Abstract
Lentiviral vectors (LVs) pseudotyped with envelope proteins of alphaviruses have recently attracted considerable interest for their potential as gene delivery tools. We report the production of human immunodeficiency virus type 1 (HIV-1)–derived LVs pseudotyped with envelope glycoproteins derived from the Aura virus (AURA). We found that the AURA-glycoprotein–pseudotyped LVs use C-type lectins (DC-SIGN and L-SIGN) as attachment factors. These interactions with DC-SIGN are specific as determined by inhibition assays and appear to facilitate transduction through a pH-dependent pathway. AURA-pseudotyped LVs were used to transduce monocyte-derived dendritic cells (DCs) and the transduction was shown to be DC-SIGN mediated, as illustrated by competitive inhibition with DC-SIGN and L-SIGN antibodies and yeast mannan. Comparisons with LVs enveloped with glycoproteins derived from vesicular stomatitis virus and Sindbis virus suggest that AURA-glycoprotein–bearing LVs might be useful to genetically modify DCs for the study of DC biology and DC-based immunotherapy.
Froelich and colleagues report that pseudotyping lentiviral vectors (LVs) with envelope glycoproteins from the Aura virus alphavirus results in LVs that use C-type lectins such as DC-SIGN as attachment factors. These pseudotyped LVs exhibit marked preferential transduction of DC-SIGN receptor-expressing dendritic cells (DCs) over other cell types, highlighting their potential for genetically modifying DCs.
Introduction
The introduction of a functional gene into specific cell types has emerged as a useful tool for both performing basic scientific research and developing novel therapeutics. Much effort has been focused on engineering viral vectors as gene transfer vehicles because of their high efficiency (Kootstra and Verma, 2003). Among these vectors, lentiviral vectors (LVs) derived from human immunodeficiency virus type 1 (HIV-1) are promising because they have the ability to produce stable transduction, maintain long-term transgene expression, and transduce both dividing and nondividing cells (Naldini et al., 1996). Using such viruses to transduce particular cell types has been restricted due to the natural tropism of HIV-1 for CD4+ T cells and macrophages. One mechanism for altering the tropism of LVs is through pseudotyping, incorporating envelope glycoproteins from other viruses into the lentiviral surface (Cronin et al., 2005). Due to its very broad tropism and stability, LVs are commonly pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G). However, the VSV-G envelope comes with a number of limitations including the susceptibility to inactivation in human complement, toxicity of constitutively expressed glycoprotein in producer cell lines, and broad tropism with unknown entry receptor(s) (DePolo et al., 2000; Coil and Miller, 2004).
There is a growing supply of alternative glycoproteins and strategies to engineer envelope glycoproteins for pseudotyping LVs, each with specific advantages and disadvantages. A number of recent reports have focused on enveloping LVs with glycoproteins derived from alphaviruses (Cronin et al., 2005). These pseudotypes are particularly promising because they can be concentrated to obtain high-titer viral preparations, resistant to inactivation by components in human sera, and stable packaging cell lines have previously been generated that could be scaled up to meet the larger volumes for clinical demand (Strang et al., 2005). Recently, pseudotyping of HIV-1 vectors has been shown with glycoproteins from alphaviruses including Western and Venezuelan equine encephalitis viruses, Chikungunya virus, Ross River virus, Semliki Forest virus, and Sindbis virus (SIN) (Morizono et al., 2001; Kahl et al., 2004; Kolokoltsov et al., 2005; Poluri et al., 2008; Akahata et al., 2010). In addition, SIN-pseudotyped LVs have been directed to specific cell types by inserting ligands (Aires da Silva et al., 2005) or attaching ligand recognition domains into the glycoprotein (Morizono et al., 2001) and by co-displaying membrane-bound antibodies (Yang et al., 2006; Lei et al., 2009; Yang et al., 2009) or ligands (Ziegler et al., 2008; Froelich et al., 2009). In this study, we show for the first time that the Aura virus glycoprotein (AURA-G) can be incorporated into HIV-1–derived LVs and form infectious pseudotyped particles.
Although the cell surface receptors used by alphaviruses to infect a broad variety of species have not yet been determined, it has been suggested that several receptors are involved in virus entry. Alphaviruses are mosquito-borne, enveloped, positive-sense RNA viruses that appear to have evolved to exploit cells of the macrophage–dendritic cell (DC) lineage for early dissemination from the site of inoculation in the skin (Gardner et al., 2000; MacDonald and Johnston, 2000; Klimstra et al., 2003). The alphaviral glycoprotein complex consists of heterodimers of the E1 and E2 glycoproteins that are organized into trimers upon exposure to low pH (Strauss and Strauss, 1994). The E2 glycoprotein mediates interactions with target cell receptors, whereas E1 is thought to mediate endosomal fusion with the viral membrane in an acidic environment (Garoff et al., 1980; Dubuisson and Rice, 1993; Kielian, 1995). The structural proteins of Aura virus are closely related to those of SIN with 56% identity in glycoprotein E2 and 61% identity in glycoprotein E1 (Rumenapf et al., 1995). Through adaptive mutation in tissue culture, laboratory alphaviral strains have been shown to acquire binding affinity for cell surface heparan sulfate, which results in broad cellular tropisms (Klimstra et al., 1998; Bernard et al., 2000; Heil et al., 2001; Zhu et al., 2010). As opposed to heparan sulfate, the mosquito-produced Sindbis alphavirus (Klimstra et al., 2003) and LVs with modified SIN glycoproteins (Yang et al., 2008) were shown to use C-type lectins as attachment receptors leading to productive transduction of DCs. Therefore, we reasoned that C-type lectins DC-SIGN and L-SIGN, together known as DC-SIGN(R), may be important attachment factors for AURA-G–bearing LVs as well.
In this study, we found that AURA-G–pseudotyped LVs have an enhanced preferential transduction for DC-SIGN(R)–expressing cells and a low transduction efficiency towards other cell types, which would make AURA-G pseudotypes very useful for gene delivery to DCs. We conducted a comparative study of the efficiency of incorporation of the VSV-, SIN-, and AURA-glycoproteins into LVs, and we demonstrated the efficient pseudotyping of LVs with the envelope proteins of Aura virus. When the conditions were optimized, we achieved titers of approximately 1 × 105 transduction units (TU)/ml for both AURA and SIN pseudotypes on cells expressing DC-SIGN with approximately 10 times less transduction units per milliliter of parental (DC-SIGN−) cells, whereas VSV-pseudotypes produced titers of approximately 10 × 106 TU/ml on both cell lines. We showed that AURA-G–pseudotyped lentivectors have an intrinsic tropism for DC-SIGN(R)–expressing cells, including monocyte-derived DCs (MoDCs). These interactions with DC-SIGN were specific as determined by inhibition assays and appeared to transduce cells through a pH-dependent pathway similar to that of SIN-G–pseudotyped LVs. Our findings indicate that the AURA-G might be an attractive envelope to pseudotype LVs for transduction of DCs for gene-transfer applications.
Materials and Methods
Cell lines
The human embryonic kidney cell line 293T was used to derive 293T.DCSIGN as previously described (Yang et al., 2008). Mouse fibroblast NIH 3T3 cells were obtained from the American Tissue Culture Collection. 3T3/MX-L-SIGN and 3T3/MX-DC-SIGN (Wu et al., 2002) were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. These cell lines were maintained in D10 medium (Dulbecco modified Eagle medium, Invitrogen) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 2 mM l-glutamine, and 100 U/ml of penicillin and 100 μg/ml of streptomycin.
Plasmid construction
The glycoprotein expression plasmids were constructed similarly to a previously reported procedure (Morizono et al., 2001). The cDNA of SIN-G was amplified from the AR 339 strain, which contains the full-length cDNA of the SIN genome (GenBank sequence J02363). The cDNA of AURA-G was amplified from the full-length cDNA of the Aura virus genome (GenBank sequence AF126284). The amplified fragments for both glycoproteins contained the E3-E2-6K-E1 coding regions with a Kozak sequence at the translational start site. These fragments were subcloned using BamH1 restriction endonuclease into the VSV-G expression plasmid containing the rabbit β-globin intron and poly (A) signal (Cell Genesys). The resulting plasmids were designated pSIN-G and pAURA-G (Fig. 1). The lentiviral backbone plasmid (FUGW and its derivatives) used in this study are the third generation HIV-based LVs in which most of the U3 region of the 3′ long terminal repeat (LTR) was deleted, resulting in a self-inactivating 3′-LTR (Fig. 1) and has been previously described (Yang et al., 2006). The integrity of the DNA sequences was confirmed by DNA sequencing.
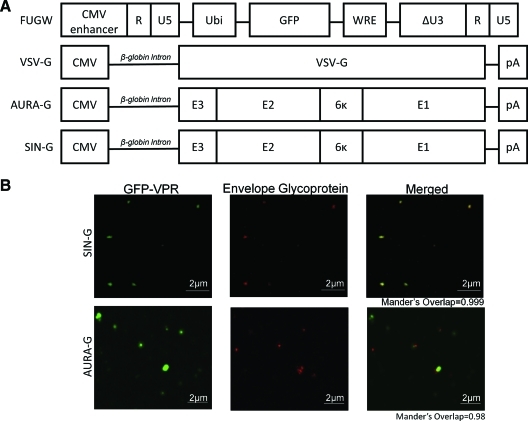
FIG. 1.
Virus-producing constructs used to make pseudotyped lentiviral vectors (LVs). (A) Schematic diagrams of constructs encoding lentiviral backbone FUGW and alphaviral envelope glycoproteins. Ubi, the human ubiquitin-C promoter; GFP, enhanced green fluorescence protein; WRE, woodchuck responsive element; ΔU3, deleted U3 region that results in the transcriptional activation of the integrated viral long terminal repeat promoter; pA, polyadenylation signal. E1, E2, 6k, and E3 are alphavirus glycoproteins: E1 and E2 for fusion and receptor binding, respectively; 6k is a linker; and E3 is a signal sequence. The VSV-G–expressing plasmid contains the rabbit β-globin intron and poly(A) signal. Aura virus (AURA) and Sindbis virus (SIN) glycoprotein (AURA-G and SIN-G) expression plasmids were constructed with both having the rabbit β-globin intron and poly(A) signal from vesicular stomatitis virus glycoprotein (VSV-G) expression plasmid to standardize glycoprotein expression cassettes. (B) Viral supernatants harvested from virus-producing cells transiently transfected with GFP-vpr, AURA-G, or SIN-G, and other necessary packaging constructs, were coated onto a poly-lysine containing coverslip by centrifugation. The resulting coverslips were then rinsed and immunostained with Sindbis virus immune ascitic fluid to label the glycoproteins (red) and imaged using a laser confocal microscope. Color images available online at www.liebertonline.com/hum
Production of pseudotyped viral particles
Recombinant LVs were prepared by transient transfection of 293T cells using a standard calcium phosphate precipitation protocol (Pear et al., 1993). 293T cells cultured in 6-cm tissue culture dishes (BD Biosciences) were transfected with the lentiviral backbone plasmid FUGW (5 μg), along with 2.5 μg of the envelope plasmid (pSIN-G, pAURA-G, or pVSV-G) and 2.5 μg of the packaging plasmids (pMDLg/pRRE and pRSV-REV). The viral supernatants were harvested 48 and 72 hr post-transfection and filtered through a 0.45-μm filter. Their titers were determined by flow cytometry analysis of enhanced green fluorescence protein (GFP) expression. To prepare concentrated viruses, 293T cells cultured in a 15-cm tissue culture dish (BD Biosciences) were transfected with the following plasmids: FUGW (37.5 μg), the envelope plasmid (18.75 μg), and the packaging plasmids (pMDLg/pRRE and pRSV-Rev, 18.75 μg for each). The viral supernatants (30 ml) were harvested 48 and 72 hr post-transfection and filtered through a 0.45-μm filter before being concentrated 300-fold by ultracentrifugation at 4°C (Optima L-80K preparative ultracentrifuge, Beckman Coulter) at 50,000 × g for 90 min with a > 80% recovery. The pellets were then resuspended in an appropriate volume of Hank's Buffered Salt Solution (Lonza).
Confocal imaging of GFP-vpr–labeled virions
GFP-vpr–labeled lentiviral particles were produced as described above with an additional plasmid encoding GFP-vpr (2.5 μg). Fresh viral supernatant was overlaid on polylysine-coated coverslips in a six-well culture dish and centrifuged at 3700 × g at 4°C for 2 hr using an RT Legend centrifuge. The coverslips were washed with cold phosphate-buffered saline (PBS) twice and incubated with SIN immune ascitic fluid (ATCC number VR-1248AF) for 40 min at 4°C. After three washes with PBS, the cells were incubated for 40 min at 4°C with 1:250 dilutions of secondary antibodies consisting of species-specific Cy5-conjugated anti-immunoglobulin G (Santa Cruz Biotechnology). Fluorescent images were acquired by using a Zeiss LSM 510 laser scanning confocal microscope equipped with filter sets for fluorescein and Cy5. A plan-apochromat oil immersion objective (63 × /1.4) was used for imaging.
Virus attachment assays
Production of [35S]methionine-labeled viruses were carried out by transfection of 293T as described above. Cells were maintained in Dulbecco modified Eagle medium complement for 4 hr. Cells were then depleted of methionine for an additional 3 hr by replacement of media with antibiotics and methionine-free minimum essential medium α (αMEM). At 8 hr post-transfection, [35S]methionine was added to a final concentration of 20 μCi/ml and cells were incubated at 37°C for an additional 12 hr. 35S-radiolabeled virus was purified from cell supernatants by using a discontinuous sucrose gradient (20%/60% [wt/wt] in TNE buffer [50 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA]), followed by pelleting through 20% sucrose in TNE buffer. Radiolabeled virus particles were resuspended in PBS. Approximately 105 cpm of each radiolabeled virus, diluted in pH = 7.4 PBS, was mixed with 106 cells in 1.5-ml microcentrifuge tubes for a total volume of 50 μl, and this mixture was incubated at 4°C for 1 hr with gentle agitation. Cells were washed three times with PBS then resuspended in PBS. Radioactivity of 35S for the resuspended cells was quantitated with a liquid scintillation counter. The experiments were performed in triplicate and the percentage of bound radiolabeled particles was calculated from a cell-free virus-only control.
Determination of p24 and infectious titers
To determine p24 titers, supernatants were diluted 1:10,000 and assayed by an enzyme-linked immunosorbent assay (ELISA) using the p24 ELISA kit from ImmunoDiagnostics, according to the manufacturer's instructions. AURA-G–producing cells made 360 ± 38 ng/μl of p24, whereas SIN-G transfected cells produced 348 ± 19 ng/μl and VSV-G supernatant yielded 308 ± 42 ng/μl of p24. To determine infectious titer, 2 × 104 293T or 293T.DCSIGN cells were transduced in triplicate with 100 μl of serially diluted viral supernatants with 8 μg/ml of polybrene (Sigma-Aldrich) for 1.5 hr by spin-inoculation at 2500 rpm and 25°C using a RT Legend centrifuge. Following the spin-infection, the supernatants were replaced with fresh culture medium and incubated for an additional 48 hr at 37°C with 5% CO2. The GFP expression was evaluated by flow cytometry analysis.
Vector-mediated transduction of cell lines in vitro
Target cells (293T.DCSIGN, 293T, 3T3-LSIGN, 3T3-DCSIGN, or 3T3 cells; 2 × 104 per well) were seeded in 96-well culture dishes and spin-infected with viral supernatants (150 μl per well with 50 ng of p24-normalized virus) at 2500 rpm and 25°C for 90 min using a RT Legend centrifuge. Subsequently, the supernatants were replaced with fresh culture medium and incubated for 48 hr at 37°C with 5% CO2. The GFP expression was evaluated by flow cytometry.
Assays to inhibit pseudotyped virus–mediated infection
In dose–response experiments, triplicate wells of 293T.DCSIGN were incubated with 0.2 to 200 μg/ml of yeast mannan (Sigma-Aldrich) at 37°C for 30 min. AURA-G–, SIN-G–, or VSV-G–bearing LVs were incubated for 8 hr, and then supernatant was replaced with fresh medium. For bafilomycin and NH4Cl inhibitions, pseudotyped viral particles were spin-inoculated in the presence of bafilomycin A1 (Sigma-Aldrich) or increasing concentrations of NH4Cl for 90 min at 25°C. Subsequently, the supernatants were replaced with medium containing bafilomycin A1 or NH4Cl and incubated for 2 hr before being replaced with fresh medium. 293T.DCSIGN cells were incubated with 5 μg/ml of anti-DC-SIGN antibodies (14E3G7, 19F7, DC-28, and isotype control antibody, Santa Cruz Biotechnology), 5 mM EDTA at 37°C for 30 min, and then inoculated with 293T-produced AURA-G–, SIN-G–, or VSV-G–bearing LVs at a multiplicity of infection (MOI) <1 for 8 hr. The cells were analyzed for GFP expression 2 days post-transduction.
Transduction of human peripheral blood mononuclear cell–derived DCs
Peripheral blood mononuclear cells (PBMCs) from healthy human donors were purchased from AllCells. PBMCs were allowed to adhere for 2 hr on non–tissue culture–treated 24-well plates and washed extensively. Adherent monocytes were plated at a density of 1 million/well and grown in RPMI medium plus 10% fetal bovine serum, antibiotics, interleukin (IL)-4 (500 U/ml), and granulocyte macrophage colony-stimulating factor (GM-CSF) (1000 U/ml) as described previously (Obermaier et al., 2003). After 2 days of culture, DCs were identified by examining the surface markers (CD11C+, DC-SIGN+) using flow cytometry analysis. Every 3 days new cytokines were added. MoDCs at day 2 cells were exposed to virus at the indicated MOI based on 293T cells. For inhibition of DC-SIGN–mediated transduction, DCs were incubated with 20 μg/ml of anti-DC-SIGN(R) antibody (14E2G7, Santa Cruz Biotechnology) or 200 μg/ml yeast mannan (Sigma-Aldrich) at 37°C for 30 min, and then inoculated with 293T-produced FUGW/AURAG or FUGW/SING LVs at an MOI = 10 based on titer on 293T cells for 8 hr before the media was changed. The cells were analyzed for GFP expression 5 days post-transduction.
Results
Generation of AURA-G-pseudotyped lentiviral vectors
It has not been previously shown that AURA-G can pseudotype HIV-1–derived LVs. We first investigated the pseudotyping efficiency of HIV-1 vectors with AURA-G by transient transfection. We standardized the glycoprotein expression plasmids used in this study by subcloning the AURA-G and SIN-G expression cassettes into the VSV-G expression vector containing the rabbit β-globin intron and the poly(A) signal sequence (Fig. 1A); the plasmid used for VSV-G expression has been used extensively for pseudotyping LVs (Kahl et al., 2004). The production of LVs pseudotyped with AURA-, SIN-, or VSV-glycoproteins were generated by the co-transfection of 293T cells with lentiviral construct FUGW (Lois et al., 2002); plasmids encoding viral gag, pol, and rev genes (Klages et al., 2000); and the respective glycoprotein expression plasmid. FUGW carries the GFP reporter gene under control of the human ubiquitin-C promoter (Fig. 1A) (Lois et al., 2002). GFP-vpr-labeled LVs were produced as described above with transfection of an additional plasmid that encoded GFP fused to the HIV-1 vpr protein (Joo and Wang, 2008). Antibody staining of GFP-vpr-labeled virus with an AURA and SIN cross-reactive antiserum revealed significant overlap (Mander's overlap coefficient >0.9) for both AURA-G– and SIN-G–pseudotyped viruses (Fig. 1B). These results indicate a similar level of incorporation of the respective glycoproteins onto lentiviral particles. In contrast VSV-G-pseudotyped viruses exhibited no glycoprotein staining when the antiserum was used (data not shown).
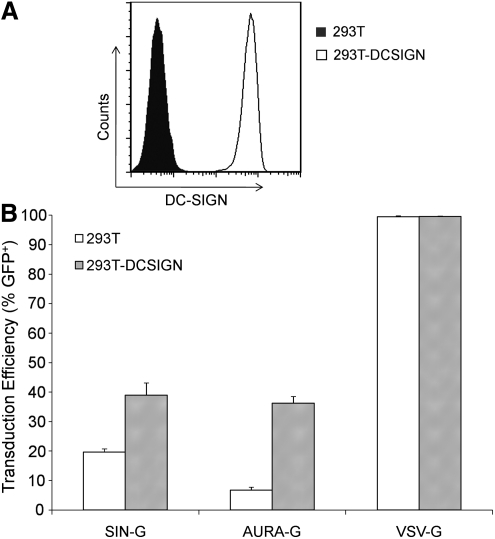
To compare the infectivity of AURA-G–, SIN-G–, and VSV-G–bearing LVs, the infectious titer was determined on 293T cells and the 293T.DCSIGN cell line, which stably expresses human DC-SIGN (Fig. 2A). Differences in viral titer may be attributed to several factors: differences in virus–receptor interactions, the efficiency of production of functional particles into the supernatant, as well as the amount of defective particles, which may serve as interfering particles. As shown in Table 1, AURA-G can effectively pseudotype HIV-1 vectors to produce infectious particles; these viruses are designated FUGW/AURAG. For comparison the infectious titer of SIN-G–pseudotyped LV (FUGW/SING) and VSV-G–pseudotyped LV (FUGW/VSVG) were produced. The production of FUGW/VSVG was the most efficient and estimated to be approximately 10 × 106 TU/ml on both 293T and 293T.DCSIGN cells. SIN-G–pseudotyped LVs exhibited a lower infectious titer on both cell lines but with an observable increase in infectivity with cells expressing DC-SIGN. The titer on 293T cells was approximately 6 × 104 TU/ml versus about 1.7 times higher on 293T cells expressing DC-SIGN (Table 1). Similarly, when AURA-G was used as the envelope glycoprotein, the infectious titer was higher with cells expressing DC-SIGN. Based on 293T.DCSIGN cells, the FUGW/AURAG titer was approximately 0.8 × 105 TU/ml versus a 10-fold decrease on 293T cells (Table 1). The AURA-G–bearing LV was much less infectious with 293T cells; the titer was approximately 7.5 and 125 times lower than that of SIN-G and VSV-G, respectively. Furthermore, the difference in infectious units between cell types is clear evidence that the transduction by SIN-G– and AURA-G–bearing LVs is enhanced by the presence of DC-SIGN.
FIG. 2.
Lentiviral transduction of DC-SIGN–expressing 293T cells. (A) The 293T.DCSIGN cell line, which stably expresses human DC-SIGN, was stained with anti-DC-SIGN- phycoerythrin (PE) (1:5). Flow cytometry revealed significant DC-SIGN expression on 293T.DCSIGN (no-fill) but not parental 293T (filled). (B) Fresh viral supernatants of FUGW/AURAG, FUGW/SING, and FUGW/VSVG were normalized by p24 (70 ng) and used to transduce 2 × 104 293T.DC-SIGN (open bars) or parental 293T cells (solid bars) lacking the expression of DC-SIGN. Three days later, the transduction efficiency was measured by analyzing GFP expression using flow cytometry. Values are given as the mean of triplicates ± SE.
Table 1.
Infectious Titers of AURA-G-, SIN-G-, and VSV-G-Pseudotyped Lentivectors
| |
293T |
293T.DCSIGN |
|---|---|---|
| Glycoprotein | Mean infectious titer (TU/ml) ± SDa | |
| AURA-G | (0.80 ± 0.05) × 104 | (0.80 ± 0.01) × 105 |
| SIN-G | (6.00 ± 0.50) × 104 | (1.00 ± 0.09) × 105 |
| VSV-G | (10.00 ± 0.1) × 106 | (9.00 ± 0.10) × 106 |
TU, transduction units.
Cell-surface expression of DC-SIGN correlates with the infection of LV pseudotyped with AURA-G and SIN-G
To evaluate the effect of DC-SIGN on infectivity per particle, virus preparations were normalized by p24 measurement and used to infect 293T and 293T.DCSIGN cells. When the same amount of virus was used, there was little difference between 293T and 293T.DCSIGN in their susceptibility to transduction by VSV-G–pseudotyped viruses (Fig. 2B). FUGW/VSVG had a similar level of transduction toward both cell lines (Fig. 2B), indicating that VSV-G has a high infectivity per particle toward both 293T and cells expressing DC-SIGN on the cell surface. In contrast, the AURA-G–bearing virus transduced 293T cells with a very low infectivity, but the infectivity was significantly enhanced in the presence of DC-SIGN (Fig. 2); FUGW/AURAG could transduce 293T.DCSIGN cells, with an efficiency of approximately 36%, but with only about 6% transduction of 293T cells. FUGW/SING had a higher infectivity per particle than AURA-G–bearing LVs towards 293T cells (approximately 20%). Similar to FUGW/AURAG, SIN-G–pseudotyped LV exhibited an increased infectivity towards 293T cells expressing DC-SIGN (Fig. 2B). Although the transductions were normalized by p24, based on the MOI the ratio of 293T.DCSIGN cells transduced generally corresponded closely to 1 − e(−MOI), as predicted from a Poisson distribution of transduction of the entire cellular population. The p24-normalized transduction demonstrates differences in the infectivity per lentiviral particle and further reveals the natural tropism of SIN-G– and AURA-G–pseudotyped LVs for DC-SIGN–expressing cells.
Cell-surface expression of DC-SIGN and L-SIGN on 3T3 cells mediates binding and transduction of pseudotyped lentiviral vectors
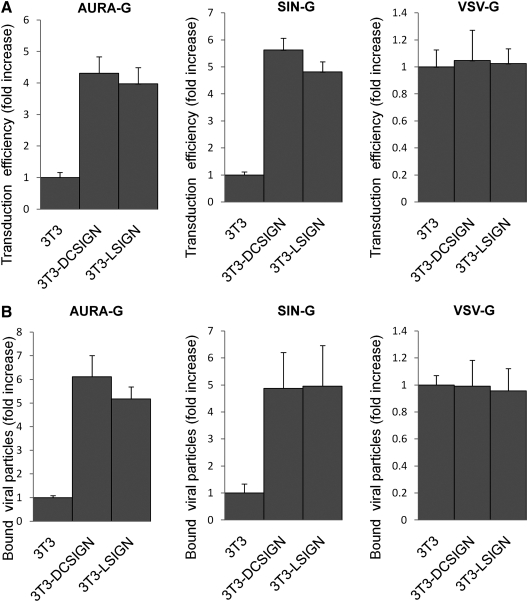
Previous studies have indicated that the cell type in which DC-SIGN(R) is expressed can have a significant impact on the efficiency of these lectins to promote viral infection (Trumpfheller et al., 2003; Wu et al., 2004). To study the function of C-type lectins as AURA-G attachment factors in other cell types, we transduced 3T3/MX-L-SIGN and 3T3/MX-DC-SIGN cell lines and the corresponding parental 3T3 cells as controls. Previously, Wu et al. (2002) examined the levels of DC-SIGN/L-SIGN expression on these cell lines using several cross-reactive monoclonal antibodies and found similar levels of expression of both C-type lectins. These cell lines were exposed to AURA-, SIN-, and VSV-glycoprotein–bearing LVs. After 48 hr, cells were analyzed by flow cytometry for GFP expression. The levels of transduction were normalized based on 3T3 transduction, and then the magnitude of the increase in transduction was assessed. The transduction efficiency in 3T3 cells of pseudotyped LVs were AURA-G, 12.1 ± 1.3; SIN-G, 10.6 ± 0.8; and VSV-G, 72.2 ± 1.0. AURA-G–pseudotyped virus showed an approximate fourfold increase in transduction with cells expressing L-SIGN and DC-SIGN (Fig. 3a). Similarly, SIN-G–enveloped LVs exhibited an increased transduction of both L-SIGN and DC-SIGN, whereas VSV-G–bearing particles did not display a significant difference (Fig. 3A).
FIG. 3.
Effects of DC-SIGN or L-SIGN expression on the infectivity of pseudotyped LVs. (A) AURA-G–, SIN-G–, and VSV-G–pseudotyped LVs were produced by transient transfection of 293T cells. Viral supernatants were normalized by p24 and spin-inoculated with L-SIGN– or DC-SIGN–expressing 3T3 cells; the parental 3T3 cells were included as controls. Three days later, the transduction efficiency was measured by analyzing GFP expression. Fold increase in percentage of GFP-positive cells is shown based on 3T3 cells where values are given as the mean of triplicates ± SE. (B) Specificity of binding to DC-SIGN. [35S]methionine-labeled virus was produced by transfection of 293T cells. Approximately 105 cpm of each radiolabeled virus diluted in phosphate-buffered saline (PBS) was mixed with 106 3T3 or DC-SIGN/L-SIGN–expressing cells. This mixture was incubated at 4°C for 1 hr with gentle agitation. Cells were washed three times with PBS and resuspended in PBS, and 35S radioactivity of the resuspended cells was quantitated with a liquid scintillation counter. Fold increase in percentage of bound 35S (cpm) is shown based on 3T3 cells where values are given as the mean of triplicates ± SE.
To determine if the increase in transduction of pseudotyped LVs for 3T3 cells expressing DC-SIGN or L-SIGN was due to greater cell binding, in vitro attachment assays were performed with [35S]methionine-radiolabeled virus. The AURA-G–bearing particles bound six- to fivefold more efficiently to DC/L-SIGN–expressing cells than to 3T3 controls. Consistent with the infection data, the increase virus binding to L-SIGN– and DC-SIGN–expressing cells correlates to an increase in transduction (Fig. 3). Results of assays with SIN-G–bearing particles showed a direct correlation between an increase in binding and the observed increase in infectivity (Fig. 3B). In the absence of DC-SIGN expression, AURA-G and SIN-G pseudotypes (approximately 2224 cpm) bound poorly to 3T3 cells, as compared with VSV-G–radiolabeled particles (approximately 13,054 CPM). VSV-G–pseudotyped virus exhibited similar levels of attachment to all cell types (Fig. 3B). This suggests that the improved virus infectivity is due to the binding of the virus to DC-SIGN(R). Together, these results reveal that the mammalian-produced AURA- and SIN-G–bearing LVs are able to bind DC-SIGN(R) receptors, resulting in enhanced transduction of DC-SIGN–expressing cells.
AURA-G–mediated infectivity requires acidification
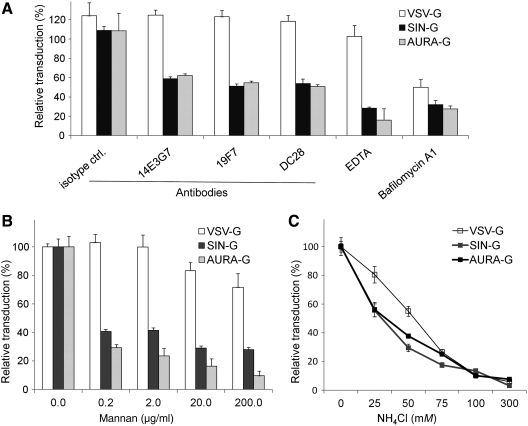
Transduction by VSV-G as well as some alphaviruses is known to involve the endocytic pathway, in which an acidic environment of endosomal vesicles is required upon internalization (Yamada and Ohnishi, 1986; Strauss and Strauss, 1994). Treatment with pH-interfering bafilomycin A1 and NH4Cl, which can neutralize acidic endosomal compartments, abolished the infectivity in 293T.DCSIGN cells for AURA-G–, SIN-G–, and VSV-G–pseudotyped LVs (Fig. 4A,C). These results are consistent with the low pH requirement of alphaviral and VSV glycoproteins to trigger membrane fusion. More direct evidence for pH-dependent fusion was provided by a dose study of increasing concentrations of NH4Cl (Fig. 4C). This suggests that both DC-SIGN–mediated transduction with FUGW/AURAG and FUGW/SING have similar inhibition kinetics due to equivalent acidification requirement for the virus–endosome fusion process. Similarly, VSV-G–mediated transduction was inhibited by increased concentrations of NH4Cl albeit less than alphaviral glycoproteins at concentrations up to 50 mM.
FIG. 4.
Specific inhibitors prevent DC-SIGN–mediated enhancement of infection. (A) In inhibition experiments, 293T.DCSIGN cells were incubated with antibodies at a concentration of 5 μg/ml, 5 mM EDTA, or bafilomycin A1 at 37°C for 30 min, and then inoculated with LVs bearing VSV-G (no fill), SIN-G (dark fill), or AURA-G (gray fill) at a multiplicity of infection (MOI) <1 for 8 hr. In dose-dependent inhibition assays 293T.DCSIGN cells were incubated with LVs in the presence of increasing concentrations of (B) yeast mannan or (C) NH4Cl. Subsequently the supernatants were replaced and incubated with fresh medium for 2 days before being analyzed for GFP expression where values are given as the mean of triplicates ± SE.
Infectivity can be blocked with inhibitors of DC-SIGN
We observed that the infection efficiency of AURA-G– and SIN-G–bearing LVs correlates with the expression of DC-SIGN on target cells. To further examine the specificity of virus interaction with these molecules, we performed infectivity experiments with an MOI of approximately 0.9 in the presence of increasing concentrations of yeast mannan, EDTA, anti-DC-SIGN monoclonal antibodies, or an isotype-matched control (Fig. 4A,B). These treatments disrupt interactions with DC-SIGN molecules (Klimstra et al., 2003; Davis et al., 2006; Lozach et al., 2007). Incubation with mannan, a natural ligand for DC-SIGN, during viral inoculation of FUGW/AURAG and FUGW/SING with 293T.DCSIGN cells resulted in a dose-dependent reduction in the amount of GFP-positive cells (Fig. 4B). However, mannan was not effective at inhibition of VSV-G–bearing LV, suggesting a different receptor-mediated interaction for VSV-G transduction of 293T.DCSIGN cells. The principal characteristic of C-type lectins is that they interact with viral glycoproteins in a calcium-dependent manner via their C-terminal carbohydrate recognition domain. EDTA is a calcium chelator and treatment during transduction with FUGW/AURAG and FUGW/SING resulted in a >70% reduction in GFP-positive cells (Fig. 4A). In contrast, FUGW/VSVG virus was not dependent on calcium for infection as revealed by the EDTA inhibition experiment. Incubation with anti-DC-SIGN antibodies during infection also resulted in a reduction in numbers of GFP-positive cells with AURA-G– and SIN-G–mediated transduction, whereas the isotype control antibody had little inhibitory effect. Again, the FUGW/VSVG virus did not exhibit significant competition with the antibody treatment of DC-SIGN–expressing cells (Fig. 4A). The results of these experiments indicate that the infectivity of AURA-G– and SIN-G–pseudotyped virus is dependent on DC-SIGN expression as well as calcium for entry, whereas FUGW/VSVG transduction is not dependent upon DC-SIGN or calcium.
AURA-G and SIN-G transduce DCs through DC-SIGN(R)
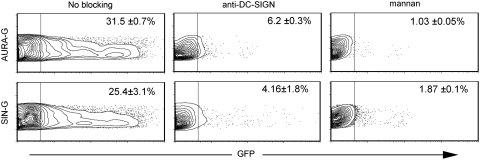
We further evaluated how efficiently AURA-G– and SIN-G–pseudotyped LVs could transduce primary immune cell targets. Human MoDCs were prepared from the PBMCs of healthy human donors and cultured with GM-CSF and IL-4 to generate DC-SIGN+ DCs (Obermaier et al., 2003), which were then challenged with MOI = 10 of AURA- and SIN-G–pseudotyped LVs. More than 80% of the cultured DCs were positive for DC-SIGN expression before infection (data not shown). Transduction of MoDCs by FUGW/AURAG was slightly more efficient than FUGW/SING, transducing approximately 31% versus about 25% DCs (Fig. 5).
FIG. 5.
LVs bearing AURA-G and SIN-G transduce DCs and transduction is mediated by DC-SIGN(R). Human monocyte-derived DCs (MoDCs) were generated by culturing respective precursor cells in the presence of GM-CSF and IL-4. The adherent cells (1 × 106) were cultured for 2 days and then incubated for 1 hr with mannan (200 μg/ml), anti-DC-SIGN(R) antibody (20 μg/ml), or without any reagents. The cells were then infected with FUGW/AURAG or FUGW/SING (MOI = 10) for 8 hr in the presence of blocking reagents. GFP expression was assayed by flow cytometry 5 days post-transduction where one representative figure is shown with values given as the mean of duplicates ± SE.
We further investigated the viral envelope–receptor interactions that facilitate MoDC transduction by AURA-G and SIN-G LVs. Based on our previous results with DC-SIGN(R)–expressing cell lines, we chose to evaluate the ability of inhibitors of DC-SIGN to block infection of human MoDCs. MoDCs were challenged with the same MOI (10) of AURA-G– and SIN-G–pseudotyped LVs incubated in the presence of mannan or anti-DC-SIGN(R) 14E3G7 antibody. Transduction of MoDCs by FUGW/AURAG decreased to approximately 6% with anti-DC-SIGN antibody and decreased to about 1% in the presence of yeast mannan (Fig. 5). Similar to FUGW/AURAG, when FUGW/SING was incubated with MoDCs in the presence of anti-DC-SIGN(R) antibody, the transduction dropped to 4%; with mannan, transduction dropped to 2% (Fig. 5). These results suggest that AURA-G and SIN-G transduction of MoDCs is mediated at least in part by interactions with DC-SIGN(R) but could also require additional cellular lectins.
Discussion
In this study, we demonstrated that glycoproteins derived from the Aura virus can pseudotype HIV-1–derived LVs. Aura virus is a New World alphavirus that shares stereological cross-reactivity with the SIN lineage (Strauss and Strauss, 1994). Since AURA-G has a 56% amino acid sequence identity in the E2-binding region compared with SIN-G, we chose to compare these pseudotyped LVs. Staining of SIN- and AURA-G–pseudotyped LVs using cross-reactive SIN immune ascitic fluid revealed a similar level of LV particles that incorporated the envelope glycoprotein. This is the first time that AURA-G has been shown to be able to incorporate into HIV-1–derived LVs. We have demonstrated that the incorporation of AURA-G into pseudotyped lentiviral particles can render them capable of infecting human cell types. Although the titer of vectors pseudotyped with AURA-G was consistently lower than that obtained with the VSV-G, AURA-G's restricted transduction was sufficient to warrant further studies to elicit specific virus–receptor interactions that may enhance transduction of specific cells types. One potential class of receptors that has been shown to act as a principal attachment factor for a broad range of enveloped viruses is C-type lectins (Lozach et al., 2007). The C-type lectins DC-SIGN and L-SIGN are important targets because they are promiscuous receptors capable of capturing viruses on antigen-presenting cells located throughout the body (Bashirova et al., 2001; Braet and Wisse 2002). Both lectins are tetrameric type II transmembrane proteins composed of a carbohydrate recognition domain, which binds in a calcium-dependent manner, and a short cytoplasmic tail responsible for signaling and internalization that can activate antigen-presenting cells leading to an immune response to the viral vectors or their transgene products (Mitchell et al., 2001; Figdor et al., 2002; Soilleux, 2003; Lozach et al., 2007). The mosquito-produced SIN (Klimstra et al., 2003) and LVs with modified Sindbis glycoproteins (Yang et al., 2008) were shown to use DC-SIGN(R) as attachment receptors leading to productive transduction of DCs. Therefore, we reasoned that C-type lectins DC-SIGN and L-SIGN may be important attachment factors for SIN-G– and AURA-G–bearing LVs as well.
In light of previous findings, we reasoned that the use of C-type lectins for DC entry might be a general property of AURA-G and that the virus–receptor interactions might be achieved with pseudotyped LVs derived from 293T cells. Cells stably expressing C-type lectins DC-SIGN and L-SIGN were used to quantify the interactions between the viral envelope and the receptor. When the conditions were optimized, FUGW/AURAG achieved titers similar to those of the SIN-G pseudotypes on DC-SIGN–expressing cells but much lower on parental cells without DC-SIGN expression. The obtained 36% transduction of 293T.DCSIGN by FUGW/AURAG corresponds closely to a predicted infectivity based on a Poisson distribution [1 − e(−MOI); MOI = 0.6] (Strang et al., 2005). Radiolabeled virus cell binding revealed a significant increase in bound viral particles to cells expressing DC-SIGN and L-SIGN for AURA-G–bearing LV particles. The interactions of AURA-G with DC-SIGN were determined to mediate transduction as revealed by competitive inhibition assays and to transduce cells through a pH-dependent pathway similar to that of SIN-G-pseudotyped LVs. Like other alphaviral pseudotypes, FUGW/AURAG was stable during ultracentrifugation, and when viral supernatant was concentrated 300-fold by using ultracentrifugation, a titer of approximately 2.0 × 106 TU/ml was obtained. Concentrated FUGW/AURAG vectors were used to transduce MoDCs, and the transduction was determined to be DC-SIGN mediated, as demonstrated by the competitive inhibition using both DC-SIGN(R) antibodies and the yeast mannan. Our findings indicate that the AURA-G might be an attractive alternative to the commonly used VSV-G for transduction of DCs for certain gene transfer applications.
The VSV-G–pseudotyped LVs exhibited similar binding and transduction of cells expressing DC-SIGN, L-SIGN, or parental 3T3 and 293T cells. Although we have shown that DC-SIGN and L-SIGN are not major mediators for the infectivity of FUGW/VSVG vectors, it remains possible that these glycoproteins may utilize other ubiquitous attachment factors to infect the same cell types. The broad tropism of FUGW/VSVG has proven useful for many investigators developing LVs; however, VSV-G LVs transduce multiple cell types when administered in vivo and could result in “off-target” gene transfer. In contrast to the broad high titer of VSV-G–pseudotyped LV, the FUGW/AURAG vector has a much lower infectivity towards cell types lacking DC-SIGN(R). The low infectivity observed in 293T and 3T3 cell types clearly reflects a restricted tropism of the AURA-G–pseudotyped LVs and may be useful to alleviate certain concerns of the “off-target” effect for gene delivery.
In this study we identified DC-SIGN and L-SIGN interactions with AURA-G and SIN-G to mediate the transduction of DCs. Genetically modified DCs have been used to elicit antigen-specific, major histocompatibility complex–restricted cytotoxic T-lymphocyte responses (Song et al., 1997; Yang et al., 2008). Transduction of human PBMC-derived MoDCs by the same MOI of FUGW/AURAG was slightly more efficient than that by FUGW/SING. There are likely cellular factors other than DC-SIGN expression that affect susceptibility to infection. Previous studies with an engineered SIN-G–pseudotyped LV have demonstrated the dynamics of virus fusion with endosomes and endosome-associated transport of viruses in target cells are important post-entry events that can affect transduction (Joo and Wang, 2008). Future studies are warranted to investigate the cellular factors in MoDCs that affect susceptibility to infection with the pseudotyped LVs and the underlying mechanisms of viral transduction at a molecular level.
The transduction of human PBMC-derived MoDCs by both AURA-G– and SIN-G–pseudotyped LVs was DC-SIGN(R) specific and could be inhibited by both yeast mannan and anti-DC-SIGN(R) antibodies. Although these results suggest that DC-SIGN(R) mediates transduction, it remains possible that additional cellular lectins could play a role in the transduction of DCs. Further experiments are required to investigate other possible attachment factors. One group recently observed that LVs pseudotyped with the same wild-type SIN envelope proteins and produced by mammalian cells could not use DC-SIGN as a receptor (Morizono et al., 2010). The SIN-G strain used in that study was identical to that used in our study, but our study clearly shows that SIN-G–bearing LVs are able to efficiently bind DC-SIGN and employ it to transduce target cells. One possibility is that transduction in their experimental setting may not have been optimized to observe differences in DC-SIGN–mediated transduction. Previous studies by this group have demonstrated that the wild-type SIN envelope (5.4 × 10−4 ng HIVp24/cell) forms infectious pseudotypes with HIV-1 on 293T cells (Morizono et al., 2001). However, when attempting to quantify the effects of DC-SIGN–mediated transduction with wild-type unmodified SIN-G pseudotypes, this group later observed negligible infection towards both the 293T cell line and DC-SIGN–expressing cells (Morizono et al., 2010). One possible reason why they observed no effect of DC-SIGN–mediated transduction is that the amount of virus used (4 × 10−5 ng HIVp24/cell) was outside the observable infectious range. In our studies we used a higher amount of SIN-G–pseudotyped LV (3.5 × 10−3 ng HIVp24/cell) and observed a significant level of infection of 293T cells and an increase in transduction with cells expressing DC-SIGN(R). We found that AURA-G and SIN-G pseudotypes have an innate tropism towards DC-SIGN(R)–expressing cells. The specific infectivity of Aura virons is naturally low (Rumenapf et al., 1995), but we found that AURA-G–pseudotyped LVs have an innate ability to transduce DC-SIGN(R)–expressing cells, which can be utilized for directing the cellular transduction of LVs towards antigen-presenting cells.
In conclusion, Aura virus glycoproteins can pseudotype HIV-1–derived LVs and can restrict the tropism towards DC-SIGN–expressing cells. LVs bearing either SIN-G or AURA-G are able to interact with DC-SIGN(R) to mediate preferential transduction of these cell types. Although these LVs preferentially transduce DCs through DC-SIGN(R), it remains possible that other DC-SIGN(R)–expressing cells such as macrophages and liver and lymph node endothelial cells could be targeted for transduction. Targeting of LVs to C-type lectin–expressing cells such as DCs can be increased by choice of suitable envelope glycoprotein with a low unspecific infectivity and preferential transduction towards DC-SIGN(R). Further measures are ongoing to improve AURA-G pseudotyping utilizing strategies to mutate the receptor-binding sites and mutations in the fusion loop region of the glycoprotein to enhance the transduction efficiency. Our findings indicate that AURA-G–pseudotyped LVs may serve as promising tools for selected gene transfer to DCs.
Acknowledgments
This work was supported by a grant from the U.S. National Institutes of Health. We thank Dr. Kye-Il Joo for assistance with confocal imaging.
Author Disclosure Statement
No competing financial interests exist.
References
- Aires da Silva F. Costa M.J. Corte-Real S. Goncalves J. Cell type-specific targeting with sindbis pseudotyped lentiviral vectors displaying anti-CCR5 single-chain antibodies. Hum. Gene Ther. 2005;16:223–234. doi: 10.1089/hum.2005.16.223. [DOI] [PubMed] [Google Scholar]
- Akahata W. Yang Z.Y. Andersen H., et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirova A.A. Geijtenbeek T.B. Van Duijnhoven G.C., et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K.A. Klimstra W.B. Johnston R.E. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology. 2000;276:93–103. doi: 10.1006/viro.2000.0546. [DOI] [PubMed] [Google Scholar]
- Braet F. Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Hepatol. Comp. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil D.A. Miller A.D. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J. Zhang X.Y. Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.W. Nguyen H.Y. Hanna S.L., et al. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePolo N.J. Reed J.D. Sheridan P.L., et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Dubuisson J. Rice C.M. Sindbis virus attachment: isolation and characterization of mutants with impaired binding to vertebrate cells. J. Virol. 1993;67:3363–3374. doi: 10.1128/jvi.67.6.3363-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figdor C.G. Van Kooyk Y. Adema G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Froelich S. Ziegler L. Stroup K. Wang P. Targeted gene delivery to CD117-expressing cells in vivo with lentiviral vectors co-displaying stem cell factor and a fusogenic molecule. Biotechnol. Bioeng. 2009;104:206–215. doi: 10.1002/bit.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J.P. Frolov I. Perri S., et al. Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 2000;74:11849–11857. doi: 10.1128/jvi.74.24.11849-11857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Frischauf A.M. Simons K., et al. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature. 1980;288:236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Heil M.L. Albee A. Strauss J.H. Kuhn R.J. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 2001;75:6303–6309. doi: 10.1128/JVI.75.14.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo K.I. Wang P. Visualization of targeted transduction by engineered lentiviral vectors. Gene Ther. 2008;15:1384–1396. doi: 10.1038/gt.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl C.A. Marsh J. Fyffe J., et al. Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J. Virol. 2004;78:1421–1430. doi: 10.1128/JVI.78.3.1421-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 1995;45:113–151. doi: 10.1016/s0065-3527(08)60059-7. [DOI] [PubMed] [Google Scholar]
- Klages N. Zufferey R. Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol. Ther. 2000;2:170–176. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- Klimstra W.B. Ryman K.D. Johnston R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra W.B. Nangle E.M. Smith M.S., et al. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokoltsov A.A. Weaver S.C. Davey R.A. Efficient functional pseudotyping of oncoretroviral and lentiviral vectors by Venezuelan equine encephalitis virus envelope proteins. J. Virol. 2005;79:756–763. doi: 10.1128/JVI.79.2.756-763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootstra N.A. Verma I.M. Gene therapy with viral vectors. Annu. Rev. Pharmacol. Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- Lei Y. Joo K.I. Wang P. Engineering fusogenic molecules to achieve targeted transduction of enveloped lentiviral vectors. J. Biol. Eng. 2009;3:8. doi: 10.1186/1754-1611-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C. Hong E.J. Pease S., et al. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lozach P.Y. Burleigh L. Staropoli I. Amara A. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol. Biol. 2007;379:51–68. doi: 10.1007/978-1-59745-393-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G.H. Johnston R.E. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A. Fadden A.J. Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGN.R. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- Morizono K. Bristol G. Xie Y.M., et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Ku A. Xie Y., et al. Redirecting lentiviral vectors pseudotyped with Sindbis virus-derived envelope proteins to DC-SIGN by modification of N-linked glycans of envelope proteins. J. Virol. 2010;84:6923–6934. doi: 10.1128/JVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. Blomer U. Gallay P., et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Obermaier B. Dauer M. Herten J., et al. Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol. Proced. Online. 2003;5:197–203. doi: 10.1251/bpo62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W.S. Nolan G.P. Scott M.L. Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluri A. Ainsworth R. Weaver S.C. Sutton R.E. Functional pseudotyping of human immunodeficiency virus type 1 vectors by Western equine encephalitis virus envelope glycoprotein. J. Virol. 2008;82:12580–12584. doi: 10.1128/JVI.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumenapf T. Strauss E.G. Strauss J.H. Aura virus is a New World representative of Sindbis-like viruses. Virology. 1995;208:621–633. doi: 10.1006/viro.1995.1193. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J. DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe? Clin. Sci. (Lond.) 2003;104:437–446. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Song W. Kong H.L. Carpenter H., et al. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang B.L. Takeuchi Y. Relander T., et al. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J. Virol. 2005;79:1765–1771. doi: 10.1128/JVI.79.3.1765-1771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H. Strauss E.G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C. Park C.G. Finke J., et al. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 2003;15:289–298. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- Wu L. Martin T.D. Vazeux R., et al. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Martin T.D. Carrington M. Kewalramani V.N. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology. 2004;318:17–23. doi: 10.1016/j.virol.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Yamada S. Ohnishi S. Vesicular stomatitis virus binds and fuses with phospholipid domain in target cell membranes. Biochemistry. 1986;25:3703–3708. doi: 10.1021/bi00360a034. [DOI] [PubMed] [Google Scholar]
- Yang H. Joo K.I. Ziegler L. Wang P. Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharm. Res. 2009;26:1432–1445. doi: 10.1007/s11095-009-9853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Bailey L. Baltimore D. Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Yang H. Rideout K., et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W. Wang L. Yang Y., et al. Interaction of E2 glycoprotein with heparan sulfate is crucial for cellular infection of Sindbis virus. PLoS One. 2010;5:e9656. doi: 10.1371/journal.pone.0009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler L. Yang L. Joo K., et al. Targeting lentiviral vectors to antigen-specific immunoglobulins. Hum. Gene Ther. 2008;19:861–872. doi: 10.1089/hum.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]