9].
Tumour-related pitfalls with US include mistaking a heavily calcified mass for bowel gas (Fig. 7) and confusing the stomach distended with air and food for an abdominal mass. Assuming a cystic lesion in the pelvis is the bladder when in fact the bladder is collapsed or compressed by a large cystic ovarian tumour is a recognized pitfall in paediatric and adolescent US scanning as it is in adults. Paratesticular rhabdomyosarcoma has typically increased vascularity at Doppler assessment and can mimic infection at initial diagnosis[10]. Multiple renal veins are difficult to visualize at US and limit the usefulness of US in diagnosing and excluding renal vein thrombus.
Figure 7.
Neuroblastoma. (a) US shows a heavily calcified treated neuroblastoma mass (between callipers) in a 2-year-old boy which could easily be mistaken for bowel gas. (b) Coronal reformatted CT at soft tissue settings in another patient demonstrates the dense calcification that may be seen after chemotherapy in neuroblastoma (arrow), in addition to some residual para-aortic tumour tissue.
Paediatric tumours tend not to invade other organs but may be adherent to other viscera. The best way to evaluate the relationship of a mass to an adjacent viscus is with dynamic real-time US. Visceral or psoas muscle invasion from retroperitoneal tumours is easily overlooked, however, if not actually formally evaluated. In the case of limb primaries, assessment for regional adenopathy can be overlooked.
Computed tomography
Major improvements in technology have improved resolution and reduced scanning times in CT examinations, which have helped to minimize the need for sedation in children. However, one of the major disadvantages of CT is the exposure to ionizing radiation with a much higher radiation dose than most other modalities, which is particularly relevant if follow-up imaging is required to monitor response or in the rare situation where multiphase imaging (of a liver mass, for example) is necessary. It is alleged that the estimated increase in lifetime cancer mortality attributable to the radiation exposure from a single abdominal CT examination in a 1-year-old child is of the order of 1 in 550[3].
The use of sedation and contrast medium are other important issues. Although scan times are shorter than MRI, sedation or anaesthesia is still often necessary in the young child. Unenhanced CT of the chest and abdomen is usually of very limited usefulness due to lack of mediastinal and intra-abdominal fat and best avoided when evaluating new mass lesions in a paediatric patient (Fig. 8)[11]. The use of contrast medium carries specific, albeit low, risks of extravasation, anaphylaxis and nephrotoxicity. Patient positioning requires careful consideration in those children with mediastinal lymphoma who virtually all present with airway difficulties. Some of these children have learnt to compensate for tracheal compression by remaining upright and forcing them to lie flat for CT examination could have tragic consequences, due to potentially irreversible compression of the trachea by an anterior mediastinal mass. Safer alternatives include scanning in the prone or lateral position or delaying the scan until after a course of steroid therapy.
Figure 8.
Wilms tumour. (a) A left renal tumour is clearly discernible as a low attenuating mass after intravenous contrast enhancement in a 6-year-old girl (arrow). (b) The same lesion is not visible on the earlier non-contrast-enhanced image, emphasizing the poor sensitivity of non-contrast CT for abdominal scanning in children (arrow).
CT images, unlike MRI, traditionally rely on axial imaging for disease assessment. The ability to reformat images in the coronal and sagittal planes reduces the risk of missing pathology, particularly bone lesions such as collapsed vertebrae (Fig. 9). CT is inferior to MRI in defining the extent of soft tissue masses and, for skull base lesions, a combination of CT and MRI at diagnosis is often needed to fully appreciate disease extent (Fig. 10).
Figure 9.
Coronal reformatted CT at bone window settings in a 7-year-old boy who had previously been treated for leukaemia with chemotherapy and a bone marrow transplant. Routine follow-up axial CT during a febrile episode had failed to demonstrate the partial vertebral collapse of T4 due to treatment-induced osteopenia. The T4 vertebral collapse is clearly evident on coronal reformatted images (arrow).
Figure 10.
Rhabdomyosarcoma. (a) Axial CT at bone window settings showing mild lateral displacement of the lateral pterygoid plate, erosion of the medial plate and some lysis also involving the left aspect of the hard palate (arrow). (b) Axial CT at soft tissue window settings in the same patient shows a large nasopharyngeal mass. (c) Axial T2-weighted MRI in a different patient clearly depicts the soft tissue extent of tumour, more obviously demonstrable from the adjacent musculature.
Pitfalls with CT in oncological imaging include contrast medium injected into a foot vein leading to streaming artefact from unopacified blood mimicking an inferior vena cava (IVC) thrombus; the low sensitivity of CT for marrow invasion and thus skeletal metastases; and the relatively poor soft tissue resolution resulting in, for example, spinal invasion being easily missed. Skull base erosion in rhabdomyosarcoma, which may manifest as subtle thinning of a pterygoid plate, can unfortunately be overlooked. Skull base erosion in rhabdomyosarcoma denotes parameningeal disease, which has a worse prognosis than other head and neck sites, which is thus crucial information that needs to be conveyed to the oncologist as early as possible. Skull base erosion indicating parameningeal disease until recently was an indication for earlier local irradiation but that is deemed less urgent in current international studies on rhabdomyosarcoma[12].
Thymic hyperplasia occurs in some younger children after cessation of chemotherapy. Thymic hyperplasia is probably only seen in the first 3–4 months after completing treatment, and thymic enlargement beyond this period, particularly in lymphoma patients, should be regarded as suspicious for tumour relapse (Fig. 11)[13]. The normal and hyperplastic thymus should be homogeneous on all imaging modalities including US, CT and MRI, and a heterogeneous thymus is always abnormal (Fig. 12).
Figure 11.
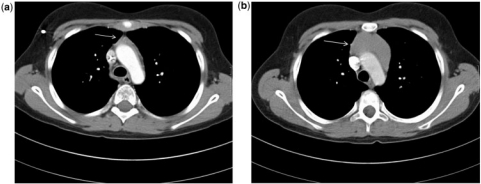
Hodgkin disease (HD). (a) Axial CT at mediastinal window settings at the level of the aortic arch, 2 months after finishing chemotherapy for HD, shows a small involuted thymus (arrow). (b) Four months later the thymus has enlarged (arrow). This enlargement was initially mistaken for thymic rebound hyperplasia but PET and later biopsy confirmed HD relapse.
Figure 12.
Normal thymus on axial CT at mediastinal window settings in a 6-month-old girl. The normal thymus is homogeneous, continuous with the superior heart border and can occupy both upper lung lobes. The normal thymus does not displace the trachea or vessels.
The identification of pulmonary nodules in children with solid primary malignancies raises the important question of when is a pulmonary nodule significant. The imaging features of pulmonary nodules in paediatrics have not been assessed to the same extent as in the adult population. There is evidence to suggest that, unlike in adults, sharply defined nodules are more likely to be malignant and small nodules (<0.5 cm) are as likely to be malignant as larger nodules[14]. Attempts are now being made in a number of studies to record and identify patients with small lung nodules not deemed to be metastases to assess their long-term outcome, and risk of relapse[15]. For single nodules in particular, as no nodule feature reliably differentiates benign from malignant lesions, biopsy of indeterminate lung lesions is necessary at times to detect or rule out malignant disease (Figs. 13 and 14)[16].
Figure 13.
Multiple lung metastases. Axial CT at lung window settings in a 3-year-old girl demonstrates multiple lung metastases. The child was under investigation for a newly discovered abdominal mass and no chest symptoms.
Figure 14.
Small solitary lung nodule. Axial CT at lung window settings in a 5-year-old asymptomatic boy undergoing routine follow-up demonstrates a parenchymal nodule less than 1 cm in the right lower lobe (arrow).
Magnetic resonance imaging
One of the major advantages of MRI is the lack of radiation exposure. MRI also produces superior soft tissue resolution compared with many other forms of imaging and improvements in technology have produced special techniques such as magnetic resonance angiography (MRA) and diffusion-weighted imaging (DWI) (Fig. 15). MRA allows delineation of vascular anatomy without the need for conventional angiography which in oncologic imaging can be used to plan liver resections and limb salvage procedures and help differentiate vascular malformations from malignant solid tumours. DWI utilizes the molecular motion of water, and although a motion-sensitive technique, DWI can now be used in the assessment of extracranial tumours in the neck, chest, abdomen and limbs[17]. MRI is superior to any other modality in assessment of the spinal cord for cord compression and MRI is the preferred modality for imaging musculoskeletal tumours and assessing bone marrow disease. MRI is generally regarded as the ideal modality for evaluating primary liver tumours and genito-urinary tract malignancies.
Figure 15.
Neuroblastoma. (a) Axial T2-weighted MRI shows residual tumour at the left paraspinal and intraspinal foramen area (3 smaller arrows). The spleen (larger single arrow) has an unusual position consequent to previous displacement by tumour and later surgical removal of the large infiltrating upper abdominal tumour. (b) Restricted diffusion is seen in the paraspinal tumour mass (black arrow), suggesting high cellularity and residual viable tumour. The spleen also has restricted diffusion which is a normal finding (white arrow).
Significant disadvantages with respect to MRI are that it is an expensive modality and a limited resource with often long waiting times. MRI is highly susceptible to cardiac and respiratory motion and the assessment of calcification, bowel, lung parenchyma, bone cortex, base of skull and lymphadenopathy is suboptimal compared with other modalities such as US or CT. The main disadvantage of MRI is the long scanning times, which in children, particularly those under the age of 6 years, necessitates the use of sedation or anaesthesia. Repeat imaging must be performed carefully to ensure the same sequences are performed to allow direct comparison.
It is important to recognize that both abscesses and tumours can demonstrate similar restricted diffusion on DWI[18]. Abscesses, however, do not show contrast enhancement after gadolinium administration on T1-weighted sequences. Marrow metastases are easily demonstrated with MRI, but the changes tend to persist on follow-up such that marrow changes cannot be relied on to monitor response to treatment or disease progression. Whole-body MRI is sensitive in the detection of lymph nodes but has poor specificity in children for predicting which nodes are infiltrated by cancer cells. It may be useful in searching for metastases when a neuroblastoma primary tumour is not metaiodobenzylguanidine (MIBG) avid. Calcification is underestimated or missed at MRI. Calcification is typical of myositis ossificans, a benign process, but is easily missed when evaluating the lesion solely with MRI, leading to unnecessary biopsy and potentially serious treatment errors. Pulsation artefact in vessels may mimic tumour thrombus (Fig. 16). With renal tumours, for example, evaluation for IVC thrombus is most reliably performed with US. Contrast medium may not be needed at MRI to evaluate all solid tumours but all renal tumours in childhood merit gadolinium-enhanced sequences to assess for contralateral tumour. In addition, in cases of Wilms tumour, gadolinium-enhanced images are necessary to detect foci of nephroblastomatosis. Children with residual area of nephroblastomatosis after treatment of the primary Wilms tumour are more likely to re-present later with metachronous tumours. Most soft tissue tumours do not have characteristic features on MRI[19]. Soft tissue sarcomas can look similar to venous malformations (Fig. 17) and synovial sarcoma is often initially misinterpreted as a benign cystic mass or even a haematoma. A biopsy is therefore an essential aspect of diagnosis whenever there is diagnostic uncertainty, as imaging cannot differentiate benign from malignant processes, and imaging cannot reliably predict the histological diagnosis.
Figure 16.
Wilms tumour. (a) Axial T2-weighted MRI shows a large right renal mass and signal void due to flowing blood in a patent IVC (arrow). US had also confirmed IVC patency, and no tumour thrombus. (b) A pseudothrombus appearance is created from pulsation artefact in the IVC on a fat-suppressed T1-weighted MRI after gadolinium enhancement (arrow).
Figure 17.
Venous malformation (VM). (a) A 2-year-old girl with a heterogeneous gluteal mass lesion on T2-weighted images, which (b) shows a fluid-fluid level (arrow) on a fat-saturated T1-weighted image. The MRI appearances were very suggestive of a VM but on clinical evaluation the mass was a firm hard lump, which is unusual for any vascular malformation. Biopsy confirmed VM with a predominant fibrous component which accounted for the clinical findings.
Nuclear medicine
Positron emission tomography/computed tomography
The use of PET/CT in childhood malignancies is currently being validated. The most widely used tracer is [18F]fluorodeoxyglucose (FDG). FDG-PET can be used in staging the disease, in evaluating the response to chemotherapy and as a surveillance test. The advantages of the technique include its ability to upstage or downstage disease and the ability to assess chemotherapy response in some conditions. The disadvantages are that FDG is not specific for cancer, it has a significant radiation burden and a lower sensitivity in certain areas such as the brain or the lungs.
Normal physiologic uptake of FDG in many parts of the body can be mistakenly interpreted as disease. Increased FDG uptake is related to enhanced glycolysis and this can occur in benign processes such as infection, inflammation and granulomatous disease. Thus, biopsy of critical areas may be required to establish the diagnosis. Since hybrid PET/CT systems have become available, co-registration with CT has helped to clarify doubts regarding the location and the nature of a finding. Physiologic FDG uptake can be seen within lymphoid tissue, salivary glands, muscles, heart, liver, spleen, glandular breast tissue, bowel, renal excretory tract and bladder. Brown fat, normally distributed in the neck, perinephric space, para-aortic and intercostals regions, can show increased FDG uptake because of increased glucose metabolism when it generates heat. Co-registration with CT is valuable to correctly interpret the finding as an area of physiologic uptake in otherwise unremarkable adipose tissue, and not as disease. Keeping the child warm during the FDG uptake period prior to the scan can be very helpful in minimizing this artefact.
Thymic rebound can occur during and after completion of chemotherapy. If this represents a physiologic thymic reaction, it most likely demonstrates homogeneously increased FDG uptake and not a patchy FDG distribution.
Radiotherapy can cause moderately increased FDG activity and at least 2 months should elapse between radiotherapy administration and a follow-up FDG-PET scan, to avoid an artefactual appearance. An FDG-PET scan to evaluate response to chemotherapy is normally performed 1 month after chemotherapy, to avoid a flare response. During or following chemotherapy, a pattern of diffusely increased FDG uptake within the skeleton can be seen, in keeping with bone marrow hyperplasia secondary to chemotherapy effect on the rapidly replicating bone marrow haematopoietic cells.
Granulocyte colony-stimulating factor is used to treat neutropenia after bone marrow transplantation and induces proliferation, differentiation and activation of neutrophil–granulocyte cells. This causes homogeneously increased FDG uptake in the skeleton, making the demonstration of disease in the bones/bone marrow more challenging.
In the context of lymphoma, FDG-PET cannot distinguish between active infection and lymphoma[20].
Single photon emission computed tomography
SPECT tracers used in paediatric oncology are mainly [123I]MIBG, [99mTc]methylendiphosphonates (MDP) and, to a much lesser extent, [111In]pentetreotide.
[123I]MIBG scan can be used at diagnosis, in monitoring response to treatment and in post-treatment evaluation of neurogenic tumours such as neuroblastomas, phaeochromocytomas, paragangliomas, ganglioneuroblastomas and ganglioneuromas. This tracer gives a radiation burden of approximately 5 mSv. Radiolabelling of MIBG with 123I is highly preferable to 131I, as it gives much better quality images and a lower radiation burden. MIBG is taken up by presynaptic noradrenergic nerve fibres. A number of drugs can reduce MIBG uptake[21], mainly anti-hypertensives, anti-depressants and sympathomimetics. MIBG shows a sensitivity of approximately 85% for neuroblastomas. The avidity of uptake seems to be in relation to the secretory activity of the tumour. False-negatives can occur when a lesion is very small or shows low-grade MIBG uptake. False-positives are very uncommon as MIBG is a very specific molecule.
The main value of [123I]MIBG scan in the management of children with neuroblastoma is in providing a whole-body depiction of the extent of disease, both at staging, in the evaluation of response to chemotherapy and at the end of treatment (Fig. 18). Approximately 5% of neuroblastomas are MIBG negative and either FDG-PET or (where available) [68Ga]DOTATE-PET are valuable alternative tracers.
Figure 18.
A 5-year-old boy with a history of a sarcoma of the right buttock. At the end of treatment, cross-sectional imaging showed a new mass in the right adrenal. Histology suggested a neuroblastic tumour. Planar images of an [123I]MIBG scan show massive metastatic infiltration within the skeleton. There are two focal areas of increased tracer uptake within the liver, in keeping with two liver metastases (arrows). The primary tumour in the right suprarenal region is not clearly visible. The findings are compatible with stage 4 neuroblastoma, with massive skeletal infiltration and at least two liver metastases. The child died after induction chemotherapy.
More differentiated neurogenic tumours such as ganglioneuromas show a much lower avidity for MIBG; only approximately 50% are MIBG avid. Ganglioneuroblastomas contain both undifferentiated neuroblasts and mature ganglion cells. They can demonstrate low-grade or absent MIBG uptake within the differentiated ganglion cells, with only some punctuate focal areas of more avid MIBG uptake within the undifferentiated neuroblasts. MIBG has shown an overall sensitivity of approximately 75% in a large series of both non-adrenal (58% sensitivity) and adrenal (85% sensitivity) phaeochromocytomas[22].
The bone scan tracer is very sensitive but not specific. Therefore, an area of abnormal uptake only represents osteoblastic reaction to an injury, of whatever nature. The specificity of the finding is increased by obtaining a detailed clinical history, both of the past events and of the current symptoms. Images of the highest possible quality are essential. This is obtained by positioning the child as well as possible (straight on the camera couch, so that perfect symmetry is achieved between the right and the left sides of the body), acquiring each image for long enough, using a high-resolution collimator and minimizing the distance between the child and the gamma camera surface.
The bone scan is not particularly good at assessing response to chemotherapy or radiotherapy, as the reactive changes can persist even when viable tumour is no longer present.
Conclusion
The investigation of malignancy in a child is initially guided by age and clinical presentation. Appropriate imaging is crucial not only for initial diagnosis but also for monitoring response to treatment. Awareness of the potential pitfalls in the various imaging modalities enables the radiologist to do the right test and interpret findings appropriately and with improved diagnostic accuracy.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Cancer Research UK. Childhood cancer statistics. 2009. http://info.cancerresearchuk.org/cancerstats/childhoodcancer/ [Google Scholar]
- 2.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:840–50. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DJ, Elliston CD, Hall EJ, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–96. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 4.Yu DC, Grabowski MJ, Kozakewich HP, et al. Primary lung tumors in children and adolescents: a 90-year experience. J Pediatr Surg. 2010;45:1090–5. doi: 10.1016/j.jpedsurg.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 5.Zerizer I, Humphries PD. Imaging ‘the lost tribe’: a review of adolescent cancer imaging. Part 2: imaging of complications of cancer treatment. Cancer Imaging. 2009;9:82–8. doi: 10.1102/1470-7330.2009.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slovis TL, Roebuck DJ. Hepatoblastoma: why so many low-birth-weight infants? Pediatr Radiol. 2006;36:173–4. doi: 10.1007/s00247-006-0128-z. [DOI] [PubMed] [Google Scholar]
- 7.Roebuck DJ, Perilongo G. Hepatoblastoma: an oncological review. Pediatr Radiol. 2006;36:183–6. doi: 10.1007/s00247-005-0064-3. [DOI] [PubMed] [Google Scholar]
- 8.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) Staging System: an INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury T, Barnacle A, Haque S, et al. Ultrasound-guided core needle biopsy for the diagnosis of rhabdomyosarcoma in childhood. Pediatr Blood Cancer. 2009;53:356–60. doi: 10.1002/pbc.22059. [DOI] [PubMed] [Google Scholar]
- 10.Agrons GA, Wagner BJ, Lonergan GJ, Dickey GE, Kaufman MS. Genitourinary rhabdomyosarcoma in children: radiologic-pathologic correlation. Radiographics. 1997;17:919–37. doi: 10.1148/radiographics.17.4.9225391. [DOI] [PubMed] [Google Scholar]
- 11.McHugh K, Disini L. Commentary: For the childrens’ sake, avoid non-contrast CT! Cancer Imaging. 2011;11:16–18. doi: 10.1102/1470-7330.2011.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defachelles AS, Rey A, Oberlin O, Stevens MC. Treatment of nonmetastatic cranial parameningeal rhabdomyosarcoma in children younger than 3 years old: International society of Paediatric Oncology Studies MMT 89 and 95. J Clin Oncol. 2009;27:1310–15. doi: 10.1200/JCO.2008.19.5701. [DOI] [PubMed] [Google Scholar]
- 13.Ford EG, Lockhart SK, Sullivan MP, Andrassy RJ. Mediastinal mass following chemotherapeutic treatment of Hodgkin's disease: recurrent tumour or thymic hyperplasia? J Pediatr Surg. 1987;22:1155–9. doi: 10.1016/S0022-3468(87)80727-3. [DOI] [PubMed] [Google Scholar]
- 14.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumours. Radiology. 2006;239:514–20. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 15.European Pediatric Soft Tissue Sarcoma Studies Group. EpSSG clinical trials. http://epssg.cineca.org/clinical_trials.htm. [Google Scholar]
- 16.Silva CT, Amaral JG, Moineddin R, Doda W, Babyn P. CT characteristics of lung nodules at diagnosis of extrapulmonary malignancy in children. AJR Am J Roengenol. 2010;194:772–8. doi: 10.2214/AJR.09.2490. [DOI] [PubMed] [Google Scholar]
- 17.Abdel Razek AA, Gaballa G, Elhawarey G, Megahed AS, Hafez M, Nada N. Characterization of pediatric head and neck masses with diffusion-weighted MR imaging. Eur Radiol. 2009;19:201–8. doi: 10.1007/s00330-008-1123-6. [DOI] [PubMed] [Google Scholar]
- 18.Feuerelein S, Pauls S, Juchems MS, et al. Pitfalls in abdominal diffusion weighted imaging: how predictive is restricted water diffusion for malignancy? AJR Am J Roentgenol. 2009;193:1070–6. doi: 10.2214/AJR.08.2093. [DOI] [PubMed] [Google Scholar]
- 19.Kellenberger C. Pitfalls in pediatric musculoskeletal imaging. Pediatr Radiol. 2009;39:S372–81. doi: 10.1007/s00247-009-1220-y. [DOI] [PubMed] [Google Scholar]
- 20.Krasin MJ, Hudson MM, Kaste SC. Positron emission tomography in paediatric radiation oncology: integration in the treatment-planning process. Pediatr Radiol. 2004;34:214–21. doi: 10.1007/s00247-003-1113-4. [DOI] [PubMed] [Google Scholar]
- 21.Solanki KK, Bomanji JB, Moyes J, et al. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG) Nucl Med Commun. 1992;13:513–21. doi: 10.1097/00006231-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia KS, Ismail MM, Sahdev A, et al. 123I-metaiodobenzyl-guanidine (MIBG) sensitivity for the detection of extra-adrenal and adrenal phaeochromocytomas: CT and MRI correlation. Clin Endocrinol (Oxf) 2008;69:181–8. doi: 10.1111/j.1365-2265.2008.03256.x. [DOI] [PubMed] [Google Scholar]