Abstract
Recombinant adeno-associated virus (rAAV) vectors offer promise for the gene therapy of α1-antitrypsin (AAT) deficiency. In our prior trial, an rAAV vector expressing human AAT (rAAV1-CB-hAAT) provided sustained, vector-derived AAT expression for >1 year. In the current phase 2 clinical trial, this same vector, produced by a herpes simplex virus complementation method, was administered to nine AAT-deficient individuals by intramuscular injection at doses of 6.0×1011, 1.9×1012, and 6.0×1012 vector genomes/kg (n=3 subjects/dose). Vector-derived expression of normal (M-type) AAT in serum was dose dependent, peaked on day 30, and persisted for at least 90 days. Vector administration was well tolerated, with only mild injection site reactions and no serious adverse events. Serum creatine kinase was transiently elevated on day 30 in five of six subjects in the two higher dose groups and normalized by day 45. As expected, all subjects developed anti-AAV antibodies and interferon-γ enzyme-linked immunospot responses to AAV peptides, and no subjects developed antibodies to AAT. One subject in the mid-dose group developed T cell responses to a single AAT peptide unassociated with any clinical effects. Muscle biopsies obtained on day 90 showed strong immunostaining for AAT and moderate to marked inflammatory cell infiltrates composed primarily of CD3-reactive T lymphocytes that were primarily of the CD8+ subtype. These results support the feasibility and safety of AAV gene therapy for AAT deficiency, and indicate that serum levels of vector-derived normal human AAT >20 μg/ml can be achieved. However, further improvements in the design or delivery of rAAV-AAT vectors will be required to achieve therapeutic target serum AAT concentrations.
Flotte and colleagues report on a phase 2 trial in which the same α1-antitrypsin (AAT) AAV vector as in phase 1 is administered intramuscularly to nine AAT-deficient individuals at one of three doses. Vector-derived expression of normal (M-type) AAT in serum is shown to be dose dependent, peaks on day 30, and persists for at least 90 days, although AAT levels were sub-therapeutic.
Introduction
Individuals with α1-antitrypsin (AAT) deficiency have mutations in the SERPINA1 gene that cause reduced secretion of AAT from the liver and consequent impaired antiprotease activity in the lung, resulting in early-onset pulmonary emphysema (Silverman and Sandhaus, 2009). More than 95% of AAT-deficient individuals have the Z-type of AAT instead of the normal M-type AAT (Brantly et al., 1991). Gene therapy approaches to treatment of AAT deficiency, using recombinant adeno-associated viral (rAAV) vectors expressing AAT, have been evaluated in preclinical and clinical studies (Song et al., 1998, 2002; Poirier et al., 2004; Brantly et al., 2006, 2009; De et al., 2006; Liqun Wang et al., 2009; Halbert et al., 2010; Chulay et al., 2011). We previously conducted a phase 1 clinical trial with an rAAV vector expressing human AAT (rAAV1-CB-hAAT), produced by a plasmid transfection method, in which sustained expression of AAT was achieved, but serum levels were substantially below the levels considered to be therapeutic (Brantly et al., 2009). Production of rAAV1-CB-hAAT using a recombinant herpes simplex virus (HSV) complementation system (Kang et al., 2009; Thomas et al., 2009) can generate much higher yields, enabling a substantial increase in dose in clinical studies, and the HSV-produced vector is more potent when given by intramuscular injection in mice (Chulay et al., 2011). We report here preliminary results from a phase 2 clinical trial of rAAV1-CB-hAAT produced by the HSV-based method.
Research Design and Methods
Participants
Individuals of either gender were eligible for study inclusion if they had a diagnosis of AAT deficiency, a serum AAT level <11 μM, a forced expiratory volume in 1 sec (FEV1) >25% of predicted, and had not received AAT augmentation therapy in the 3 months before enrollment and planned not to receive it for 12 months after enrollment.
Vector production and characterization
The rAAV1-CB-hAAT vector was identical to the vector used in a phase 1 clinical trial (Brantly et al., 2009) except that it was made using a recombinant HSV complementation system in suspension baby hamster kidney (BHK) cells (Kang et al., 2009; Thomas et al., 2009), based on the method of Conway and colleagues (1999) and purified by Convective Interaction Media (CIM) QA Monolith anion-exchange chromatography (BIA Separations, Villach, Austria) followed by AVB Sepharose affinity chromatography (GE Healthcare Life Sciences, Piscataway, NJ). It was produced in compliance with current Good Manufacturing Practice at SAFC Pharma (Carlsbad, CA) and characterized in compendial assays or product-specific assays as described previously (Chulay et al., 2011).
Study design and conduct
This is a nonrandomized, open-label, multicenter, sequential, three-arm, phase 2 clinical trial evaluating the safety and efficacy of administration of rAAV1-CB-hAAT conducted under an investigational new drug (IND) application with approval by institutional review boards and in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained before any study procedures were performed. Three cohorts of three subjects each received rAAV1-CB-hAAT at dose levels of 6×1011, 1.9×1012, or 6×1012 vector genomes (VG)/kg body weight by intramuscular injection on a single occasion. Subjects in cohort 1 received 10 intramuscular injections distributed across a single muscle site, subjects in cohort 2 received 32 intramuscular injections distributed across three muscle sites, and subjects in cohort 3 received 100 intramuscular injections distributed across 10 muscle sites. Each injection was given in a volume of 1.35 ml, at the appropriate vector concentration to achieve the desired total vector dose. The clinical protocol specified that subjects could choose to have injections administered using topical anesthetic cream or conscious sedation with intravenous midazolam.
A data and safety monitoring board reviewed safety data for the first two dose level cohorts before the next higher dose cohort was enrolled. Safety is being monitored by evaluation of adverse events, hematology (complete blood count with white cell differential) and clinical chemistry parameters (electrolytes, glucose, albumin, globulin, blood urea nitrogen, creatinine, creatine kinase, bilirubin, and hepatic enzymes), histological examination of muscle biopsies at 3 and 12 months, and measurement of serum antibodies to AAT. Efficacy is being evaluated by measurement of serum concentrations of M-type AAT and total AAT. Additional information being collected includes changes in antibody responses to AAV and T cell responses to AAV and AAT.
Laboratory assessments
Antibodies to AAT were measured by ELISA, using a modification of a previously described method (Brantly et al., 2006) in which serum from a cynomolgus macaque immunized against human AAT by injection with rAAV1-CB-hAAT was used as the reference standard. All other assays were performed as previously described (Brantly et al., 2009), including measurement of antibodies to AAV1 using a neutralization assay, T cell responses by ex vivo interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay and polychromatic flow cytometry of peripheral blood mononuclear cells (PBMCs) after stimulation with AAV or AAT peptides, and M-specific AAT by ELISA. Epitope mapping of the ELISPOT response to an AAT peptide pool was performed with a matrix of subpools to identify the single peptide that was reactive.
Muscle biopsies were performed with a disposable Price muscle biopsy clamp (V. Mueller catalog #SU20910; Cardinal Health Medical Products, McGaw Park, IL) to obtain two approximately 2×1×0.5 cm specimens. Half of each specimen was immediately frozen and the other half was prepared for histopathology by fixation in 10% neutral buffered formalin for 4 to 24 hr and then transferred to 70% ethanol until processing. Paraffin serial sections (4 μm) were stained with hematoxylin and eosin (H&E) and by immunohistochemistry for human AAT as previously described (Poirier et al., 2004). Inflammatory cell immunophenotype was determined with monoclonal antibodies that recognize CD3, CD20, CD68 (Dako, Carpinteria, CA), and CD4 (Cellmarque, Rocklin, CA) following standard clinical validation on an autostainer (Dako).
Results
Characterization of rAAV1-CB-hAAT
The four 25-liter batches of crude cell lysate yielded a total of 1.0×1016 VG, with an overall product recovery during purification of 23%. The study drug met all release criteria, including sterility, endotoxin (<0.3 EU/ml), and absence of detectable mycoplasma, adventitious viruses, replication-competent AAV, and replication-competent HSV. Residual BHK DNA and protein were 4.1 and 15.9 ng/ml, respectively, and residual HSV DNA and protein were 76 and 834 ng/ml, respectively. Vector concentration measured by quantitative PCR was 5.0×1012 VG/ml, vector infectivity measured as median tissue culture infective dose (TCID50) was 4.7×1010 IU/ml, and hAAT expression measured by ELISA in infected HEK 293 cell supernatant was 2.2 μg/ml.
Preliminary clinical trial results
Nine subjects were enrolled and received intramuscular injections of rAAV1-CB-hAAT between June 2010 and October 2010. All were white and seven were female, with a mean age of 51.1 (range, 20–68) years and mean weight of 71.2 (range, 55–90) kg. The AAT phenotype was ZZ for eight subjects and SZ for one.
Administration of rAAV1-CB-hAAT by multiple intramuscular injections was well tolerated in all subjects. On the basis of subject preferences, injections were performed with topical anesthetic cream for the three subjects in cohort 1 and for two subjects in each of cohorts 2 and 3, and under conscious sedation using intravenous midazolam for one subject in each of cohorts 2 and 3.
All subjects reported at least one adverse event of mild to moderate intensity. The most frequently reported adverse events were injection site reactions (discomfort, erythema, bruising, or pain) of mild intensity, which occurred in eight of nine subjects. A list of all adverse events is provided in Table 1. No serious adverse events were reported.
Table 1.
Summary of Adverse Events
| Mild | Moderate | Severe | Total | |
|---|---|---|---|---|
| Events related to study agent or its administration General disorders and administration site conditions | ||||
| Influenza-like illness | 1 | 1 | ||
| Injection site discomfort | 6 | 6 | ||
| Injection site erythema | 2 | 2 | ||
| Injection site hemorrhage | 7 | 7 | ||
| Injection site pain | 1 | 1 | 2 | |
| Malaise | 1 | 1 | ||
| Investigations | ||||
| Blood creatine phosphokinase increased | 2 | 1 | 3 | |
| Musculoskeletal and connective tissue disorders | ||||
| Muscle strain | 1 | 1 | ||
| Vascular disorders | ||||
| Phlebitis, superficial | 1 | 1 | ||
| Events not related to study agent or its administration Gastrointestinal disorders | ||||
| Nausea | 1 | 1 | ||
| General disorders and administration site conditions | ||||
| Pyrexia | 1 | 1 | ||
| Infections and infestations | ||||
| Gastroenteritis, viral | 1 | 1 | ||
| Oral candidiasis | 1 | 1 | ||
| Urinary tract infection | 1 | 1 | ||
| Injury, poisoning, and procedural complications | ||||
| Arthropod bite | 1 | 1 | ||
| Postprocedural discomfort | 3 | 3 | ||
| Procedural pain | 2 | 2 | 4 | |
| Metabolism and nutrition disorders | ||||
| Dehydration | 1 | 1 | ||
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 1 | 1 | ||
| Joint sprain | 1 | 1 | ||
| Limb discomfort | 1 | 1 | ||
| Muscle spasms | 1 | 1 | ||
| Muscle strain | 1 | 1 | ||
| Myalgias | 2 | 2 | ||
| Pain in extremity | 1 | 1 | ||
| Nervous system disorders | ||||
| Headache | 2 | 2 | ||
| Respiratory, thoracic and mediastinal disorders | ||||
| Sinus headache | 1 | 1 | ||
| Throat irritation | 1 | 1 | ||
| Upper respiratory tract infection | 1 | 1 | ||
| Skin and subcutaneous tissue disorder | ||||
| Furuncle | 1 | 1 | ||
| Rash, erythematous | 1 | 1 | ||
| Skin hemorrhage | 1 | 1 | ||
| Vascular disorders | ||||
| Orthostatic hypotension | 1 | 1 | ||
| Phlebitis, superficial | 2 | 2 | ||
| Postmastectomy lymphedema syndrome | 1 | 1 | ||
Data represent the number of subjects who reported the listed adverse event on one or more occasions.
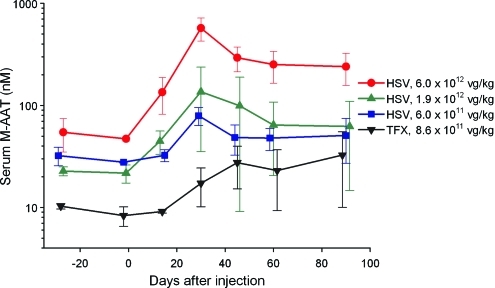
Measurement of serum M-specific AAT levels demonstrated a dose-dependent increase after injection of rAAV1-CB-hAAT, which peaked on day 30 (mean value of 572 nM in the highest dose cohort) and then declined on day 45 with little change thereafter (mean value of 240 nM on day 90 in the highest dose cohort). The average peak serum M-AAT level in the lowest dose cohort in this study was more than 2-fold higher than the average peak serum M-AAT level in the highest dose cohort in the previous phase 1 study with transfection-produced vector (Fig. 1). Serum M-AAT levels for individual subjects are shown in Fig. 2.
FIG. 1.
Serum M-specific α1-antitrypsin (AAT) concentration after injection of rAAV1-CB-hAAT produced by plasmid transfection (TFX) or the herpes simplex virus (HSV) method. Values shown represent means±SD. The dose of vector administered to subjects is indicated in the figure legend. Values for the TFX group are from a previous study (Brantly et al., 2009). Values for the 6×1011 VG/kg HSV group do not include results for subject 303, who had an AAT phenotype of SZ; the monoclonal antibody used to determine serum M-specific AAT concentrations has little cross-reactivity with Z-type AAT but cross-reacts strongly with S-type AAT, causing results for this assay in this subject to be spuriously high. Color images available online at www.liebertonline.com/hum
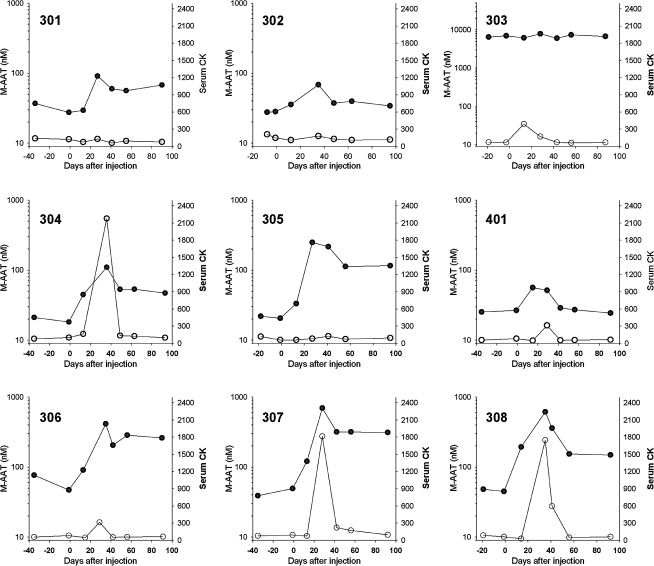
FIG. 2.
Serum M-specific α1-antitrypsin (AAT) concentration (solid symbols) and serum creatine kinase (CK) levels (open symbols) after injection of rAAV1-CB-hAAT in individual subjects. Subject 303 had an AAT phenotype of SZ, and results for the M-specific AAT ELISA in this subject are spuriously high. Subjects 305 and 307 were the two male subjects.
Serum creatine kinase (CK) was transiently elevated on day 30 in two of three subjects in cohort 2 and in three of three subjects in cohort 3, and had normalized by day 45 in four subjects and by day 60 in one subject (Fig. 2). There were no clinically significant changes in any other clinical chemistry or hematology parameter.
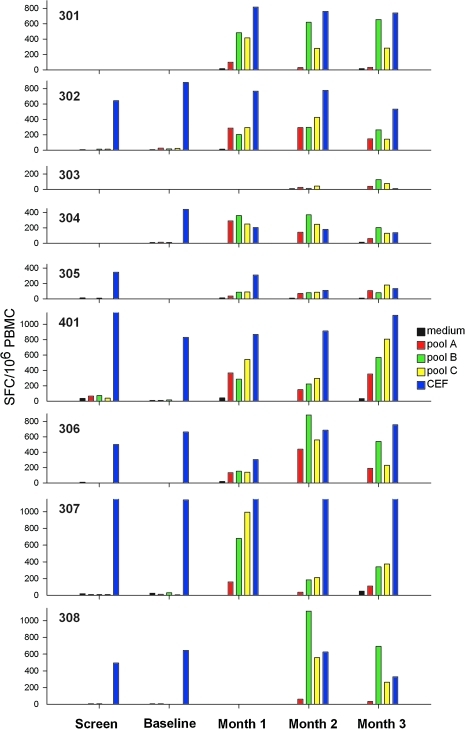
As expected on the basis of previous preclinical and clinical testing, all subjects in all three dose level cohorts developed neutralizing antibodies against AAV (Table 2) and IFN-γ ELISPOT responses to AAV peptides (Fig. 3). There was no apparent relationship between the dose of vector administered and the magnitude of the IFN-γ ELISPOT responses to AAV peptides. Both AAV1-specfic CD8+ and CD4+ T cell responses were detected and had a cytokine profile similar to that seen in the previous clinical trial with this vector (Brantly et al., 2009).
Table 2.
Neutralizing Antibody Responses to AAV
| |
|
Low dose |
Middle dose |
High dose |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serotype | Visit | Subject 301 | Subject 302 | Subject 303 | Subject 304 | Subject 305 | Subject 401 | Subject 306 | Subject 307 | Subject 308 |
| AAV1 | Screen | <5 | <5 | 80 | <5 | <5 | <5 | <5 | <5 | 160 |
| Day 14 | 5,120 | 5,120 | 40,960 | 10,240 | 5,120 | 5,120 | 20,480 | 5,120 | 20,480 | |
| Month 1 | 5,120 | 2,560 | 20,480 | 10,240 | 10,240 | 10,240 | 10,240 | 2,560 | 20,480 | |
| Month 3 | 20,480 | 40,960 | 20,480 | 40,960 | 20,480 | 40,960 | 81,920 | 40,960 | 81,920 | |
| AAV2 | Screen | <5 | <5 | 80 | <5 | <5 | <5 | <5 | <5 | 80 |
| Month 3 | 160 | <5 | 10,240 | 160 | 80 | 320 | 640 | 320 | 81,920 | |
| AAV8 | Screen | <5 | <5 | 80 | <5 | <5 | <5 | <5 | <5 | 80 |
| Month 3 | 80 | 20 | 5,120 | 1,280 | 80 | 2,560 | 1,280 | 80 | 10,240 | |
rAAV-lacZ vectors mixed with serial dilutions of serum were used to infect Huh7 cells. Results are expressed as the reciprocal of the highest serum dilution that inhibited β-galactosidase expression by 50%.
FIG. 3.
Time course of IFN-γ ELISPOT responses to pools of AAV1 capsid peptides or controls. PBMCs were obtained at screening, baseline, and 1, 2, and 3 months after vector administration and were stimulated with each of three pools (A, B, and C) of AAV1 capsid peptides (15-mers overlapping by 10 amino acids) or with a positive control peptide pool (CEF). SFC, spot-forming cells.
None of the subjects has developed antibodies to AAT, but one subject in the mid-dose cohort (subject 401) developed IFN-γ ELISPOT responses to a pool of AAT peptides at month 1 that persisted at months 2 and 3 (baseline and screening samples were negative). Epitope mapping identified a single peptide (peptide 46, DTEEEDFHVDQVTTV) distant from the site of the PI*Z mutation that was the target of the ELISPOT response. Cytokine flow cytometry after stimulation with the AAT peptide pool showed that this subject had a response to AAT mediated by CD4+ T cells expressing IFN-γ but not tumor necrosis factor (TNF)-α and by CD8+ T cells expressing both IFN-γ and TNF-α. On the basis of review of history and physical examination findings at each visit and hematology and clinical chemistry data, there was no evidence that the T cell response to this AAT peptide was associated with any untoward clinical effects.
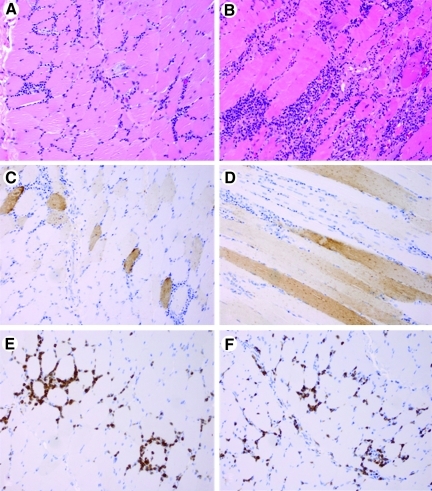
Histological examination of muscle biopsies, obtained on day 90 from eight subjects, showed moderate to marked mononuclear endomysial and perivascular inflammatory infiltrates composed primarily of mature lymphocytes and smaller populations of monocytes and plasma cells (Fig. 4). There was also prominent myofiber regeneration, as evidenced by numerous basophilic myofibers with large vesicular nuclei containing prominent nucleoli. Occasional myofibers undergoing active necrosis with phagocytosis by macrophage-like cells were identified. No significant endomysial fibrosis was seen. Immunohistochemical staining indicated that CD3-immunoreactive T lymphocytes comprised the most abundant single subset of mononuclear cells, with CD8+ cells accounting for slightly more of the total inflammatory cell population than CD4-reactive cells. Scattered CD20-immunoreactive B lymphocytes and CD68-reactive macrophages were also seen. There was strong immunostaining of AAT within endomysial and perimysial blood vessels, not associated with cell components, and in perimysial and endomysial connective tissue. Focal individual myofibers showed weaker AAT immunoreactivity of the sarcoplasm, with some myofibers having a dispersed granular pattern of reactivity.
FIG. 4.
Histology and immunohistochemical study of skeletal muscle. (A) H&E-stained section showing a moderate endomysial inflammatory reaction composed primarily of mononuclear cells. (B) H&E-stained section showing a marked endomysial inflammatory reaction. (C) Immunohistochemistry for AAT, showing individual weak to moderate granular reactivity in individual myofibers on cross-section. (D) Immunohistochemistry for AAT, showing individual weak to moderate granular reactivity in individual myofibers cut longitudinally. (E) Immunohistochemistry for CD3, showing a high proportion of T lymphocytes comprising the inflammatory infiltrate. (F) CD8-immunoreactive T cells comprise a significant subset of the total lymphocytic infiltrate.
Discussion
The most significant finding in this study was a clear demonstration of a linear dose–response relationship. This is the first time, to the authors' knowledge, that such a linear relationship between physical dose of a gene therapy product and expression of a therapeutic protein has been shown in humans. Previous clinical trials with the rAAV2-CB-hAAT vector (Brantly et al., 2006, 2009) and with an AAV2 vector expressing clotting factor IX (Manno et al., 2003, 2006) did not have sufficient numbers of subjects with sustained expression at various doses to enable demonstration of a linear dose response, and in clinical trials with an AAV1 vector expressing lipoprotein lipase (Stroes et al., 2008) the end point was indirect, and thus cannot be compared directly with our results. Our observation indicates that human rAAV gene therapy for AAT deficiency behaves in a predictable fashion and is an important step in the ability to design and conduct appropriate safety and efficacy studies in support of product licensure.
However, AAT is one of the most abundant serum proteins (normal concentration, 20 to 50 μM or about 1,000 to 2,500 μg/ml), and the peak serum AAT levels achieved after delivery of 6×1012 VG/kg by multiple intramuscular injections (between 412 and 694 nM, equivalent to 21 to 36 μg/ml) were below the target therapeutic concentration (>11 μM, equivalent to 572 μg/ml) required to reduce the risk of emphysema. Further improvements in product delivery or product design will therefore be required to achieve therapeutic target serum AAT concentrations. For example, administration of an rAAV1 vector expressing a CTLA4Ig transgene by a regional intravenous method achieved serum concentrations 5- to 8-fold higher than multiple intramuscular injections of the same vector in cynomolgus macaques (Toromanoff et al., 2008), and suggests that regional vascular delivery of rAAV1-CB-hAAT might result in higher serum AAT concentrations. It is also possible that regional vascular delivery may elevate expression levels by reducing anti-AAV immune responses (Toromanoff et al., 2010), or that a similar result could be achieved by short-term administration of immunosuppressive drugs. The use of alternative AAV serotypes should also be considered. AAV1 was selected for use in the current clinical trial on the basis of evidence of improved transduction efficiency with AAV1 compared with AAV2 after intramuscular injection in mice (Xiao et al., 1999; Chao et al., 2000; Gao et al., 2002; Rabinowitz et al., 2002; Lu et al., 2006). However, more recent data indicate that other serotypes, including recombinant serotypes and other nonnaturally occurring serotypes, may transduce muscle cells more efficiently than AAV1 (Rodino-Klapac et al., 2007; Asokan et al., 2010; Qiao et al., 2010; Pulicherla et al., 2011). A combination of these approaches may be necessary in order to ultimately achieve the goal of effective gene therapy for AAT deficiency. Results of the current study provide a foundation for designing rational therapeutic protocols.
Results of this study provide additional evidence of the safety of AAV gene therapy. In the highest dose cohort (6×1012 VG/kg), subjects received a total of between 3.3×1014 and 4.3×1014 VG, administered in a total of 135 ml distributed over 100 intramuscular injections, with only mild and transient discomfort at the injection sites.
As expected, all subjects developed anti-AAV antibodies and IFN-γ ELISPOT responses to AAV peptides. In a previous clinical trial with the same vector, anti-AAV immune responses were not associated with any significant decline in peak AAT expression; expression rose irregularly during the first 30 to 180 days and was then sustained at similar levels through day 365. In the present study, serum CK levels were elevated in most subjects in the higher two dose level cohorts on day 30 after injection, which corresponded to the time of peak serum AAT expression, and there was histological evidence of inflammatory cells in muscle biopsy samples 3 months after injections, but no clinical symptoms suggestive of ongoing myositis. It is not known if the T cells seen in muscle biopsies are AAV specific, or if antivector immune responses are responsible for the observed decline in AAT expression after day 30.
We documented T cell response to a single AAT peptide in one subject but found no evidence of untoward clinical effects (no symptoms, no antibody response to AAT, no abnormal liver function tests, and no change in total AAT concentration). The fact that the epitope eliciting this T cell response was distant from the site of the missense mutation is puzzling. Although there is no evidence that the glycosylation pattern of AAT expressed from muscle is different from that of AAT expressed from liver, it is possible that altered glycosylation of AAT may break tolerance and trigger adaptive immune responses in some individuals under certain circumstances. Alternatively, this subject may have had low levels of preexisting reactive T cells that were not detected in the peripheral blood before vector administration. It is reassuring that the three subjects in the highest dose cohort did not mount any detectable T cell responses to AAT peptides.
In summary, results from this clinical trial support the feasibility and safety of AAV gene therapy of AAT deficiency, although further improvements in the design or delivery of rAAV-AAT vectors will be required to achieve therapeutic target serum AAT concentrations.
Acknowledgments
This study was supported in part by grant R01FD003896 from the Office of Orphan Products Development, U.S. Food and Drug Administration, and by grant R01HL069877 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. The authors thank the technical staff of SAFC Pharma (Carlsbad, CA) for contract manufacture of the study drug, the NHLBI DSMB for review of clinical safety data, and Dr. Ann Fu for histology services.
Author Disclosure Statement
D.R.K., G.Y., and J.D.C. are employees of and hold share options in Applied Genetic Technologies Corporation, and have a conflict of interest to the extent that this work potentially increases their personal financial interests. J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. None of the other authors has a competing financial interest.
References
- Asokan A. Conway J.C. Phillips J.L., et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly M.L. Wittes J.T. Vogelmeier C.F., et al. Use of a highly purified α1-antitrypsin standard to establish ranges for the common normal and deficient α1-antitrypsin phenotypes. Chest. 1991;100:703–708. doi: 10.1378/chest.100.3.703. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 α1-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H. Liu Y. Rabinowitz J., et al. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Chulay J.D. Ye G.J. Thomas D.L., et al. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human α1-antitrypsin made using a recombinant herpes simplex virus production method. Hum. Gene Ther. 2011;22:155–165. doi: 10.1089/hum.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J.E. Rhys C.M. Zolotukhin I., et al. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999;6:986–993. doi: 10.1038/sj.gt.3300937. [DOI] [PubMed] [Google Scholar]
- De B.P. Heguy A. Hackett N.R., et al. High levels of persistent expression of α1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol. Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C.L. Madtes D.K. Vaughan A.E., et al. Expression of human α1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol. Ther. 2010;18:1165–1172. doi: 10.1038/mt.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W. Wang L. Harrell H., et al. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- Liqun Wang R. McLaughlin T. Cossette T., et al. Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of α1-antitrypsin using invasive and noninvasive delivery. Mol. Ther. 2009;17:81–87. doi: 10.1038/mt.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Choi Y.K. Campbell-Thompson M., et al. Therapeutic level of functional human α1-antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J. Gene Med. 2006;8:730–735. doi: 10.1002/jgm.896. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Poirier A.E. Combee L.A. Martino A.T. Flotte T.R. Toxicology and biodistribution studies of a recombinant adeno-associated virus 2 (rAAV2) alpha-1 antitrypsin (AAT) vector. Mol. Ther. 2004;9:S40. [Google Scholar]
- Pulicherla N. Shen S. Yadav S., et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 2011;19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C. Zhang W. Yuan Z., et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum. Gene Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J.E. Rolling F. Li C., et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac L.R. Janssen P.M. Montgomery C.L., et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J. Transl. Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman E.K. Sandhaus R.A. Clinical practice: α1-Antitrypsin deficiency. N. Engl. J. Med. 2009;360:2749–2757. doi: 10.1056/NEJMcp0900449. [DOI] [PubMed] [Google Scholar]
- Song S. Morgan M. Ellis T., et al. Sustained secretion of human α1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S. Scott-Jorgensen M. Wang J., et al. Intramuscular administration of recombinant adeno-associated virus 2 α1-antitrypsin (rAAV-SERPINA1) vectors in a nonhuman primate model: Safety and immunologic aspects. Mol. Ther. 2002;6:329–335. doi: 10.1006/mthe.2002.0673. [DOI] [PubMed] [Google Scholar]
- Stroes E.S. Nierman M.C. Meulenberg J.J., et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler. Thromb. Vasc. Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Thomas D.L. Wang L. Niamke J., et al. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009;20:861–870. doi: 10.1089/hum.2009.004. [DOI] [PubMed] [Google Scholar]
- Toromanoff A. Cherel Y. Guilbaud M., et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol. Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Toromanoff A. Adjali O. Larcher T., et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol. Ther. 2010;18:151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W. Chirmule N. Berta S.C., et al. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]