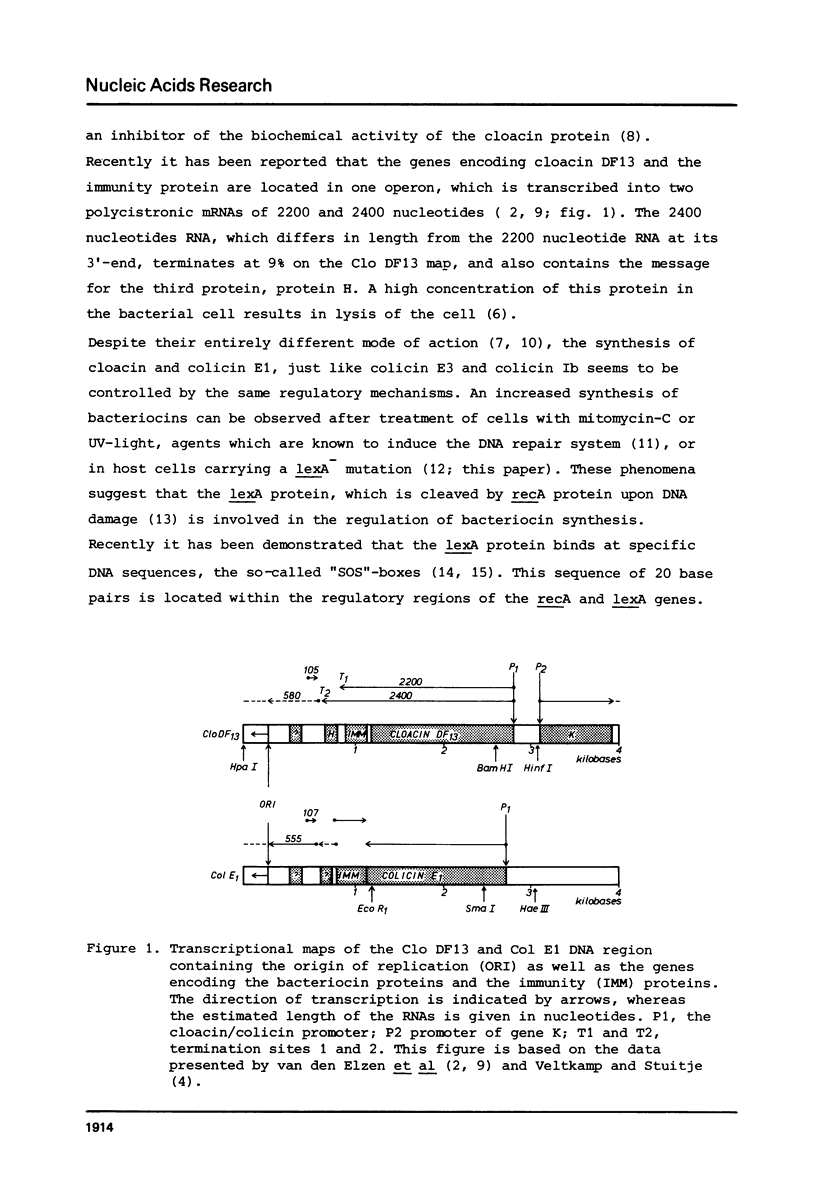
Abstract
Treatment of cells, harbouring the bacteriocinogenic plasmic Clo DF13 with mitomycin-C, which induces the cellular SOS response, results in a significantly increased transcription of the operon encoding the bacteriocin cloacin DF13, the immunity protein and the lysis protein H. The nucleotide sequences of the promoter regions and N-terminal parts of the bacteriocin genes of Clo DF13, Col E1 and the pMB1 derivative pBR324 have been determined. A comparison of these sequences with those of corresponding regions of the lexA, recA and uvrB genes revealed that the promoter regions of the bacteriocin genes studied contain binding sites for the lexA protein, which is the repressor of the E. coli DNA-repair system. Using both, a thermosensitive lexA host strain and a host with pACYC184 into which the lexA gene had been cloned, we were able to demonstrate, that in vivo the lexA protein is involved in the regulation of bacteriocin synthesis. From the data presented, we conclude that bacteriocin synthesis is controlled at least by the lexA repressor. It has been reported that also catabolite repression might play an essential role in the control of bacteriocin synthesis. Computer analysis of the DNA sequence data indicated that the promoter regions of both, the cloacin DF13 and colicin E1 genes contain potential binding sites for the cyclic AMP-cyclic AMP Receptor Protein complex.
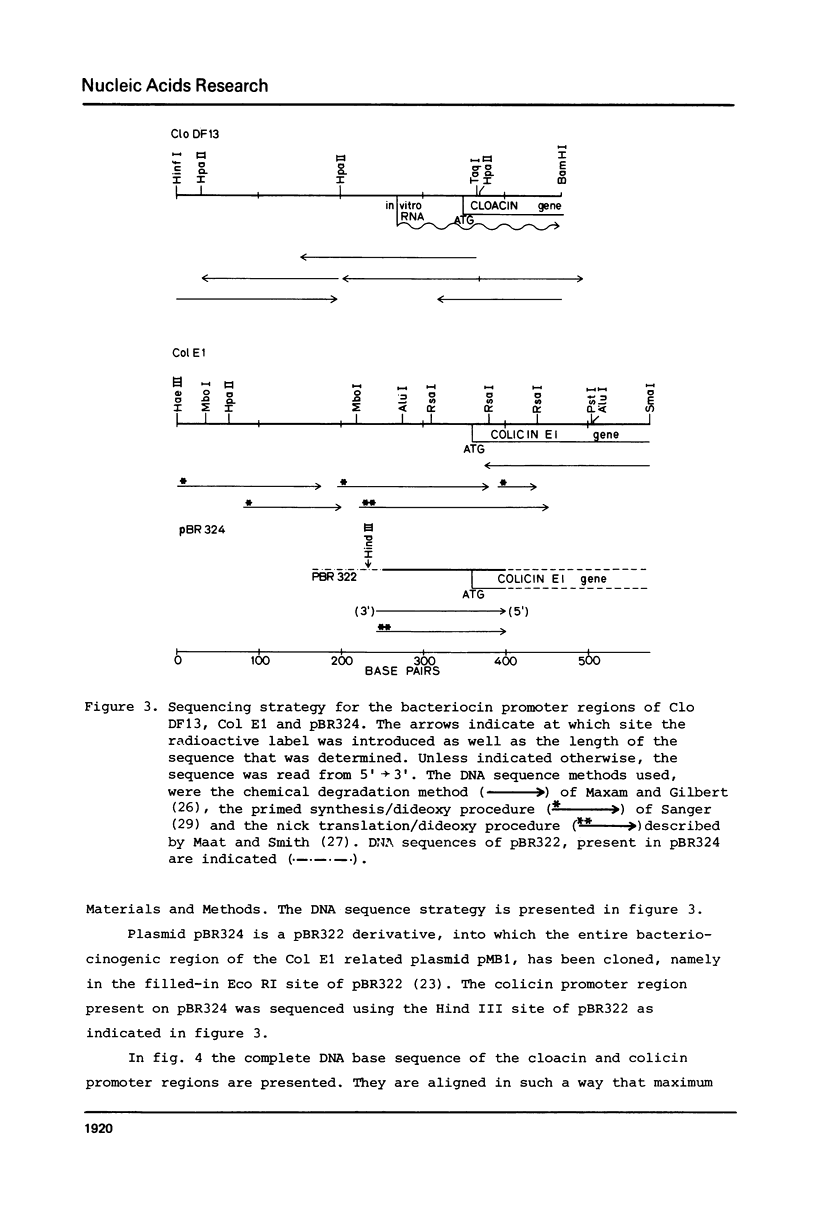
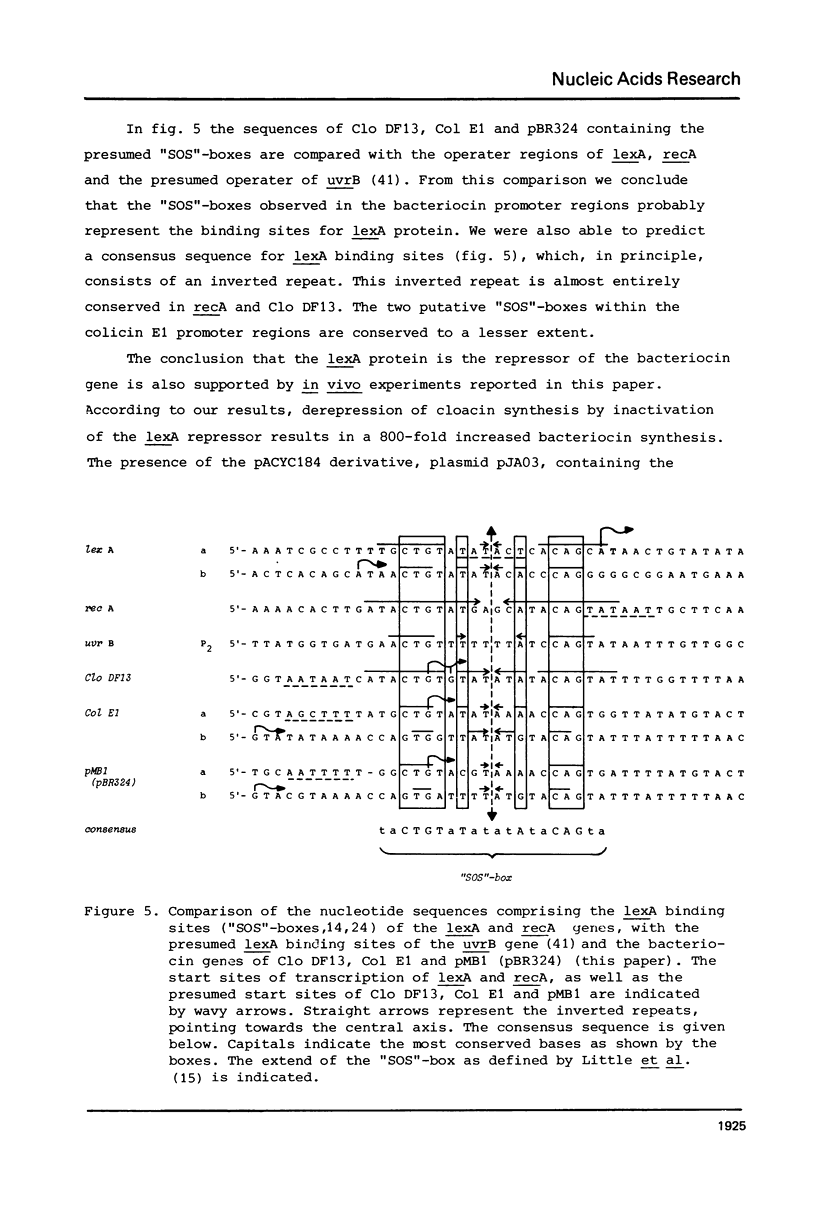
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli P. M., Overbeeke N., Veltkamp E., van Embden J. D., Nijkamp H. J. Genetic map of the bacteriocinogenic plasmid CLO DF13 derived by insertion of the transposon Tn901. Mol Gen Genet. 1978 Mar 20;160(1):1–11. doi: 10.1007/BF00275113. [DOI] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar F. Molecular cloning vectors derived from the CoLE1 type plasmid pMB1. Life Sci. 1979 Sep 3;25(10):807–817. doi: 10.1016/0024-3205(79)90538-1. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Klaasen-Boor P. Purification and characterization of a complex between cloacin and its immunity protein isolated from Enterobacter cloacae (Clo DF13). Dissociation and reconstitution of the complex. Eur J Biochem. 1977 Feb 15;73(1):107–114. doi: 10.1111/j.1432-1033.1977.tb11296.x. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Kennedy C. K., Sherratt D. J. Plasmid replication and the induced synthesis of colicins E1 and E2 in Escherichia coli. J Bacteriol. 1974 Mar;117(3):940–946. doi: 10.1128/jb.117.3.940-946.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Miki T., Kagamiyama H., Nakazawa T., Nakazawa A. The nucleotide sequence surrounding the promoter region of colicin E1 gene. Gene. 1981 Nov;15(2-3):119–126. doi: 10.1016/0378-1119(81)90121-9. [DOI] [PubMed] [Google Scholar]
- Farid-Sabet S. Colicins and bacterial membranes: structures and functions. I. Effects of colicins on the protein composition of the membranes of sensitive and tolerant Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):982–989. [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. II. Functions and regulation of synthesis of the gene H product. Mol Gen Genet. 1981;183(2):326–332. doi: 10.1007/BF00270636. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Pirkl E., Zentgraf H., Schaller H. Conversion of bacteriophage fd into an efficient single-stranded DNA vector system. Mol Gen Genet. 1980 Jan;177(2):231–242. doi: 10.1007/BF00267434. [DOI] [PubMed] [Google Scholar]
- Hull R. A. Effect of tsl mutations on Col E1 expression in a recA strain of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):775–776. doi: 10.1128/jb.123.2.775-776.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Konisky J. Studies of the regulation of colicin Ib synthesis. II. The synthesis of col Ib-P9 specific RNA in vivo. Mol Gen Genet. 1974;132(3):223–232. doi: 10.1007/BF00269395. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. Localization of the Tn5 transposase promoter using the cycling reaction of RNA polymerase. Nucleic Acids Res. 1981 Apr 24;9(8):1873–1883. doi: 10.1093/nar/9.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Borstlap A. J., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF16: action of the immunity protein in vivo and in vitro. Antimicrob Agents Chemother. 1975 Jul;8(1):76–85. doi: 10.1128/aac.8.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat J., Smith A. J. A method for sequencing restriction fragments with dideoxynucleoside triphosphates. Nucleic Acids Res. 1978 Dec;5(12):4537–4545. doi: 10.1093/nar/5.12.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M. C., Amass K., de Crombrugghe B. Molecuar model of the DNA interaction site for the cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2213–2217. doi: 10.1073/pnas.78.4.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo H., Shimada K., Takagi Y. Isolation and characterization of a mutant ColE1 plasmid that allows constitutive colicin E1 synthesis. J Bacteriol. 1979 Jul;139(1):311–315. doi: 10.1128/jb.139.1.311-315.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Patient R. K. Characterization of in vitro transcription initiation and termination sites in Col E1 DNA. Nucleic Acids Res. 1979 Jun 25;6(8):2647–2665. doi: 10.1093/nar/6.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Van Tiel-Menkvled G. J., Rezee A., De Graaf F. K. Production and excretion of cloacin DF13 by Escherichia coli harboring plasmid CloDF13. J Bacteriol. 1979 Nov;140(2):415–423. doi: 10.1128/jb.140.2.415-423.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp E., Stuitje A. R. Replication and structure of the bacteriocinogenic plasmids Clo DF13 and CoI E1. Plasmid. 1981 Jan;5(1):76–99. doi: 10.1016/0147-619x(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Spanjaerdt Speckman E. A., Stouthamer A. H. Mode of action of a bacteriocin produced by Enterobacter cloacae DF13. Antonie Van Leeuwenhoek. 1969;35(3):287–306. doi: 10.1007/BF02219150. [DOI] [PubMed] [Google Scholar]
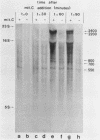
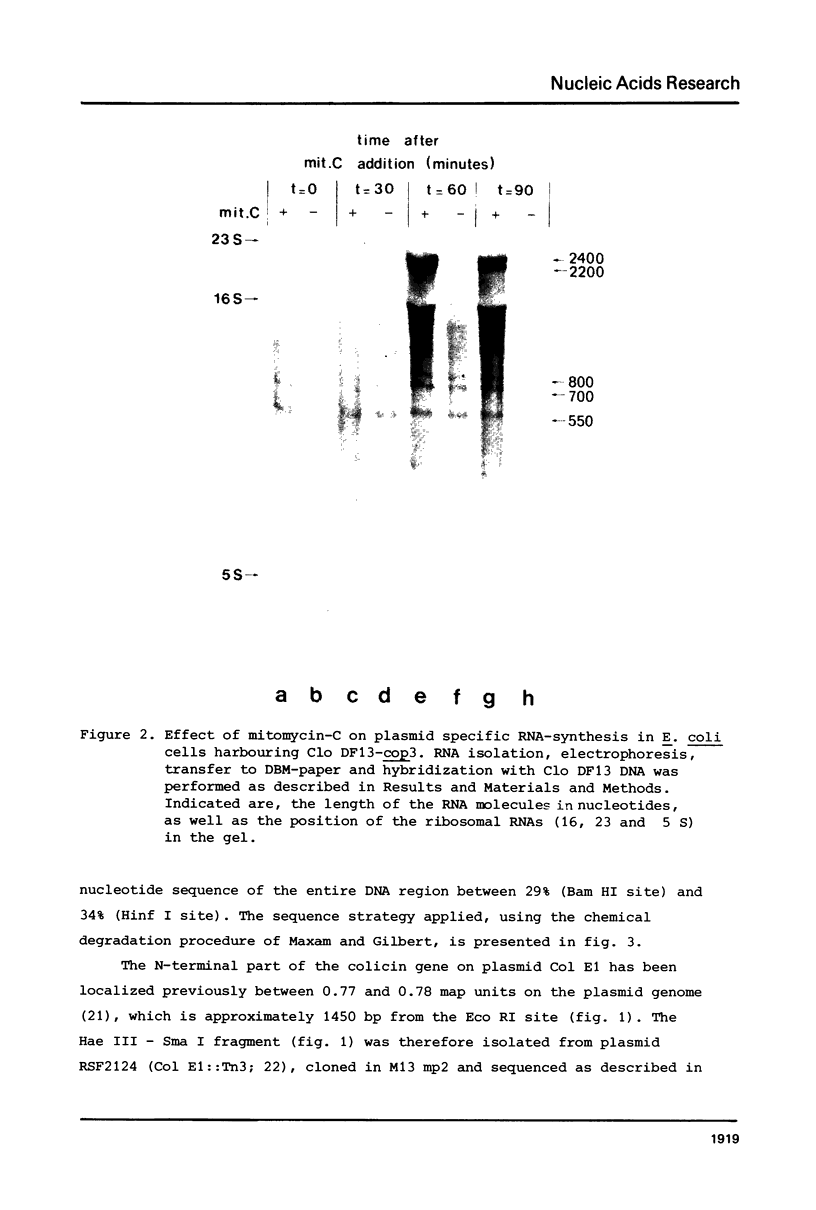
- van Tiel-Menkveld G. J., Veltkamp E., De Graaf F. K. Mitomycin C-induced synthesis of cloacin DF13 and lethality in cloacinogenic Escherichia coli cells. J Bacteriol. 1981 Apr;146(1):41–48. doi: 10.1128/jb.146.1.41-48.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E., Zwetsloot J., Noordermeer I., Pannekoek H., Dekker B., Dijkema R., van Ormondt H. The structure and function of the regulatory elements of the Escherichia coli uvrB gene. Nucleic Acids Res. 1981 Nov 11;9(21):5623–5643. doi: 10.1093/nar/9.21.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Gaastra W., Spelt C. E., de Graaf F. K., Veltkamp E., Nijkamp H. J. Molecular structure of the immunity gene and immunity protein of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1980 Oct 10;8(19):4349–4363. doi: 10.1093/nar/8.19.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Konings R. N., Veltkamp E., Nijkamp H. J. Transcription of bacteriocinogenic plasmid CloDF13 in vivo and in vitro: structure of the cloacin immunity operon. J Bacteriol. 1980 Nov;144(2):579–591. doi: 10.1128/jb.144.2.579-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]