Abstract
A slow progressive death of neurons is the hallmark of neurodegenerative diseases, such as glaucoma. A therapeutic candidate, erythropoietin (EPO), has shown promise in many models of these diseases; however, it also causes polycythemia, a potentially lethal side effect. We have developed a novel mutant form of EPO that is neuroprotective but no longer erythropoietic by altering a single amino acid (arginine to glutamate at position 76; R76E). We hypothesized that a single intramuscular injection of recombinant adeno-associated virus carrying EpoR76E (rAAV2/5.CMV.EpoR76E) would protect retinal ganglion cells in a mouse model of glaucoma without inducing polycythemia. This systemic treatment not only protected the retinal ganglion cell somata located within the retina; it also preserved axonal projections within the optic nerve, while maintaining the hematocrit within normal limits. The rescued retinal ganglion cells retained their visual function demonstrated by flash visual evoked potentials. To our knowledge, this is the first demonstration of a therapy that protects neurons from death and prevents loss of visual function from the slow neurodegenerative effects of glaucoma. Because of its broad range of cellular targets, EpoR76E is likely to be successful in treating other neurodegenerative diseases as well.
Erythropoietin (EPO) is a therapeutic candidate in many models of neurodegenerative diseases, but it also leads to potentially lethal polycythemia. In the present report, Sullivan and colleagues show that a single intramuscular injection of recombinant adeno-associated virus serotype 5 carrying a point-mutated EPO (EpoR76E) was able to protect retinal ganglion cells in a mouse model of glaucoma without inducing polycythemia.
Introduction
Many neurodegenerative diseases such as Alzheimer's, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson's, and glaucoma are characterized by the slow, progressive loss of neurons. In most of these cases, neuronal death is triggered by a variety of factors that are disease specific. Autoimmunity plays a key role in MS causing severe axonal damage, as well as in ALS, where it leads to loss of motor neurons. Alzheimer's is often associated with plaques and tangles in the brain and in glaucoma it appears that an increase in intraocular pressure (IOP) causes axonal damage and retinal ganglion cell (RGC) death. Whereas many laboratories are working to develop disease-specific interventions, we hope to develop general neuroprotective treatments, such as erythropoietin (EPO), that may ameliorate neurodegenerative diseases regardless of their initiating factors.
Several investigations have established EPO as a neuroprotective agent, protecting multiple neuronal cell types from various injuries (Konishi et al., 1993; Sakanaka et al., 1998; Grimm et al., 2002, 2004; van der Kooij et al., 2008). Previously, our laboratory focused on delivery of a mutated form of erythropoietin (EPO) to rescue photoreceptor cells in a mouse model of retinal degeneration (Sullivan et al., 2011). EPO and EPO-R76E can cross the blood–brain barrier, allowing direct access to vulnerable neurons (Brines et al., 2000; Ehrenreich et al., 2004). We demonstrated that this mutant form of EPO was able to provide high levels of protection without significantly increasing hematocrit. The success of EPO in protecting photoreceptors compelled us to test this approach as a treatment for the slow progressive loss of neurons that occurs in other types of neurodegenerative disease.
The present study uses gene therapy and systemic delivery of EPO-R76E to assess treatment potential for degenerating neurons in the DBA/2J model of glaucoma. We have selected glaucoma as a model system in part because of the anatomical separation between RGC somata (located in the proximal retina) and their axons (located in the optic nerve), which allows us to differentially monitor two aspects of neuronal degeneration: (1) cell body loss through apoptosis or necrosis and (2) axonal (Wallerian) degeneration. A further advantage is that a functional visual test exists (flash visual evoked potential, F-VEP) that assesses communication from the RGC soma, through the axon, to the visual cortex, allowing us to measure functional protection of the treatments.
A well-characterized model of pigmentary glaucoma, the DBA/2J mouse was used in our experiments (John et al., 1998). The initial neuronal injury occurs at the optic nerve head (Howell et al., 2007; Buckingham et al., 2008), initiating anterograde Wallerian degeneration of the ganglion cell axons projecting to the brain, resulting in vision loss. The axonal injury also leads to programmed cell death of the RGC somata within the retina (Howell et al., 2007; Beirowski et al., 2008). The progressive pathology of this pigmentary glaucoma model, although asynchronous between eyes, can be predicted by monitoring IOP levels (John et al., 1998). The IOP increases between 6 and 12 months of age, with optic nerve damage beginning at 8 months and increasing in severity with age (Libby et al., 2005). We studied the effects of treatment with the candidate drug, recombinant adeno-associated virus (rAAV) carrying Epo-R76E, on both the anatomical loss and functional integrity of RGC somata and axons in this model.
Materials and Methods
Injections
DBA/2J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Vectors were produced by the University of Iowa Vector Core (Iowa City, IA). A Hamilton syringe was used to deliver 10 μl containing 1×1011 genome copies of rAAV2/5.CMV.eGFP, rAAV2/5.CMV.Epo, or rAAV2/5.CMV.EpoR76E into the quadriceps of 1-month-old mice. The Epo transgene was derived from rhesus; this was used in previous studies and therefore, for consistency, was used in this study as well (Sullivan et al., 2011). All experimental procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO, Rockville, MD) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center (Memphis, TN).
Intraocular pressure
Mice were anesthetized with ketamine/xylazine/urethane (25/10/800 μg/g body weight). IOP was measured monthly from 5 to 8 months of age, using a TonoLab tonometer (Colonial Medical Supply, Franconia, NH). The TonoLab rebound tonometer was used because it is noninvasive, accurate, and reproducible in DBA/2J mice as well as other mouse strains (Wang et al., 2005; Filippopoulos et al., 2006). Mice that developed an IOP of 13 mmHg or greater were used in the analysis.
Immunohistochemistry
At 10 months of age mice were euthanized and eyes were enucleated and stored in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C. Retinas were isolated and incubated in phosphate-buffered saline (PBS) and blocked in 20% normal donkey serum in PBS containing 0.1% Triton X-100 and 0.5% bovine serum albumin (BSA) for a minimum of 2 hr at 4°C. The primary antibody, anti-neuronal nuclei (NeuN monoclonal antibody; Chemicon, Temecula, CA), was used at a 1:500 dilution and the secondary antibody (Alexa 488; Invitrogen, Carlsbad, CA) was used at a 1:200 dilution. Retinas were placed RGC side up, mounted with VECTASHIELD containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and viewed with a Nikon Eclipse TE2000 confocal microscope (Nikon, Tokyo, Japan).
RGC imaging and counts
Retinas were first imaged at 4× magnification and a grid was placed over each retina to randomly select eight locations to be imaged at 40× magnification. The number of NeuN-positive cells in each region was counted manually with ImageJ software (available at http://rsbweb.nih.gov/ij/; developed by W. Rasband, National Institutes of Health, Bethesda, MD) and MetaMorph (Universal Imaging/Molecular Devices, Sunnyvale, CA).
EPO enzyme-linked immunosorbent analysis and hematocrit
Serum from blood samples was probed for EPO and EPO-R76E, using the human EPO Quantikine IVD ELISA kit according to the protocol of the manufacturer (R&D Systems, Minneapolis, MN). It should be noted that the ELISA kit is calibrated against human EPO and has been shown to be 4-fold less sensitive for rhesus versus human EPO (Rivera et al., 2005), and this was taken into account for calculations presented in this paper. The absorbance at 450 nm with 600 nm reference was detected with a BioTek μQuant plate reader (BioTek, Winooski, VT). In some mice the serum samples were pooled in order to obtain sufficient material for the ELISA. Hematocrit was measured by capillary centrifugation.
Optic nerve damage
Optic nerves were isolated and placed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 week at 4°C. Next, samples were postfixed in 1% osmium tetroxide in 0.1 M cacodylate buffer, dehydrated in a graded ethanol series, further dehydrated in propylene oxide, and embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield PA), cut into 1-μm sections (Reichert-Jung Ultracut E; Leica Microsystems, Vienna, Austria) and stained with 1% phenylenediamine in 50% methanol (Sigma-Aldrich, St. Louis, MO). Sections were viewed by light microscopy, using an Olympus BX51 microscope (Olympus America, Center Valley, PA). Before beginning axon counts, the optic nerve was traced at 10× magnification and the cross-sectional area was automatically calculated with ImageJ software. The entire length from top to bottom of the same cross-section was then imaged, using a 60× oil immersion lens. Approximately the same region of 0.010 mm2 was selected from each ×60 image; both live and dead axons were manually counted with ImageJ software. Measurements of the cross-sectional area of the optic nerve were used with axon density to estimate the total number of axons.
F-VEP
After overnight dark adaptation, mice were anesthetized with an intraperitoneal injection of ketamine/xylazine/urethane (25/10/800 μg/g body weight). Although urethane potentiates some ion channels (Hara and Harris, 2002), it was used consistently in all the mice in this study, and therefore it did not affect the relative F-VEP results between groups. Body temperature was maintained at 37°C with a heading pad. Pupils were dilated with 1% atropine. Platinum needle electrodes (Grass Technologies, West Warwick, RI) were placed approximately 3 mm lateral to lambda over the left and right cortex. Flashes of white light at an intensity of 1.0 cd·sec/m2 were presented in a Ganzfeld dome (Diagnosys, Lowell, MA). The flash frequency was 1 Hz with an inner sweep delay of 500 msec. Each result was an average of 200 sweeps.
Phlebotomy
Mice treated with rAAV2/5.CMV.Epo developed a hematocrit above 90% and therefore had to be phlebotomized weekly. Mice treated with rAAV2/5.CMV.eGFP or rAAV2/5.CMV.EpoR76E were not phlebotomized. Phlebotomy was performed by tail vein clipping or facial vein puncture according to a slightly modified published protocol (Golde et al., 2005). Briefly, approximately 0.2 ml of blood was collected weekly from the mice. The collection site was alternated between the tail vein and the facial vein. A 20-gauge needle (Becton Dickinson, Franklin Lakes, NJ) instead of a lancet was used to penetrate the facial vein.
Statistical analysis
One-way analysis of variance (ANOVA) followed by a pair-wise Bonferroni post hoc comparison test, with a p value≤0.01 considered statistically significant, was used to compare RGC counts, axon counts, N1 amplitude, and P1 amplitude. Statistical analysis was performed with Prism 4.0 software (GraphPad, San Diego, CA).
Results
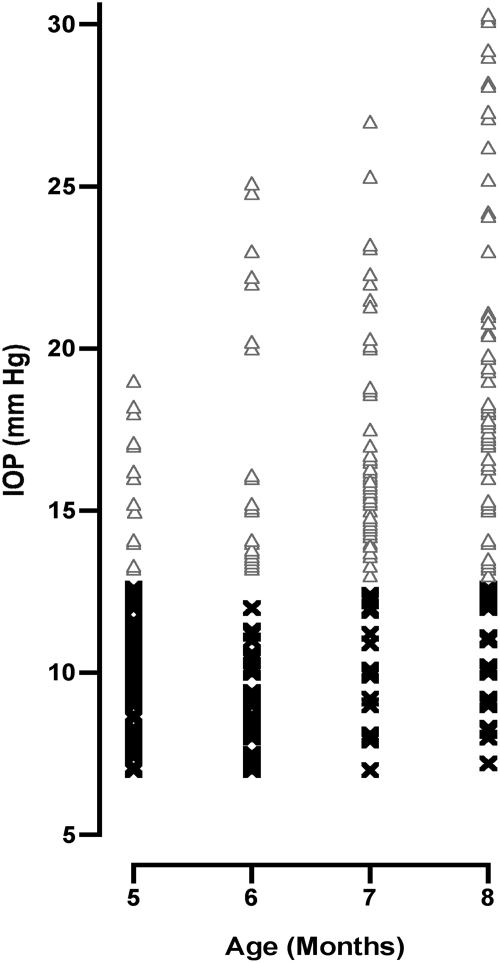
The DBA/2J mouse strain has a variable response in the elevation of IOP; however, when IOP is high neuronal loss occurs. To identify animals with elevated IOP and, therefore, the potential for developing glaucoma, we measured the IOP between the ages of 5 and 8 months (Fig. 1). The baseline IOP we observed at 3 months of age (12.5 mmHg) was in agreement with previous reports (John et al., 1998; Savinova et al., 2001). Once the baseline data were collected we aged a cohort of 47 mice. At 8 months of age, 73 of the 94 eyes had an IOP that exceeded the baseline. All of the eyes with an elevated IOP of ≥13 mmHg were included in the study to test the effects of EPO and EPO-R76E.
FIG. 1.
The intraocular pressure (IOP) was increased in 73 of 94 DBA/2J mouse eyes by 8 months of age. The scatter plot shows IOP measured monthly by rebound tonometry from DBA/2J mice. Only mice that exhibited an increased IOP above 13 mmHg (open triangles) were considered glaucomatous and entered into the study for further analysis. Mice that exhibited an IOP below 13 mmHg (×) were not likely to have appreciable RGC loss and were not included within this study.
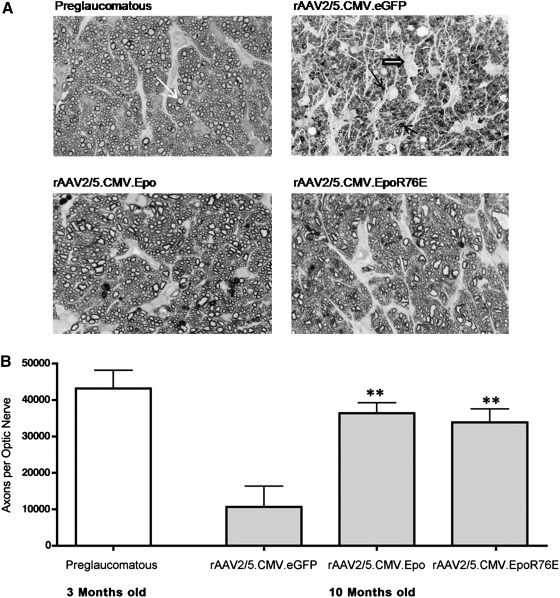
To assess the neuroprotective effects of EPO, we examined the optic nerves of four groups of mice: 3-month-old mice (before the onset of glaucoma) and 10-month-old mice receiving either rAAV2/5.CMV.eGFP (control vector), rAAV2/5.CMV.Epo (Epo vector), or rAAV2/5.CMV.EpoR76E (mutant Epo vector). An obvious difference was observed when examining the cross-sections of the optic nerves (Fig. 2A). As expected (Libby et al., 2005; Buckingham et al., 2008), there was little degeneration in the optic nerves from young mice and a significant amount of degeneration in the nerves from the older control mice. The effects of both normal EPO and mutant EPO were apparent even at low magnification. Mice treated with either Epo vector had optic nerves that resembled those in young mice without the degeneration observed in aged-matched control mice. To confirm these observations, axons in the optic nerve were counted (Fig. 2B). This quantification confirmed our initial observation, with 70% more surviving axons (p≤0.001) in mice treated with the Epo vector (36,366 axons per nerve) and with the mutant Epo vector (33,841 axons per nerve), than in age-matched control mice (10,648 axons per nerve). There was no significant difference in the number of surviving axons between the EPO-treated optic nerves and nerves from young untreated controls.
FIG. 2.
Treatment with rAAV2/5.CMV.Epo or rAAV2/5.CMV.EpoR76E protects the optic nerve of DBA/2J mice with exceedingly high IOP (≥25 mmHg) from glaucomatous axonal degeneration. (A) Bright-field micrographs of optic nerve cross-sections (original magnification, 60×) stained with p-phenylenediamine (PPD). Nerves from preglaucomatous mice (3 months old) have a high density of healthy axons (top left, white arrow). At 10 months of age optic nerves from control mice (rAAV2/5.CMV.eGFP) had profound axonal degeneration marked by the loss of healthy axons, increased sick/dying axons (top right, solid arrows), and gliosis (top right, open arrow). The majority of optic nerves from mice that were treated with either Epo vector (bottom left and right) had little or no axonal degeneration. (B) Bar graph of the number of healthy axons per optic nerve. Data represent means±SEM, and statistical analysis was performed by one-way analysis of variance with pair-wise Bonferroni post hoc test; **p≤0.001 versus rAAV2/5.CMV.eGFP. There was no statistically significant difference between the preglaucomatous, rAAV2/5.CMV.Epo, and rAAV2/5.CMV.EpoR76E groups.
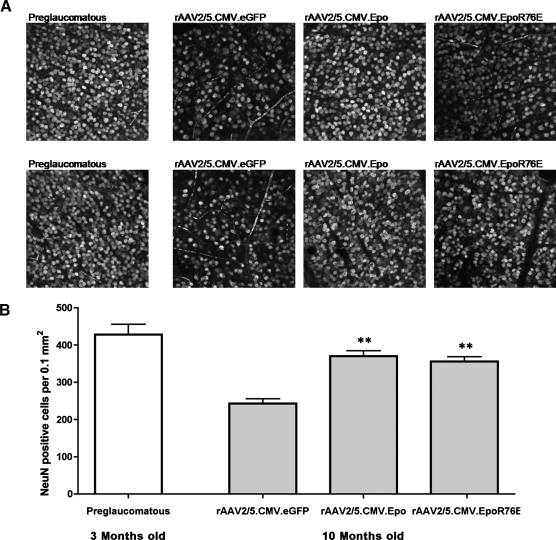
The preservation of the optic nerve mimics the protective effect of both Epo vectors on RGC somata. Retinal flat mounts from all four groups were probed with anti-NeuN (Fig. 3A), a marker for RGCs (Canola et al., 2007; Dijk et al., 2007; Zhong et al., 2007; Buckingham et al., 2008) and some displaced amacrine cells. Young mice had a high NeuN-positive cell density throughout the entire retina whereas older control mice had some areas that were completely devoid of cells. The ganglion cell layer (GCL) of mice treated with either Epo vector closely resembled that of younger mice having a dense population of cells throughout. To directly assess the effects of our treatment, NeuN-positive cells in the GCL were counted (Fig. 3B). The average cell density in young mice (406 cells per 0.1 mm2) was almost 2-fold greater than the density in older control (eGFP-treated) mice (243 NeuN-positive cells per 0.1 mm2). Treatment with the Epo vector (384 cells per 0.1 mm2) or the mutant Epo vector (344 cells per 0.1 mm2) provided a significant level of protection to RGC somata (p≤0.001). In addition, there was no significant difference in the number of NeuN-positive cells in the Epo-treated groups as compared with the young controls.
FIG. 3.
Treatment with rAAV2/5.CMV.Epo or rAAV2/5.CMV.EpoR76E protects NeuN-positive cells from glaucomatous cell death. (A) Confocal micrographs (original magnification, 40×) of retinal flat mounts from DBA/2J mice and labeled with anti-NeuN. The first column contains representative images from 3-month-old preglaucomatous mice. Columns 2–4 contain representative images from 10-month-old DBA/2J mice treated with rAAV2/5.CMV.eGFP, rAAV2/5.CMV.Epo, or rAAV2/5.CMV.EpoR76E, respectively. All images come from independent eyes. (B) Bar graph showing average density of NeuN-positive cells. Data represent means±SEM, and statistical analysis was done by one-way analysis of variance with pair-wise Bonferroni post hoc test; **p≤0.001 versus rAAV2/5.CMV.eGFP. There was no statistically significant difference between the preglaucomatous, rAAV2/5.CMV.Epo, and rAAV2/5.CMV.EpoR76E groups.
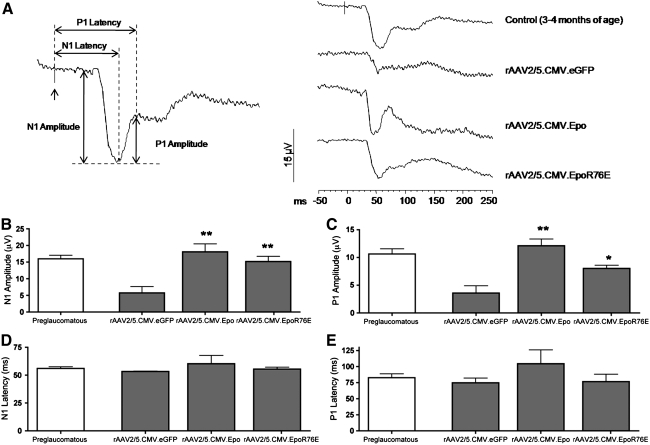
In addition to anatomical preservation of both axons and somata, we found that gene therapy using either Epo vector also preserved visual function in DBA/2J mice. F-VEPs were measured in the visual cortex of DBA/2J mice from all four groups. For a signal to reach the visual cortex both the RGC soma and axon must be intact. The classical N1 and P1 peaks in the F-VEP averaged waveforms were assessed in each treatment group (Fig. 4A). Young DBA/2J mice produced a robust and reproducible signal. By 10 months of age the signal had severely diminished in untreated animals. The decline was particularly notable in the N1 peak; a number of these mice had no detectable signal at all. Ten-month-old mice that received treatment with either the Epo or the mutant Epo vector had visually evoked responses that resembled those of their younger counterparts. The average amplitude and the latency of the N1 and P1 peaks were quantified (Fig. 4B–E). Young mice had normal N1 (15.7 μV) and P1 (11.3 μV) peak amplitudes (Fig. 4B and C). There was considerable attenuation of the peaks in older control (eGFP-treated) mice (N1, 7.3 μV; P1, 4.2 μV). When animals received either Epo vector there was preservation of the evoked potential amplitude. Ten-month-old mice that received the Epo vector had an N1 peak of 16 μV and a P1 peak of 14 μV. These evoked potentials were significantly higher than those observed in age-matched controls (p≤0.001). Similarly, treatment with the mutant Epo vector also rescued both peaks (N1, 14 μV; P1, 8 μV; p≤0.001 and p≤0.05, respectively). Compared with preglaucomatous controls, the latency of both the N1 and P1 peaks was unchanged in 10-month-old rAAV2/5.CMV.eGFP-treated mice with high IOP (Fig. 4D and E). Treatment with either Epo vector did not statistically significantly alter the latency of either peak, even with the inclusion of one rAAV2/5.CMV.Epo vector-treated mouse that had a large increase in the latency time of both peaks (p>0.05; Fig. 4D and E).
FIG. 4.
Visual function is preserved in 10-month-old DBA/2J mice treated with rAAV2/5.CMV.Epo or rAAV2/5.CMV.EpoR76E. (A) Average waveforms of the flash visual evoked potential (F-VEP) in control and treated mice. (B–E) Bar graphs of the peak amplitudes of (B) N1 and (C) P1 waves and the latency of the (D) N1 and (E) P1 waves. Data represent means±SEM, and statistical analysis was done by one-way analysis of variance with pair-wise Bonferroni post hoc test; *p≤0.01; **p≤0.001 versus rAAV2/5.CMV.eGFP. There was no statistically significant difference in the N1 and P1 peak amplitude between the preglaucomatous control mice, the rAAV2/5.CMV.Epo-treated mice, and the rAAV2/5.CMV.EpoR76E-treated mice. There was no statistically significant difference in the peak latency of the N1 and P1 waves between any of the treatment groups and the preglaucomatous control mice. The up arrow (↑) in (A) indicates a flash of light.
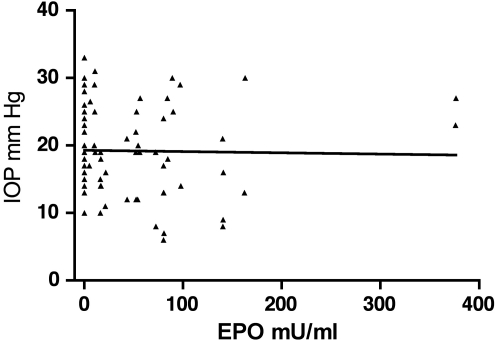
rAAV-mediated gene therapy using either the wild-type Epo vector or the EpoR76E mutant vector produced similar amounts of circulating EPO (Table 1). However, long-term treatment with the mutant vector caused little change in hematocrit whereas similar treatment with the wild-type vector caused dangerously high hematocrit levels (Table 1). Treatment with either rAAV2/5.CMV.Epo or rAAV2/5.CMV.EpoR76E had no statistically significant effect on the IOP (Fig. 5).
Table 1.
Hematocrit and Serum Erythropoietin Levels in 10-Month-Old DBA/2J Mice
| Vector treatment | Erythropoietin (mU/ml)a | Hematocrita |
|---|---|---|
| rAAV2/5.CMV.eGFP | 0 (n=6) | 46±2.5% (n=17) |
| rAAV2/5.CMV.Epo | 91.6±37 (n=8) | 81±10.1% (n=9)b |
| rAAV2/5.CMV.-EpoR76E | 67.2±89.4 (n=18) | 55±11.3% (n=23) |
Data represent means±SD.
Mice treated with rAAV2/5.CMV.Epo were phlebotomized routinely to keep hematocrit levels from exceeding 90%; no other treatment groups required this.
FIG. 5.
Erythropoietin (EPO) does not affect the IOP level. The scatter plot shows the IOP levels and serum EPO concentrations from the same 8-month-old DBA/2J mice. As demonstrated by linear regression analysis, there is no correlation between the concentration of EPO and the IOP (R2=0.0006674). The slope of the line=−0.0097.
Discussion
Glaucoma is the second leading cause of blindness worldwide, affecting nearly 70 million people (Quigley and Broman, 2006). At present, there are no neuroprotective agents clinically available for preventing RGC death or reducing visual field loss in patients with primary open angle glaucoma (Sena et al., 2010). Here we show for the first time that systemic gene delivery of Epo or EpoR76E is capable of protecting both the axons and the RGC somata from glaucomatous damage in DBA/2J mice, and that this morphological protection translates into functional rescue of the visual pathway against glaucomatous damage. In addition, the expression of mutant EPO-R76E provided this protection while maintaining the hematocrit within healthy limits.
The F-VEP is an appropriate electrophysiological test for the study of functional loss due to optic nerve diseases (Holder, 2004). If either the RGC soma or axon is compromised the signal cannot reach the visual cortex and therefore is not detected by F-VEP (Sabel and Aschoff, 1993; Heiduschka et al., 2005, 2010). At 2 years of age nearly all untreated DBA/2J mice have advanced glaucoma resulting in few remaining RGCs and almost no detectable cortical activity as measured by F-VEP (Heiduschka et al., 2010). In the current study we found that as early as 10 months of age the majority of control mice also had minimal cortical response because of glaucomatous damage. Untreated glaucomatous mice had low but detectable N1 and P1 amplitudes and normal latencies, indicating extensive cell death, but normal synaptic connectivity in the remaining cells. Treatment with either rAAV2/5.CMV.Epo or rAAV2/5.CMV.EpoR76E preserved the F-VEP, which was similar to that observed in young mice. On average, mice receiving these vectors showed complete rescue of the N1 and P1 amplitudes, demonstrating functional protection of the RGC soma and axon.
The results of this study and others (Bayer et al., 2002; Keswani et al., 2004; Campana et al., 2006; Yin et al., 2010) indicate that systemic delivery of EPO is therapeutic for a broad range of neurodegenerative diseases both in the peripheral and central nervous systems. In many neurodegenerative diseases axonal degeneration precedes death of the neuronal cell body and is often causal of disease symptoms (Coleman and Perry, 2002; Raff et al., 2002). This is true of glaucoma, in which progressive axon degeneration is the key pathogenic event causing vision loss and eventual death of the RGC soma (Howell et al., 2007; Nagaraju et al., 2007; Beirowski et al., 2008). Therefore, demonstration of protection in glaucoma may indicate that our mutant EPO will also protect against many other CNS degenerative diseases. One likely example is Alzheimer's disease, in which there appears to be a genetic link with glaucoma because the rate of glaucoma in Alzheimer's patients is five times higher than that observed in a control population (Bayer et al., 2002; McKinnon et al., 2003). Also, systemic protein therapy with EPO or nonerythropoietic EPO has been shown to rescue axons in other neurodegenerative models including optic nerve crush (Wang et al., 2009), acrylamide-induced neuropathy (Keswani et al., 2004), chronic constriction injury model (Campana et al., 2006), sciatic nerve transection (Yin et al., 2010), and ALS (Mennini et al., 2006).
In the DBA/2J model the primary insult to the optic nerve occurs in the glial lamina as a result of increased IOP (Howell et al., 2007). Although the mechanism of the pathogenesis is unknown several possibilities exist, including mechanical stress, glutamate excitotoxicity, neurotrophin deprivation, oxidative stress, glial cell modulation, and inflammation (Baltmr et al., 2010; Qu et al., 2010). Here we report that the protection afforded by EPO is independent of IOP levels. This is of key importance because all currently available therapies are targeted at lowering the IOP, and these therapies have had only mixed success due to poor patient compliance and a subpopulation of patients with normal-tensive glaucoma. In contrast, systemic treatment with EPO-R76E blocks progression of the disease at time of treatment regardless of the IOP.
There are three potential mechanisms by which EPO might provide protection to the RGCs:
1. Direct interaction with the RGCs: EPO could interact directly with the RGC somata or axons, allowing them to maintain metabolic activity and function despite increased pressure. This hypothesis is supported by the fact that EPO and its receptor are expressed in the RGC layer of both humans and mice. Furthermore, EPO treatment of the retina activates the expected signal transduction cascades (Junk et al., 2002; Shah et al., 2009; Scheerer et al., 2010). Also, in vitro evidence suggests that EPO acts as a paracrine factor (Ruscher et al., 2002) and protects neurons from excitotoxic conditions including exposure to N-methyl-d-aspartic acid (Digicaylioglu and Lipton, 2001) or nitric oxide (Yamamoto et al., 2004).
2. Interactions with surrounding cells: EPO may act on the surrounding tissue to provide indirect protection to the optic nerve. Increased glial reactivity within astrocytes and Müller cells in the retina (Inman and Horner, 2007) as well as astrocytes and microglia within the optic nerve head is associated with the glaucomatous state in the DBA/2J model (Hernandez et al., 2008; Son et al., 2010). We have shown that treatment with EPO can decrease Müller cell hypertrophy (Rex et al., 2009). In astrocytes, glutamate activation of aquaporin-4 increases water permeability and causes edema (Gunnarson et al., 2008). EPO prevents this aquaporin-mediated swelling in astrocytes by blocking the activation of group 1 metabotropic glutamate receptors (Gunnarson et al., 2009). In addition, reactive astrocytes in the optic nerve head produce nitric oxide synthase in response to increased IOP (Neufeld et al., 1997, 1999). Nitric oxide has been shown to be an “axonal injury factor” that stimulates production of endogenous EPO as a protective factor to reduce axonal degeneration in an acrylamide-induced neuropathy model (Keswani et al., 2004).
3. Indirect effects on blood flow and cerebrospinal fluid pressure: Some patients have normal-tensive glaucoma, in which IOP is normal but visual field loss from optic nerve damage still occurs. In a retrospective study of patients with normal-tensive glaucoma, many had lower cerebral spinal fluid (CSF) pressure, which resulted in a trans-pressure gradient across the optic nerve (the IOP was comparatively higher than the CSF pressure; Berdahl et al., 2008). This trans-pressure difference may be responsible for optic nerve damage in normal-tensive glaucoma patients (Berdahl et al., 2009; Yang et al., 2010). EPO may alter this trans-pressure by altering blood flow (Yamasaki et al., 2005; Lin et al., 2007) and, in our model, it might indirectly raise CSF pressure, resulting in a restored balance of pressure on the optic nerve and hence neuroprotection.
In summary, studies by our group and others have demonstrated that systemic therapy with EPO provides protection in various neurodegenerative models including photoreceptor degeneration (Sullivan et al., 2011), optic nerve crush (Wang et al., 2009), acrylamide-induced neuropathy (Keswani et al., 2004), the chronic constriction injury model (Campana et al., 2006), and sciatic nerve transection (Yin et al., 2010). Results of the current study have expanded these findings by establishing intramuscular delivery of rAAV2/5.CMV.EpoR76E as an improved therapy without hematopoietic side effects and with the potential to treat glaucoma as well as a broad range of neurodegenerative diseases both in the peripheral nervous system and central nervous system. Because systemic EPO is already approved by the U.S. Food and Drug Administration and currently the protein therapy of choice for anemic patients (Wish, 2009), EPO-R76E is a promising candidate to move quickly from bench to bedside. As a result of these exciting results, we are now testing human EPO-R76E with the goal of ultimately translating this work to the clinic. Additional future studies will further explore possible mechanisms of action of EPO-R76E as well as its therapeutic potential in treating other forms of neurodegenerative disease.
Acknowledgments
The authors thank Dr. Machelle Pardue and her student, Rachael Stewart, for their assistance and guidance in performing and analyzing the F-VEPs. This work was supported by an unrestricted grant from Research to Prevent Blindness, NIH 5P30EY13080, NIH R01EY017841, the U.S. Army Medical Research and Materiel Command, the Telemedicine & Advanced Technology Research Center (W81XWH-10-1-0528), a Research to Prevent Blindness Career Development Award, the Glaucoma Research Foundation, the UTHSC Neuroscience Institute, Hope for Vision, and the Roche Foundation for Anemia Research.
Department of Defense Nonendorsement Disclaimer
The views, opinions, and/or findings contained in this research presentation are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy, or decision unless so designated by other documentation. No official endorsement should be made.
Author Disclosure Statement
No competing financial interests exist for authors Eldon E. Geisert and Jessica Hines-Beard. Authors Timothy A. Sullivan and Tonia S. Rex have filed a patent entitled “Therapeutic Compositions and Methods for Disorders Associated with Neuronal Degeneration”; however, no licensing has occurred.
References
- Baltmr A. Duggan J. Nizari S., et al. Neuroprotection in glaucoma: Is there a future role? Exp. Eye Res. 2010;91:554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Bayer A.U. Ferrari F. Erb C. High occurrence rate of glaucoma among patients with Alzheimer's disease. Eur. Neurol. 2002;47:165–168. doi: 10.1159/000047976. [DOI] [PubMed] [Google Scholar]
- Beirowski B. Babetto E. Coleman M.P. Martin K.R. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Berdahl J.P. Allingham R.R. Johnson D.H. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115:763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Berdahl J.P. Ethier C.R. Allingham R.R. Cerebrospinal fluid pressure and glaucomatous optic disc cupping. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:1289–1290. doi: 10.1007/s00417-009-1110-x. [DOI] [PubMed] [Google Scholar]
- Brines M.L. Ghezzi P. Keenan S., et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham B.P. Inman D.M. Lambert W., et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J. Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana W.M. Li X. Shubayev V.I., et al. Erythropoietin reduces Schwann cell TNF-α, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur. J. Neurosci. 2006;23:617–626. doi: 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- Canola K. Angénieux B. Tekaya M., et al. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest. Ophthalmol. Vis. Sci. 2007;48:446–454. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M.P. Perry V.H. Axon pathology in neurological disease: A neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M. Lipton S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Dijk F. Bergen A.A. Kamphuis W. GAP-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp. Eye Res. 2007;84:858–867. doi: 10.1016/j.exer.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H. Degner D. Meller J., et al. Erythropoietin: A candidate compound for neuroprotection in schizophrenia. Mol. Psychiatry. 2004;9:42–54. doi: 10.1038/sj.mp.4001442. [DOI] [PubMed] [Google Scholar]
- Filippopoulos T. Matsubara A. Danias J., et al. Predictability and limitations of non-invasive murine tonometry: Comparison of two devices. Exp. Eye Res. 2006;83:194–201. doi: 10.1016/j.exer.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Golde W.T. Gollobin P. Rodriguez L.L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab. Anim. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Grimm C. Wenzel A. Groszer M., et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat. Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grimm C. Wenzel A. Stanescu D., et al. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J. Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarson E. Zelenina M. Axehult G., et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia. 2008;56:587–596. doi: 10.1002/glia.20627. [DOI] [PubMed] [Google Scholar]
- Gunnarson E. Song Y. Kowalewski J.M., et al. Erythropoietin modulation of astrocyte water permeability as a component of neuroprotection. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1602–1607. doi: 10.1073/pnas.0812708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K. Harris R.A. The anesthetic mechanism of urethane: The effects on neurotransmitter-gated ion channels. Anesth. Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- Heiduschka P. Fischer D. Thanos S. Recovery of visual evoked potentials after regeneration of cut retinal ganglion cell axons within the ascending visual pathway in adult rats. Restor. Neurol. Neurosci. 2005;23:303–312. [PubMed] [Google Scholar]
- Heiduschka P. Julien S. Schuettauf F. Schnichels S. Loss of retinal function in aged DBA/2J mice: New insights into retinal neurodegeneration. Exp. Eye Res. 2010;91:779–783. doi: 10.1016/j.exer.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Hernandez M.R. Miao H. Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- Holder G.E. Electrophysiological assessment of optic nerve disease. Eye (Lond) 2004;18:1133–1143. doi: 10.1038/sj.eye.6701573. [DOI] [PubMed] [Google Scholar]
- Howell G.R. Libby R.T. Jakobs T.C., et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman D.M. Horner P.J. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- John S.W. Smith R.S. Savinova O.V., et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- Junk A.K. Mammis A. Savitz S.I., et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani S.C. Buldanlioglu U. Fischer A., et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann. Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Konishi Y. Chui D.H. Hirose H., et al. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993;609:29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- Libby R.T. Anderson M.G. Pang I.H., et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Lin X. Fujita M. Kanemitsu N., et al. Sustained-release erythropoietin ameliorates cardiac function in infarcted rat-heart without inducing polycythemia. Circ. J. 2007;71:132–137. doi: 10.1253/circj.71.132. [DOI] [PubMed] [Google Scholar]
- McKinnon S.J. Glaucoma: Ocular Alzheimer's disease? Front. Biosci. 2003;8:1140–1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- Mennini T. De Paola M. Bigini P., et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol. Med. 2006;12:153–160. doi: 10.2119/2006-00045.Mennini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju M. Saleh M. Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest. Ophthalmol. Vis. Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld A.H. Hernandez M.R. Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch. Ophthalmol. 1997;115:497–503. doi: 10.1001/archopht.1997.01100150499009. [DOI] [PubMed] [Google Scholar]
- Neufeld A.H. Sawada A. Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J. Wang D. Grosskreutz C.L. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp. Eye Res. 2010;91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H.A. Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M.C. Whitmore A.V. Finn J.T. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Rex T.S. Wong Y. Kodali K. Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp. Eye Res. 2009;89:735–740. doi: 10.1016/j.exer.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V.M. Gao G.P. Grant R.L., et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Ruscher K. Freyer D. Karsch M., et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: Evidence from an in vitro model. J. Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel B.A. Aschoff A. Functional recovery and morphological changes after injury to the optic nerve. Neuropsychobiology. 1993;28:62–65. doi: 10.1159/000119001. [DOI] [PubMed] [Google Scholar]
- Sakanaka M. Wen T.C. Matsuda S., et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinova O.V. Sugiyama F. Martin J.E., et al. Intraocular pressure in genetically distinct mice: An update and strain survey. BMC Genet. 2001;2:16. doi: 10.1186/1471-2156-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer N. Dünker N. Imagawa S., et al. The anemia of the newborn induces erythropoietin expression in the developing mouse retina. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R111–R118. doi: 10.1152/ajpregu.00108.2010. [DOI] [PubMed] [Google Scholar]
- Sena D.F. Ramchand K. Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2010;2:CD006539. doi: 10.1002/14651858.CD006539.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.S. Tsang S.H. Mahajan V.B. Erythropoietin receptor expression in the human diabetic retina. BMC Res. Notes. 2009;2:234. doi: 10.1186/1756-0500-2-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J.L. Soto I. Oglesby E., et al. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780–789. doi: 10.1002/glia.20962. [DOI] [PubMed] [Google Scholar]
- Sullivan T. Kodali K. Rex T.S. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem. Res. 2011;36:613–618. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- van der Kooij M.A. Groenendaal F. Kavelaars A., et al. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res. Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Wang H. Liu Z.L. Zhuang X.T., et al. Neuroprotective effect of recombinant human erythropoietin on optic nerve injury in rats. Chin. Med. J. (Engl) 2009;122:2008–2012. [PubMed] [Google Scholar]
- Wang W.H. Millar J.C. Pang I.H., et al. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest. Ophthalmol. Vis. Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- Wish J.B. Past, present, and future of chronic kidney disease anemia management in the United States. Adv. Chronic Kidney Dis. 2009;16:101–108. doi: 10.1053/j.ackd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Koshimura K. Sohmiya M., et al. Effect of erythropoietin on nitric oxide production in the rat hippocampus using in vivo brain microdialysis. Neuroscience. 2004;128:163–168. doi: 10.1016/j.neuroscience.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Yamasaki M. Mishima H.K. Yamashita H., et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050:15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Yang Y. Yu M. Zhu J., et al. Role of cerebrospinal fluid in glaucoma: Pressure and beyond. Med. Hypotheses. 2010;74:31–34. doi: 10.1016/j.mehy.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Yin Z.S. Zhang H. Bo W. Gao W. Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. Am. J. Neuroradiol. 2010;31:509–515. doi: 10.3174/ajnr.A1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Bradley J. Schubert W., et al. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest. Ophthalmol. Vis. Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]