Abstract
Clinically relevant mature cartilage cells (chondrocytes) present challenges for use in cartilage tissue engineering applications, given their low capacity for cell division and tissue production. Since the in situ environment of chondrocytes is hypertonic relative to standard culture medium conditions, in this study we tested the hypothesis that using culture medium of a hypertonic, more physiologic osmolarity during both two-dimensional (2D) expansion of mature bovine chondrocytes (MBCs) and their subsequent encapsulation culture in three-dimensional (3D) agarose hydrogel constructs produces improved engineered tissue construct mechanical and biochemical properties. Results demonstrate that 2D expansion of MBCs in hypertonic (NaCl) medium before encapsulation yielded improved construct mechanical properties. However, 3D encapsulation culture of cells in hypertonic (NaCl) medium yielded poorer construct mechanical properties. Osmolarity-related differences in construct biochemical content and organization may have contributed to differences in mechanical properties, as construct glycosaminoglycan content correlated moderately with construct mechanical properties, and construct collagen distribution varied between 3D osmotic culture groups. Results of this study suggest that application of hypertonic (NaCl) medium during 2D mature chondrocyte expansion, but not 3D encapsulated chondrocyte culture, may serve as a convenient and inexpensive method for improving mechanical properties of expanded cell-seeded constructs.
Introduction
Engineering of cartilage tissue constructs that can successfully replace articular cartilage damaged by the progression of osteoarthritis is expected to have a significant impact on clinical treatment modalities. Our group's tissue engineering studies are guided by the premise that elaboration of construct mechanical properties and biochemical composition to near-native levels before implantation will increase the chances of construct survival in the relatively harsh biomechanical and biochemical environment of diarthrodial joints. We have previously demonstrated promising outcomes using juvenile bovine cartilage cells (chondrocytes) in agarose hydrogel to generate constructs with physiologic or supraphysiologic juvenile cartilage tissue properties.1–7 However, the lack of clinically available juvenile human chondrocytes has led our group and others to investigate cartilage tissue engineering with human and other animal chondrocytes more similar in maturity to those available for human autologous and allogenic cartilage repair strategies.8–13
These mature chondrocytes present challenges for production of robust tissue-engineered constructs. Compared to juvenile cells, they exhibit lower capacity for expansion to sufficient cell number,14–16 and they produce inadequate levels of tissue extracellular matrix (ECM) after necessary cell expansion.17 However, given mature cells' clinical relevance, researchers have explored various strategies to overcome the limitations that they pose. These include application of biomimetic chemical and physical factors during mature chondrocyte two-dimensional (2D) expansion and subsequent three-dimensional (3D) tissue culture.8–11 In one such study, our group found that application of a growth factor cocktail (GFC) during mature canine chondrocyte expansion was critical for increasing cell number to adequate levels before encapsulation in 3D hydrogel culture and for priming these cells for robust ECM production upon encapsulation.8
The success of these biomimetic approaches in promoting tissue growth by mature cells motivated us to investigate the application of another component of the in situ chondrocyte environment, the extracellular osmolarity, as a means to enhance mature chondrocyte ECM elaboration in long-term culture. This effort builds upon our group's earlier work in which we characterized the osmotic environment of cartilage tissue and used applied osmotic loading as a tool for study of chondrocyte mechanobiology and for measurement of cell and tissue properties.18–27 It also builds upon additional studies that have explored the effect of osmotic environment on chondrocyte metabolic activity, primarily by measuring precursor factors related to ECM production.28–31
The current study tests the hypothesis that application of a hypertonic, more physiologic osmotic environment (created by the addition of NaCl) during 2D expansion of mature bovine chondrocytes (MBCs) and their subsequent 3D culture in agarose hydrogel constructs improves engineered tissue construct biochemical and mechanical properties. Based on previous successes in using growth factor media supplementation to enhance the properties of tissues created from mature chondrocyte sources, in this study we supplemented all 2D cell expansion and 3D cell culture media with growth factor formulations similar to those used previously.2,8–10 Therefore, any observed effects of osmotic environment on cell and tissue properties may be the result of combined osmotic and growth factor effects.
Materials and Methods
Media preparation
All media used in this study were prepared from high-glucose (4.5 g/L) Dulbecco's modified Eagle medium (DMEM) containing 4 mM L-glutamine (Invitrogen). Tissue harvest medium was prepared from DMEM by supplementation with 5% fetal bovine serum (FBS; Atlanta Biologics), amino acids (1×minimal essential amino acids and 1×nonessential amino acids; Mediatech), buffering agents (10 mM HEPES, 10 mM sodium bicarbonate, 10 mM TES, and 10 mM BES; Mediatech), and antibiotic–antimycotic mix (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B; Invitrogen). The osmolarity of this medium was 371 mOsm, as confirmed by freezing point osmometer.
The isotonic medium osmolarity (300 mOsM) applied during the experiment was chosen to mimic the osmolarity of commonly used culture medium and the hypertonic medium osmolarity (400 mOsM) was chosen to mimic the osmotic environment of native cartilage tissue.27,30 NaCl was chosen to increase medium osmolarity because Na+ is the most prevalent ion species in the native cartilage tissue.30 To prepare isotonic passaging medium (PM), DMEM was diluted with deionized, distilled water to an appropriate osmolarity, and then supplemented with 5% FBS and antibiotic–antimycotic mix to a final osmolarity of 300 mOsM, confirmed by freezing point osmometer. Hypertonic PM was prepared to 400 mOsM by the addition of NaCl (biotechnology grade; Sigma) to isotonic PM. To prepare isotonic chemically defined medium (CDM) for use in 3D culture, DMEM was diluted with deionized, distilled water to an appropriate osmolarity, and then supplemented with 100 nM dexamethasone (Sigma), 100 μg/mL sodium pyruvate (Sigma), 50 μg/mL L-proline (Sigma), 1% ITS+ premix (Becton Dickinson), and antibiotic–antimycotic mix to a final osmolarity of 300 mOsM. Hypertonic CDM was prepared to 400 mOsM by the addition of NaCl (biotechnology grade; Sigma) to isotonic CDM.
Tissue harvesting and cell isolation
Full-depth cartilage tissue samples were harvested from the carpometacarpal joints of freshly slaughtered, developmentally mature (2–4-year-old) cows (n=2 experimental runs, n=1 joint per run). MBCs were then isolated from the tissue by enzymatic digestion in tissue harvest medium containing 195 U/mL collagenase IV (Worthington). Digestion was performed for 11 hours on an orbital shaker placed in an incubator that was maintained at 37°C and 5% CO2. The digested tissue and digestion medium were then passed through a 70 μm filter to yield a cell suspension free of undigested tissue and cell debris.
Cell passaging
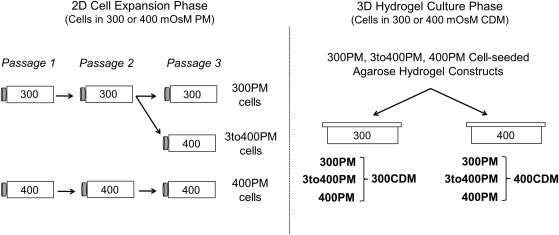
Isolated MBCs were expanded under one of three osmotic passaging conditions (Fig. 1). A 300PM cells were passaged three times in isotonic PM, and 400PM cells were passaged three times in hypertonic PM. 3to400PM cells were passaged twice in isotonic PM, and then passaged for a final passage in hypertonic PM. This condition was applied to compare the effects of one versus three passages in hypertonic medium on cell biosynthetic activity.
FIG. 1.
Schematic of experimental groups resulting from osmotic conditions applied during 2D cell passaging and 3D construct phases of the experiment. 2D, two-dimensional; 3D, three-dimensional.
At each passage, cells were plated in PM of appropriate osmolarity in tissue culture-treated flasks at ∼27,000 cells/cm2. Media for all PM conditions were supplemented with a GFC consisting of 5 ng/mL bFGF (Invitrogen), 10 ng/mL PDGF (Invitrogen), and 1 ng/mL transforming growth factor beta 3 (TGFβ3; R&D Biosystems).9 Media were changed three times a week, with fresh GFC added at each change. Cell flasks were visually evaluated for confluency. Once cells in any PM condition reached 90% confluency (∼5–6 days of culture, depending on passage number), cells in all PM conditions were released from the plating surface by treatment with a 100 U/mL collagenase V (Sigma) and 0.05% trypsin/0.53 mM EDTA (Invitrogen) solution. While 400PM and 3to400PM cells were slightly less confluent than 300PM cells at the conclusion of each passage, cells in all PM conditions were released at the same time to ensure identical processing during trypsinization (and agarose encapsulation described below). After trypsinization, released cells were plated at the same density in fresh PM of the appropriate osmolarity plus GFC, initiating a new passage.
Encapsulation of passaged cells in agarose hydrogel
Once 300PM cells had become 90% confluent at passage three, cells from all PM conditions were released from the plating surface as described previously. Cells were then resuspended at 60 million cells/mL in CDM of the same osmolarity as that used during the final passage. For each PM condition, the cell solution was then mixed at a 1:1 ratio with melted 4% Type VII agarose in phosphate-buffered saline (PBS) for a final solution concentration of 30 million cells/mL in 2% Type VII agarose. The cell-seeded agarose hydrogel was cast in a glass and rubber apparatus (thickness=2.34 mm), allowed to cure at 25°C, and then transferred to a tissue culture dish containing CDM of the same osmolarity applied during the final cell passage, supplemented with 50 μg/mL ascorbic acid.
Osmotic culture of cell-seeded agarose hydrogels
Cylindrical constructs (3.00 mm diameter×2.34 mm thick) were punched from the cell-seeded agarose hydrogels prepared from each of the passaged cell populations (300PM, 400PM, and 3to400PM). These constructs were then split into two 3D osmotic culture conditions. In the 300CDM condition, disks were maintained in isotonic CDM, whereas in the 400CDM condition, disks were maintained in hypertonic CDM. This division created the six distinct PM-CDM construct groups shown in Figure 1. Media for all groups were supplemented with 50 μg/mL ascorbic acid and 10 ng/mL of the growth factor TGFβ3.8,9 Constructs were maintained at 37°C and 5% CO2 for four weeks. Media were changed three times weekly, with fresh ascorbic acid and TGFβ3 added at each change.
Mechanical testing of agarose constructs and sample harvest
On days 0, 14, and 28 of culture, hydrogel constructs (n=2 experimental runs, n=8–10 constructs per PM-CDM group per run for each time point) were tested in unconfined compression between two impermeable platens in a custom material testing device as previously described.32 Before testing, constructs from all PM-CDM groups were equilibrated to a testing bath of PBS (300 mOsM), the volume of which was significantly greater than construct volume. Constructs were then maintained in PBS for the duration of testing. The compressive Young's modulus was determined from the equilibrium response of the stress relaxation test by dividing the equilibrium stress (minus the tare stress) by the applied static strain. After mechanical testing, construct wet weight was recorded and constructs were stored at −20°C for biochemical analysis. To examine potential secretion of ECM components into the construct culture media, at day 28 of culture, 1 mL of medium was saved from each PM-CDM group dish for analysis of glycosaminoglycan (GAG) and collagen content. Additional constructs were harvested at day 28 for histological analysis.
Biochemical and statistical analysis
Net cell doubling number in 2D culture for each passaged cell group at each passage was calculated by taking the log base two of (cell number at the end of the passage divided by cell number at the beginning of the passage) for two to four independent flasks per passage. Two-way analysis of variance (ANOVA) was performed on the 300PM and 400PM cell doubling number data for factors of passage number (three levels) and PM condition (two levels). One-way ANOVA was performed on the passage three 300PM, 400PM, and 3to400PM cell doubling number data for factor of PM condition (3 levels). If ANOVA returned significance for the main effect of the factors, a post-hoc Tukey's Honest Significant Difference test for unequal n was performed to assess significance (p<5E-02) between each passaged cell group at each time point. Significant differences between passaged cell group values are marked on the appropriate figure 5.
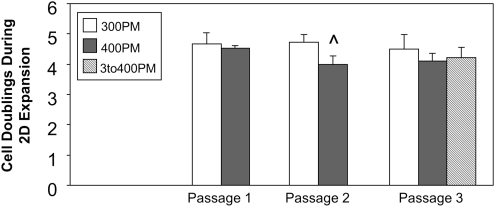
FIG. 5.
Net cell doublings during each passage of 2D cell expansion (mean±STD; n=2–4 samples/group; ^p<5E-02 with 300PM group at same passage).
Tissue constructs from days 0, 14, and 28 of culture were enzymatically digested and probed for DNA, GAG, and total (type nonspecific) collagen content according to previously established methods.33 Culture medium samples were tested directly for GAG and total collagen content using the same methods. Construct DNA content was calculated as DNA/construct (μg). 3D net cell doubling number in individual samples from each PM-CDM group from days 0 to 14 and days 14 to 28 of culture was calculated by converting DNA/construct to cell number/construct by the assumption of 7.7 pg DNA/cell and then taking the log base two of (sample cell number at the later time point divided by group average sample cell number at the earlier time point).
Construct GAG and collagen mass were normalized to construct wet weight (GAG/ww and collagen/ww) or construct DNA mass (GAG/DNA and collagen/DNA). Spearman's correlation analysis was performed between Young's modulus and GAG/ww values, and between Young's modulus and collagen/ww values, for the entire PM-CDM data set at day 14 and 28 to assess significance of correlations (p<5E-02) between these factors at these time points. Medium collagen values were negligible for all PM-CDM groups and were not further analyzed. For each PM-CDM group, the total dish medium GAG mass at day 28 was normalized to number of constructs in the dish (medium GAG/construct; μg).
Three-way ANOVA was performed on the day 14 and 28 DNA/construct, 3D cell doubling number, GAG/ww, GAG/DNA, collagen/ww, collagen/DNA, and Young's modulus data sets for factors of culture time (two levels), PM condition (three levels), and CDM condition (two levels) to determine significance (p<5E-02) of the main effect of each factor. Two-way ANOVA was performed on the day 28 medium GAG/construct data set for factors of PM condition (three levels) and CDM condition (two levels). If ANOVA returned significance for the main effect of the factors, a post-hoc Tukey's Honest Significant Difference test for unequal n was performed to assess significance (p<5E-02) between each PM-CDM group at each time point. Significant differences between PM-CDM groups are marked on the appropriate figures. Student's t-test was also performed on DNA/construct, GAG/ww, GAG/DNA, and Young's modulus data sets to determine significant differences (p<5E-02) between day 0 PM-CDM group values and their respective later time point values (collagen levels did not resolve at day 0). For ease of visual comparison of later time point data, only the all-group average day 0 value is displayed in relevant figures (represented by a dotted line). However, statistical significance markings between day 0 and later time points indicate differences between distinct PM-CDM group day 0 values and their respective later time point values.
Histological analysis
Histological samples were dehydrated, paraffin-embedded, sectioned (7 μm), and mounted onto microscope slides. Before labeling, samples were cleared and rehydrated. ECM component collagen was stained with Picrosirius Red (Sigma) for observation of total (type nonspecific) collagen distribution patterns in the construct.
Results
In this study, the effect of cell expansion in hypertonic, medium on cell-seeded 3D construct properties was similar for constructs seeded with cells passaged in this medium for three passages (400PM) or for one passage (3to400PM). Therefore, in the following Results and Discussion sections, references to hypertonic passaging conditions refer to both 400PM and 3to400PM in concert.
3D hydrogel construct mechanical properties
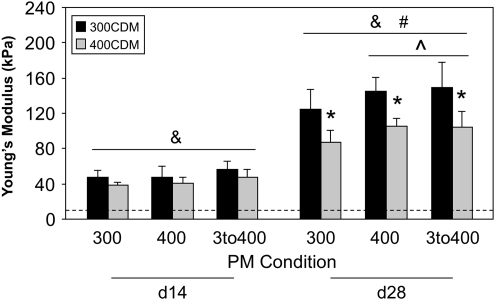
Construct Young's moduli generally increased throughout the culture period (Fig. 2), with day 14 and 28 moduli significantly greater than day 0 moduli, and day 28 moduli significantly greater than day 14 moduli, for all PM-CDM groups (p<1E-04). Passaging of cells in hypertonic medium rather than isotonic medium yielded higher construct moduli. At day 28 of culture, 400PM and 3to400PM moduli were significantly higher (∼20%) than 300PM moduli for both CDM conditions (p<5E-02). 3D culture of constructs in hypertonic medium yielded lower construct moduli than did culture in isotonic medium. At day 28 of culture, 400CDM moduli were significantly lower (∼30%) than 300CDM moduli for all PM conditions (p<1E-04).
FIG. 2.
Young's modulus (kPa) at days 14 and 28 of culture (mean±standard deviation (STD); n=16–18 constructs/group; &p<5E-02 with respective day 0 PM group; #p<5E-02 with respective day 14 PM-CDM group; *p<5E-02 with 300CDM, same PM condition, group at same culture time point; ^p<5E-02 with 300PM, same CDM condition, group at same culture time point). Dotted line indicates for reference the average day 0 value. PM, passaging medium; CDM, chemically defined medium.
3D hydrogel ECM content
Hydrogel ECM content generally increased throughout the culture period. GAG/ww and GAG/DNA were significantly higher (p<1E-09) at day 14 and 28 than at day 0 for all PM-CDM groups (Fig. 3A, B), and collagen/ww and collagen/DNA values were resolvable for all PM-CDM groups at days 14 and 28, but not at day 0 (Fig. 3C, D). Further, in most PM-CDM groups, GAG/ww, GAG/DNA, collagen/ww, and collagen/DNA were significantly greater (p<5E-02) at day 28 than at day 14 (Fig. 3A, B, C, and D, respectively).
FIG. 3.
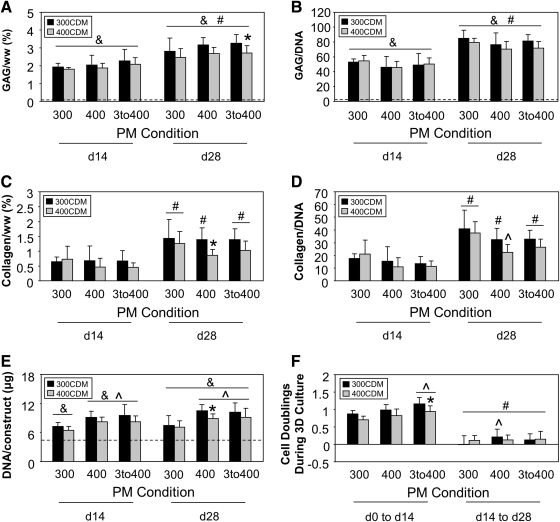
(A) GAG/ww (%), (B) GAG/DNA, (C) Collagen/ww (%), (D) Collagen/DNA, and (E) DNA/construct (μg) of constructs at days 14 and 28 of 3D culture and (F) Net cell doublings in hydrogel constructs from days 0 to 14 and days 14 to 28 of 3D culture (mean±STD; n=18–20 constructs/group for GAG, DNA, and cell doubling number measures; n=11–14 constructs/group for collagen measures; &p<5E-02 with respective day 0 PM group; #p<5E-02 with respective day 14 PM-CDM group (graphs A–D) or respective days 0 to 14 PM-CDM group (graph F); *p<5E-02 with 300CDM, same PM condition, group at same culture time point; ^p<5E-02 with 300PM, same CDM condition, group at same culture time point). Dotted lines on graphs A, B, and E indicate for reference the average day 0 value. Collagen values for day 0 are not presented, as they were below the resolution threshold of our assaying technique. GAG, glycosaminoglycan.
Passaging of cells in hypertonic medium rather than isotonic medium before encapsulation yielded slightly higher construct GAG/ww (Fig. 3A). At day 28, 400PM and 3to400PM GAG/ww was 10%–15% higher than 300PM GAG/ww. While ANOVA returned significance for PM condition (p=5.65E-05), post-hoc analysis did not return significance between PM-CDM group values compared across PM condition. 3D culture of constructs in hypertonic medium yielded lower construct GAG/ww than did culture in isotonic medium (Fig. 3A). At day 28, 400CDM GAG/ww was ∼15% lower than 300CDM GAG/ww (significant for 3to400PM-400CDM vs. 3to400PM-300CDM, p=1.36E-02).
Normalization of construct GAG mass to DNA mass rather than wet weight yielded opposite trends across PM condition, with GAG/DNA lower for 400PM and 3to400PM than for 300PM (Fig. 3B). This normalization also reduced the disparity between 400CDM and 300CDM GAG values (Fig. 3B). While ANOVA of GAG/DNA data returned significance for PM and CDM conditions (p<5E-02), post-hoc analysis did not return any significant differences between PM-CDM group GAG/DNA at day 14 or day 28. In contrast to the trends observed in the construct GAG/ww data, 400CDM medium GAG/construct values were ∼40%–50% greater than 300CDM values (no significant differences between PM-CDM groups, data not shown).
PM condition did not have a significant effect (p=7.52E-02) on construct collagen/ww (Fig. 3C). However, as in the GAG/ww data, 3D culture of constructs in hypertonic medium yielded lower collagen/ww than did culture in isotonic medium. At day 28, 400CDM collagen/ww was ∼13%–38% lower than 300CDM collagen/ww, depending on PM condition (significant for 400PM-400CDM vs. 400PM-300CDM, p=3.44E-02). Normalization of construct collagen mass to DNA mass revealed that passaging of cells in hypertonic medium rather than isotonic medium before encapsulation decreased per-cell construct collagen content (Fig. 3D). Collagen/DNA was generally lower for 400PM and 3to400PM than for 300PM (significant for 400PM-400CDM vs. 300PM-400CDM, p=1.13E-03). Normalization of construct collagen mass to DNA mass lessened the disparity between 300CDM and 400CDM collagen values but did not alter the trend observed in collagen/ww data (Fig. 3D). While ANOVA returned significance for CDM condition (p=8.95E-03), post-hoc analysis returned no significant differences between PM-CDM groups compared across CDM condition. Medium collagen levels were negligible for all PM-CDM groups (data not shown).
Spearman's correlation analysis returned a moderate and significant correlation between Young's moduli and GAG/ww at both time points, with R2=0.41 at day 14 and R2=0.48 at day 28 (p<1E-09). This analysis returned a weak but significant correlation between Young's moduli and collagen/ww at both time points, with R2=0.08 at day 14 and R2=0.28 at day 28 (p<5E-02).
3D hydrogel histological analysis
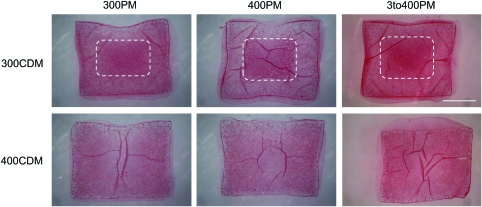
PM osmolarity did not appear to have an effect on construct collagen distribution, whereas CDM osmolarity did. Staining of day 28 hydrogel samples with picrosirius red dye for total collagen distribution indicated that for all PM conditions, 400CDM constructs demonstrated less concentrated collagen staining in their center region than did 300CDM constructs (Fig. 4).
FIG. 4.
Histological staining of extracellular matrix component collagen (type nonspecific) using picrosirius red dye on mature bovine chondrocyte-seeded agarose hydrogels at day 28 of culture. Labels on columns and rows indicate PM and CDM conditions. Region of darker staining in the center of the 300CDM constructs is denoted by the dashed white lines. Scale bar=1 mm. Color images available online at www.liebertonline.com/tec
Cell doubling and construct DNA content
Passaging of cells in hypertonic, rather than isotonic, medium decreased 2D net cell doublings, with a trend of lower cell doubling number in 400PM cell populations than in 300PM populations at each passage, and in 3to400PM populations at passage three (Fig. 5). Post-hoc analysis returned significance between 400PM and 300PM cell doubling number at passage two (p=4.97E-02). ANOVA performed on the 300PM, 400PM, and 3to400PM data at passage three returned no significance. In 3D culture, there was an overall increase in construct DNA content throughout the culture period, with both PM and CDM condition influencing content values. Day 14 and 28 construct DNA content (Fig. 3E) was significantly higher than respective day 0 PM content for all PM-CDM groups (p<1E-06). While ANOVA of DNA content returned significance for culture time (p=1.20E-04), there was no significant difference between day 14 and 28 content for any PM-CDM group. Passaging of cells in hypertonic rather than isotonic medium significantly increased construct DNA content (Fig. 3E), with 400PM and 3to400PM content significantly higher than 300PM content for both CDM conditions at both time points (p<5E-02). 3D culture of constructs in hypertonic rather than isotonic medium reduced construct DNA content (Fig. 3E), indicated by lower content for 400CDM than for 300CDM (significant for 400PM-300CDM vs. 400PM-400CDM at day 28, p=3.47E-02).
The effect of PM and CDM condition on construct DNA content was reflected in 3D cell doubling number (Fig. 3F). Net doubling of cells was greater during the first 2 weeks of 3D culture, with day 0 to 14 numbers significantly higher than day 14 to 28 numbers for all PM-CDM groups (p<1E-04). Passage of cells in hypertonic medium yielded an increase in net cell doublings in subsequent 3D culture (Fig. 3F), demonstrated by higher 3D cell doubling number for 400PM and 3to400PM than for 300PM. This effect was significant between 3to400PM and 300PM for both CDM conditions at the day 0 to 14 time point (p<1E-02), and between 400PM-300CDM and 300PM-300CDM at the day 14 to 28 time point (p=1.28E-02). 3D culture of constructs in hypertonic medium generally yielded a decrease in net cell doublings (Fig. 3F), with 400CDM cell doubling numbers lower than 300CDM numbers (significant between 3to400PM-400CDM vs. 3to400PM-300CDM at day 0 to 14, p=9.36E-03).
Discussion
From the findings of this study we accept the hypothesis that alteration of osmotic environment by addition of NaCl during 2D growth factor expansion of MBCs and their subsequent 3D culture in agarose hydrogels can modulate the biochemical and mechanical properties of the hydrogels in which cells are seeded. Osmotic alteration yielded its greatest effect on the mechanical properties of constructs. Expansion of MBCs in hypertonic medium before encapsulation (400PM and 3to400PM) yielded cell-seeded constructs with significantly higher moduli than did expansion in isotonic medium (300PM). In contrast, 3D culture of cell-seeded constructs in hypertonic medium (400CDM) yielded constructs with significantly lower moduli than did culture in isotonic medium (300CDM). The higher moduli are closer to those of native cartilage and thus represent better functional properties.
It should be noted that in this study, we solely explored the influence of hypertonic conditions created through the addition of NaCl, an ionic, physiologic osmolyte, on chondrocyte and engineered tissue properties. Review of the literature yields mixed findings on the influence of ionic versus nonionic osmolytes on chondrocyte metabolic activity. Regarding precursor measures related to chondrocyte tissue production, one study indicated a similar effect of ionic and nonionic osmolytes on chondrocyte aggrecan promoter activity in 2D culture,18 whereas another noted a similar, though not identical, effect of such osmolytes on chondrocyte sulfate incorporation in 3D culture.30 More recent work from our laboratory (unpublished) suggests that chondrocyte tissue production may vary not only with ionic strength of applied osmolytes but also with the elemental composition of ionic osmolytes. The applicability of previous results to those of the current study is uncertain, due to differences between cell culture systems used in the studies and the precursor nature of promoter activity and sulfate incorporation measures previously examined. However, there is some indication that ionic and nonionic solutes may differentially affect cell and engineered tissue properties examined in the current study. Osmotic effects described here therefore should be considered specific to application of hypertonic NaCl conditions.
Cartilage mechanical properties arise from ECM component concentration and organization.34,35 The slight influence of 2D and 3D osmotic environments on construct biochemical composition and distribution demonstrated in this study therefore may have contributed to the significant differences observed in osmotic group mechanical properties. PM osmolarity-related trends in the collagen/ww data did not closely follow trends in the Young's modulus data. Passage of cells in hypertonic medium before encapsulation yielded constructs with similar collagen/ww, but significantly higher Young's moduli, than did passage of cells in isotonic medium. The low Spearman's correlation coefficients returned between Young's moduli and collagen/ww values at days 14 and 28 of culture reflect this observation. While the overall correlation between these measures was weak, analysis of collagen data according to CDM osmolarity indicates that lower 400CDM collagen/ww values, coupled with the observed less-distinct collagen organization in 400CDM constructs, may partially explain the lower mechanical properties of constructs 3D cultured in hypertonic medium.36 The mechanism by which 3D culture medium osmolarity influences construct ECM content and organization warrants further investigation, as it is possible that osmotic effects on cell production of type II versus type I collagen, or on the distribution of quantitatively minor, yet structurally significant, ECM proteins not measured in this study (e.g., cartilage oligomeric matrix protein, link protein, and type IX collagen) contributed to the observed differences in mechanical properties.
PM and CDM osmolarity-related trends in the GAG/ww data more closely followed trends in the Young's modulus data, as reflected in the higher Spearman's correlation coefficients returned between these measures. Passage of cells in hypertonic medium yielded constructs with both higher GAG content and Young's moduli than did passage of cells in isotonic medium. Further, 3D culture of cells in hypertonic medium yielded both lower GAG content and Young's moduli than did culture in isotonic medium. A combined analysis of the GAG/ww, GAG/DNA, and medium GAG data according to PM condition suggests that the effect of PM osmolarity on construct GAG content was related to its effect on net cell doubling in subsequent 3D culture. Normalization of construct GAG mass to construct DNA mass eliminated the disparity observed between higher 400PM and 3to400PM and lower 300PM GAG/ww values, and medium GAG levels did not vary between PM conditions. Our finding that cells passaged in hypertonic media yielded slightly lower GAG/DNA in subsequent 3D agarose culture is opposite in trend to findings of another study in which human osteoarthritic chondrocytes expanded in hypertonic (NaCl) media yielded slightly higher GAG/DNA in subsequent 3D pellet culture.37 Trends in neither study were significant. The slight disparity between findings of the previous study and our study may be due to dependence of osmotic effects on age, species, or disease state of the cell source tissue; 3D culture model; or cell expansion plate coating (in the previous study, cells were expanded on fibronectin-coated plates, which may have affected cell behavior via integrin-associated signaling pathways).
Analysis of the GAG/ww, GAG/DNA, and medium GAG data according to CDM condition indicates that the lower net cell doubling yielded by 3D culture in hypertonic medium also likely played a role in the lower GAG content of 400CDM constructs, and that greater secretion of GAG into the hypertonic medium may have contributed to this disparity as well. The trend of greater secretion of GAG into hypertonic medium warrants further investigation and may be related to alterations in the size or matrix retention capability of GAGs produced by cells maintained in this culture condition. Our finding that hypertonic culture of 3D cell constructs decreased construct cell number and decreased per-cell GAG content is in contrast to another study's findings in which culture of juvenile chondrocytes in 3D alginate bead culture in hypertonic media of different osmolarities yielded lower cell content, but higher per-cell GAG production, than culture in isotonic media.29 This disparity in findings demonstrates, as discussed previously, potential dependence of observed osmotic effects on cell source tissue age, 3D culture model, or osmolyte (in this case, NaCl/KCl vs. NaCl alone). Further investigation into the influence of individual culture elements on observed osmotic effects will provide a better understanding of the applicability of our and other results to a wide variety of cartilage tissue engineering models.
Our findings on the effects of 2D expansion and 3D culture medium osmolarity on 3D construct cell number bring novel insight to our understanding of osmotic effects on cell division and/or cell death. It has been previously reported that 2D or 3D culture in hypertonic medium yields lower chondrocyte number than culture in isotonic medium,29,37 findings in agreement with our 2D cell doubling data (according to 2D culture medium osmolarity) and 3D cell doubling data for the first 14 days of culture (according to 3D culture medium osmolarity). However, we found that passage of cells in hypertonic medium yielded lower cell number in 2D culture, but higher cell number upon cell encapsulation in 3D culture (regardless of the osmolarity of 3D culture medium). This finding has not been previously reported and may be related to differences between the growth factor mixes we used in our 2D and 3D culture media. Future studies are necessary to address this topic, and that of the relative effects of osmolarity on cell division and cell death, since the overall 2D cell count and 3D construct DNA content measures performed in this study were not capable of discerning between these effects.
The results of this study provide guidelines for the use of hypertonic (NaCl) growth factor medium to yield improved cell-seeded construct properties in tissue engineering applications. While treatment of cells with hypertonic medium during passaging may yield greater cell number in subsequent 3D culture, leading to greater overall matrix production and better construct properties, the lower number of cells yielded by 2D passaging in hypertonic medium may limit the amount of engineered tissue that can be manufactured. However, given that we obtained the same beneficial results from passaging chondrocytes in hypertonic medium for one or for three passages, tissue engineering applications that begin with limited cell numbers may apply hypertonic medium for only a final passage to optimize both cell availability during the expansion phase and cell-seeded construct properties during the 3D culture phase of the protocol.
The improvement in MBC-seeded hydrogel properties that we observed with application of more-physiologic hypertonic medium during cell expansion may be compared to previously observed effects of other physiologic stimuli on engineered cartilage tissue properties. For example, the improvements observed in this study are more modest than those reported by Ng et al.,8 who compared the effect of growth factor expansion (vs. no expansion) on the properties of agarose hydrogels seeded with mature canine chondrocytes. However, the current results are on par with the beneficial effect of dynamic deformational loading on juvenile bovine chondrocyte-seeded construct mechanical properties observed by Lima et al.2 Further, the highest construct GAG content obtained in this study (from passage of MBCs in hypertonic medium followed by 3D construct culture in isotonic medium) was, after only 28 days of culture, on par with the GAG content of native mature bovine cartilage tissue (∼3.5% GAG/ww).
While each run of the current study was performed using cells from only one mature bovine joint, independent experimental runs returned similar values and osmotic condition-dependent trends for each data measure. These findings indicate low inter-animal variability for the mature bovine tissue/cells used here. The use of serum-free media during 3D culture may have contributed further to reducing variability in these runs.38 We therefore believe that the results obtained in this study are likely reproducible in further studies using individual or pooled animal cell populations.
In summary, the results of this study suggest that application of hypertonic (NaCl) medium during the final passage of mature chondrocyte growth factor expansion, followed by application of isotonic medium during subsequent encapsulated cell culture, is an easy-to-implement, inexpensive culture technique with potentially significant benefits for cartilage tissue engineering approaches that rely on clinically relevant mature chondrocyte sources.
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (AR52871 and AR46568) and by an NSF Graduate Research Fellowship (ESO).
Disclosure Statement
No competing financial interests exist for any of the authors of this article.
References
- 1.Lima E.G. Bian L. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The effect of applied compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Conf Proc IEEE Eng Med Biol Soc. 2006;1:779. doi: 10.1109/IEMBS.2006.259313. [DOI] [PubMed] [Google Scholar]
- 2.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima E.G. Tan A.R. Tai T. Bian L. Ateshian G.A. Cook J.L. Hung C.T. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41:3253. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima E.G. Tan A.R. Tai T. Marra K.G. DeFail A. Ateshian G.A. Hung C.T. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. J Biomed Mater Res A. 2009;91:692. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian L. Crivello K.M. Ng K.W. Xu D. Williams D.Y. Ateshian G.A. Hung C.T. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2009;15:2065. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers B.A. Mauck R.L. Chiang I.E. Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 8.Ng K.W. Lima E.G. Bian L. O'Conor C.J. Jayabalan P.S. Stoker A.M. Kuroki K. Cook C.R. Ateshian G.A. Cook J.L. Hung C.T. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian L. Fong J.V. Lima E.G. Stoker A.M. Ateshian G.A. Cook J.L. Hung C.T. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munirah S. Samsudin O.C. Aminuddin B.S. Ruszymah B.H. Expansion of human articular chondrocytes and formation of tissue-engineered cartilage: a step towards exploring a potential use of matrix-induced cell therapy. Tissue Cell. 2010;42:282. doi: 10.1016/j.tice.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Gavenis K. Schneider U. Groll J. Schmidt-Rohlfing B. BMP-7-loaded PGLA microspheres as a new delivery system for the cultivation of human chondrocytes in a collagen type I gel: the common nude mouse model. Int J Artif Organs. 2010;33:45. [PubMed] [Google Scholar]
- 12.Stenhamre H. Nannmark U. Lindahl A. Gatenholm P. Brittberg M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly(urethane urea) scaffolds. J Tissue Eng Regen Med. 2011;5:578. doi: 10.1002/term.350. [DOI] [PubMed] [Google Scholar]
- 13.Francioli S.E. Candrian C. Martin K. Heberer M. Martin I. Barbero A. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J Biomed Mater Res A. 2010;95:924. doi: 10.1002/jbm.a.32917. [DOI] [PubMed] [Google Scholar]
- 14.Evans C.H. Georgescu H.I. Observations on the senescence of cells derived from articular cartilage. Mech Ageing Dev. 1983;22:179. doi: 10.1016/0047-6374(83)90111-2. [DOI] [PubMed] [Google Scholar]
- 15.Guerne P.A. Blanco F. Kaelin A. Desgeorges A. Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38:960. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- 16.Bulstra S.K. Buurman W.A. Walenkamp G.H. Van der Linden A.J. Metabolic characteristics of in vitro cultured human chondrocytes in relation to the histopathologic grade of osteoarthritis. Clin Orthop Relat Res. 1989;242:294. [PubMed] [Google Scholar]
- 17.Tran-Khanh N. Hoemann C.D. McKee M.D. Henderson J.E. Buschmann M.D. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]
- 18.Palmer G.D. Chao Ph P.H. Raia F. Mauck R.L. Valhmu W.B. Hung C.T. Time-dependent aggrecan gene expression of articular chondrocytes in response to hyperosmotic loading. Osteoarthritis Cartilage. 2001;9:761. doi: 10.1053/joca.2001.0473. [DOI] [PubMed] [Google Scholar]
- 19.Hung C.T. LeRoux M.A. Palmer G.D. Chao P.H. Lo S. Valhmu W.B. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology. 2003;40:61. [PubMed] [Google Scholar]
- 20.Chao P.G. Tang Z. Angelini E. West A.C. Costa K.D. Hung C.T. Dynamic osmotic loading of chondrocytes using a novel microfluidic device. J Biomech. 2005;38:1273. doi: 10.1016/j.jbiomech.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Chao P.H. West A.C. Hung C.T. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol. 2006;291:C718. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- 22.Ateshian G.A. Likhitpanichkul M. Hung C.T. A mixture theory analysis for passive transport in osmotic loading of cells. J Biomech. 2006;39:464. doi: 10.1016/j.jbiomech.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albro M.B. Chahine N.O. Caligaris M. Wei V.I. Likhitpanichkul M. Ng K.W. Hung C.T. Ateshian G.A. Osmotic loading of spherical gels: a biomimetic study of hindered transport in the cell protoplasm. J Biomech Eng. 2007;129:503. doi: 10.1115/1.2746371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chahine N.O. Chen F.H. Hung C.T. Ateshian G.A. Direct measurement of osmotic pressure of glycosaminoglycan solutions by membrane osmometry at room temperature. Biophys J. 2005;89:1543. doi: 10.1529/biophysj.104.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahine N.O. Wang C.C. Hung C.T. Ateshian G.A. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech. 2004;37:1251. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C.C. Guo X.E. Sun D. Mow V.C. Ateshian G.A. Hung C.T. The functional environment of chondrocytes within cartilage subjected to compressive loading: a theoretical and experimental approach. Biorheology. 2002;39:11. [PubMed] [Google Scholar]
- 27.Oswald E.S. Chao P.H. Bulinski J.C. Ateshian G.A. Hung C.T. Dependence of zonal chondrocyte water transport properties on osmotic environment. Cell Mol Bioeng. 2008;1:339. doi: 10.1007/s12195-008-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopewell B. Urban J.P. Adaptation of articular chondrocytes to changes in osmolality. Biorheology. 2003;40:73. [PubMed] [Google Scholar]
- 29.Xu X. Urban J.P. Tirlapur U.K. Cui Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis Cartilage. 2010;18:433. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Urban J.P. Hall A.C. Gehl K.A. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 31.van der Windt A.E. Haak E. Das R.H. Kops N. Welting T.J. Caron M.M. van Til N.P. Verhaar J.A. Weinans H. Jahr H. Physiological tonicity improves human chondrogenic marker expression through nuclear factor of activated T-cells 5 in vitro. Arthritis Res Ther. 2010;12:R100. doi: 10.1186/ar3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauck R.L. Wang C.C. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Kelly T.A. Ng K.W. Ateshian G.A. Hung C.T. Analysis of radial variations in material properties and matrix composition of chondrocyte-seeded agarose hydrogel constructs. Osteoarthritis Cartilage. 2009;17:73. doi: 10.1016/j.joca.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong C.G. Mow V.C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88. [PubMed] [Google Scholar]
- 35.Setton L.A. Mow V.C. Muller F.J. Pita J.C. Howell D.S. Altered structure-function relationships for articular cartilage in human osteoarthritis and an experimental canine model. Agents Actions Suppl. 1993;39:27. doi: 10.1007/978-3-0348-7442-7_3. [DOI] [PubMed] [Google Scholar]
- 36.Mow V.C. Ratcliffe A. Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 37.Koo J. Kim K.I. Min B.H. Lee G.M. Controlling medium osmolality improves the expansion of human articular chondrocytes in serum-free media. Tissue Eng Part C Methods. 2010;16:957. doi: 10.1089/ten.TEC.2009.0525. [DOI] [PubMed] [Google Scholar]
- 38.Davis J.M., editor. Basic Cell Culture: A Practical Approach. Oxford: Oxford University Press; 1996. [Google Scholar]