Abstract
Objectives
The primary aim of the study was to evaluate the effectiveness and tolerability of open-label olanzapine on motor and vocal tics in children and adolescents with Tourette syndrome (TS). Secondary aims included assessing the response of TS-associated disruptive behaviors to olanzapine exposure.
Method
Twelve children and adolescents (mean age 11.3 ± 2.4 years, range 7–14 years) with Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) TS were enrolled in a single-site, 6-week, open-label, prospective, flexible-dose design in outpatients receiving monotherapy with olanzapine. Standardized ratings of tic symptoms, disruptive behaviors, and aggression were obtained, along with adverse events and safety data.
Results
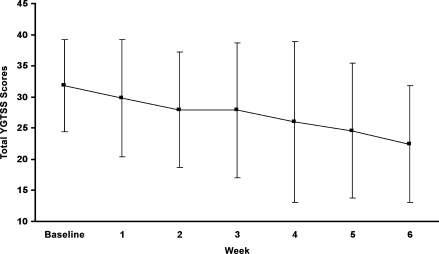
Over the 6-week trial, olanzapine administration was associated with a significant decrease in total tic severity as measured by the Yale Global Tic Severity Scale (30% reduction by week 6; effect size 0.49). A significant majority of subjects were rated as “much improved” or “very much improved” on the Clinical Global Impressions–Improvement Scale (GCI-I) by both clinicians (67%; 8/12) and parents (64%; 7/11). Attention-deficit/hyperactivity disorder (ADHD) symptoms showed significant improvements from baseline for both inattention (33% decrease) and hyperactive/impulsivity (50% decrease) scores (effect sizes 0.44 and 0.43, respectively). Aggression was also decreased as assessed by fewer numbers of aggressive episodes on the Overt Aggression Scale (OAS). Little change in anxiety symptoms was noted. The most widely reported side effects were drowsiness/sedation and weight gain; adverse events were generally well tolerated. Mean weight gain of 4.1 ± 2.0 kg was observed over the 6-week trial, a mean percent change of 8.4 ± 4.4 (p < 0.001).
Conclusions
Additional studies of the benefits of olanzapine treatment for tic control as well as the commonly associated co-morbid features of TS are indicated, especially if approaches to predict or minimize weight gain can be determined.
Introduction
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder characterized by chronic and impairing motor and vocal muscle movements or tics, which are involuntary, chronic, and repetitive in nature. Tics are likely to vary in frequency and severity over time; impairment can consist of subjective distress, interference with activities, social impact, and occasional physical morbidity (McCracken 1999; Leckman et al. 2006). Prevalence of TS has been estimated between 0.05% to 3%, and usually has a familial component (Freeman et al. 2000). Onset of TS occurs in childhood, and is more prevalent in males than females, estimated in a ratio of 4.3:1 worldwide (Freeman et al. 2000; Stephens et al. 2004). Tic severity usually peaks in preadolescent years and tends to decrease after adolescence in the majority of individuals (Leckman et al. 2006).
Increasing awareness of the clinical complexity of TS and associated features has led to the recognition of the high frequency of associated co-morbid psychopathology. Co-morbidity, including attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder, rage episodes, aggression, obsessive compulsive disorder (OCD), and other emotional disorders are often more debilitating than the tics themselves, and they compound the significant social and behavioral difficulties in children with TS (McCracken 1999; Kadesjo et al. 2000; Freeman et al. 2000; Stephens et al. 2004).
Clinical treatment of TS is also often complicated by the high frequency of difficult-to-tolerate side effects and limited behavioral improvement afforded by treatment with many agents, such as the conventional neuroleptics. Combination treatments are often employed in an effort to manage multiple problem behaviors, including co-morbid symptoms. More recently, interest in the possible benefits of atypical antipsychotics for TS has increased, with initial studies suggesting possible superiority of second-generation or atypical neuroleptics over conventional neuroleptics (Sallee et al. 2000; Scahill et al. 2003). Overall, however, few systematic studies have been performed with these agents, and fewer still have attempted to broadly assess medication effects on associated features of TS, leaving significant uncertainty as to the actual efficacy and tolerability of many of the atypical antipsychotics for TS.
Olanzapine has been suggested as another option in the treatment of TS, due to its apparent tic reduction effects and its relative tolerability at least as seen in adults (Budman et al. 2001). However, few clinical trials or reports have attempted to sensitively measure the impact of olanzapine treatment for TS on associated co-morbid behavioral and emotional symptoms in addition to the response of motor and vocal tics per se. One report noted significant olanzapine-related reductions in measures of aggression derived from the Child Behavior Checklist in 10 children with TS (Stephens et al. 2004), although the magnitude of change was small (11–16%). Therefore, the purpose of this pilot investigation was to gather additional systematic information on the potential therapeutic effects and tolerability of olanzapine as a broader treatment for the range of psychopathological behaviors associated with TS, in addition to motor and vocal tic symptoms and impairment.
Methods
Design
This was a single site, 6-week, open label flexible dose design in outpatients with Tourette disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) receiving monotherapy with olanzapine. All subjects had a comprehensive psychiatric and medical assessment, and, if enrolled, underwent a screening period of 7–14 days for completion of baseline ratings and assessment.
Inclusion and exclusion criteria
Subjects were recruited from successive referrals to the UCLA Child Obsessive Compulsive Disorder and Related Disorders Program and the UCLA Semel Institute for Neuroscience and Human Behavior, as well as through advertisement in local media and referrals from local mental health facilities and medical professionals. Enrollment took place from February, 2003, to March, 2005. All subjects were required to meet the following inclusion criteria: (1) between the ages of 7 and 17 years; (2) DSM-IV diagnosis of TS; (3) minimum body weight of greater than or equal to 25 kg; (4) outpatient status and not believed to require inpatient hospitalization during the course of the study; (5) minimum National Institute of Mental Health (NIMH) Clinical Global Impressions–Severity (CGI-S) score of moderately ill or greater severity (≥rating of 4); (6) normal screening medical history and physical examination; and (7) patient and parent/guardian must provide written informed consent (or assent).
Subjects were excluded from participation in the study if they had one or more of the following exclusion criteria: (1) Prior adequate treatment with olanzapine; (2) hypersensitivity to olanzapine; (3) need for co-morbid psychotropic medication during the study period or required concurrent medication that effects tics; (4) presence of significant co-morbid medical illness; (5) history of seizure disorder; 6) co-morbid psychotic disorder (schizophrenia disorder, schizophrenia, schizoaffective disorder, psychotic disorder, not otherwise specified); (7) history of substance or alcohol abuse or dependence within 6 months of screening; (8) pregnancy, lactation, or positive pregnancy test; (8) absence of acceptable contraceptive method for sexually active females; (9) detection of pregnancy during the study, which would have led to withdrawal from the study; (10) concurrent behavioral treatment for tic symptoms during the 6-week treatment period.
The study protocol was approved by the UCLA Human Subjects Protection Committee. All subjects and their parent or guardian were given written informed consent or assent along with a detailed explanation of the procedures before participating in the trial.
Visits and outcome measures
Subjects were diagnosed using the Anxiety Disorders Interview Schedule for DSM-IV, Research and Lifetime Version for Children and Parents (ADIS-RLV) (Silverman and Albano 2002), an expanded version of the ADIS-IV-PC (Silverman and Albano 1996) containing modules covering disruptive disorders, bipolar disorder, tic disorders, pervasive developmental disorders, and substance abuse disorders. The ADIS-IV, on which the ADIS-RLV is based, possesses favorable psychometric properties including strong test–retest reliability (κ = 0.76), interrater reliability (parent r = 0.98, child r = 0.93), and concurrent validity (McCracken 1999; Wood et al. 2002). The assessment of TS was augmented by the use of the Tic Disorders supplement of the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1996), and impairment was required for the TS diagnosis given the treatment-related nature of the study.
Symptom severity was rated by clinical personnel. Personnel used measures that included the SNAP IV (Swanson, Nolan and Pelham Questionnaire revision, Parent), the Overt Aggression Scale (OAS), Children's Psychiatric Rating Scale, the Yale Global Tic Severity Scale (YGTSS) (Leckman et al. 1989), and the Multidimensional Anxiety Scale for Children (MASC). Initial medical evaluation consisted of a detailed past medical history and physical examination to rule out medical illnesses, pregnancy, and illicit drug/alcohol use. Lab tests were conducted and included hematology, hemoglobin, hematocrit, platelet count, clinical chemistry, and routine urinalysis. In addition, an electrocardiogram (EKG) was done to rule out any preexisting cardiac conditions.
Following the baseline evaluation, subjects were seen weekly according to a 7-day plus or minus 3-day period for repeated evaluation and dosage titration. Weekly assessment schedules included the CGI-S and –Improvement Scale (CGI-I) completed by study physicians (J.M., R.S.). In addition, at baseline and on weeks 2, 4, and 6, the YGTSS was rated by one experienced rater trained to reliability (S.C.) and blinded to dosing and adverse events. The SNAP IV–Parent Scale, the IOWA Parent Conners' Inattention/Overactivity, IOWA Aggression/defiance and Conners' Index, OAS, and the MASC were conducted at the initial week and at weeks 2, 4, 5, and 6. Each weekly visit also included the recording of side effects and adverse events. Adverse events information was collected from parent and subject, via spontaneous report, and by physician examination. Laboratory tests were repeated at week 6 or end of trial.
Dosing schedule
Subjects received medication in the form of 2.5-mg olanzapine tablets, in a flexible dosing schedule as recommended by the manufacturer (see Table 2).For patients who weighed equal to or less than 40 kg, the starting dose was 2.5 mg every other day for 3 days, which was increased to 2.5 mg every day for the remainder of the week as needed. Dosing was increased to 5 mg/day by week 2 for subjects that required additional medication. Subsequent dosing adjustments were made in 5-mg increments every 7 days as needed, up to a maximum dose of 20 mg/day. For patients who weighed more than 40 kg, the starting dose was 2.5 mg every day for 3 days, which was increased to 5 mg every day for the remainder of the week as needed. Dosing was increased to 10 mg/day by week 2 for subjects that required additional medication. As with the patients weighing equal to or less than 40 kg, subsequent dosing adjustments were made in 5-mg increments every 7 days as needed, up to a maximum dose of 20 mg/day. Dosage reductions were made at any time throughout the trial for all patients, as clinically indicated based on occurrence of adverse events of moderate severity or greater. At the end of the study, subjects provided with options for subsequent treatment at UCLA or community facilities.
Table 2.
Dosing Schedule during Study Enrollment
| |
Week 1 |
|
|
|
|---|---|---|---|---|
| Weight limit | First 3 days | Next 4 days | Week 2 | Weeks 3–6 |
| Subject ≤ 40 kg | 2.5 mg QOD | 2.5 mg QD as needed | 5 mg QD as needed | 5–20 mg QD as needed |
| Subject > 40 kg | 2.5 mg QD | 5 mg QD as needed | 10 mg QD as needed | 10–20 mg QD as needed |
Olanzapine dosing schedule for subjects during study trial. Dosing was based on weight of child and severity of Tourette syndrome symptoms.
QOD = every other day; QD = daily.
Data Analyses
Objectives
The primary objective of the study was to demonstrate the open-label effectiveness of olanzapine on motor and vocal tic symptoms in children and adolescents with TS receiving olanzapine. Secondary objectives included: (1) gathering information on effective dose ranges for effects on both tic and associated behavioral symptoms; (2) evaluating the short-term safety of olanzapine in children and adolescents with TS; (3) assessing the effectiveness of olanzapine on the associated symptoms of overactivity, aggression, and other disruptive behaviors.
Statistical methods
Motor and vocal tic symptom severity, as measured by YGTSS Motor and Vocal subscale and Total Scores, were evaluated over the course of the 6-week trial using a general linear models repeated measures design, analyzing both within- and between-subjects effects. When sphericity could not be assumed, a Greenhouse–Geisser epsilon was used, based on the sample size of the study. A mixed models regression design was used to evaluate effective dosage ranges for olanzapine on tic and associated behavioral symptoms. Adverse effects were evaluated by descriptive statistics. In addition, weight gain was examined as a primary side effect and was compared from baseline to week 6, using paired t-tests and a one sample t-test based on percent change from baseline values. The efficacy of olanzapine on: (1) the SNAP-IV rating scale items of ADHD Inattention, ADHD Hyperactivity, ODD, IOWA Inattention/ overactivity, IOWA Aggression/defiance and Conner's Index and (2) child and parent reported MASC scale items of Physical symptoms, Harm Avoidance, Social Anxiety, and Separation Anxiety/Panic were both evaluated using a general linear models repeated measures design, examining within- and between-subject effects. The Bonferroni or Tukey correction method was used to correct for multiple comparisons, with calculated corrections based on number of tests per analysis subcategory (e.g., motor tic category, vocal tic category, etc.). Effect sizes (Cohen d) were also calculated to estimate the strength of the observed treatment effect using baseline versus final values. Means and standard deviations were calculated for continuous variables, such as age. A significance level of 0.05 was used for all statistical tests. Analyses were performed using SPSS version 13.0.
Results
A total of 12 subjects were enrolled (mean age ± standard deviation [SD], 11.33 ± 2.35 years; age range 7–14; 11 males, 1 female). Two subjects were Hispanic, one was African-American, and the remaining subjects were Caucasian. All 12 subjects provided data over 6 weeks of the study and were used for analyses. Of the 12 subjects enrolled in the study, 4 (33.3%) had previously taken clonidine for TS treatment, 3 (25%) had prior risperidone or haloperidol treatment, and 3 (25%) had received other forms of psychotropic medication for non-tic symptoms. Six (50%) had no prior medication treatment for TS. No subject was withdrawn from an effective treatment.
In this study, all subjects (100%) had at least one co-morbid diagnosis at baseline visit (see Table 1).Of the 12 subjects, 8 (66.7%) had two co-morbid diagnoses, 3 (25%) had three co-morbid diagnoses, and 3 (25%) had four or more comorbid diagnoses. Nine of the 12 subjects (75%) met criteria for at least one disruptive behavior disorder (ADHD, ODD, or CD). The most commonly occurring co-morbid diagnosis was ADHD (67.7%), followed by social phobia (33.3%).
Table 1.
Percentage of Tourette Syndrome Subjects by Co-Morbid Diagnoses
| Co-morbid diagnosis | Percent |
|---|---|
| Attention-deficit/hyperactivity disorder | 66.67 |
| Social phobia | 33.33 |
| Generalized anxiety disorder | 25.00 |
| Obsessive compulsive disorder | 25.00 |
| Oppositional defiant disorder | 25.00 |
| Pervasive developmental disorder/other | 16.67 |
| Anxiety disorder NOS | 8.33 |
| Conduct disorder | 8.33 |
| Dysthymia | 8.33 |
Reported co-morbid diagnoses and percentage occurrence in study sample (n = 12).
NOS = not otherwise specified.
Primary outcome measure: Effectiveness of olanzapine of motor and vocal tic symptoms
Statistically significant pre–post treatment decreases were noted for all motor tic category variables with repeated measures analyses: number (f = 2.91, df = 6, p = 0.015), frequency (f = 3.69, df = 6, p = 0.003), intensity (f = 2.55, df = 6, p = 0.029), complexity (f = 2.41, df = 6, p = 0.037) interference (f = 2.71, df = 6, p = 0.021) and severity (f = 3.36, df = 6, p = 0.006). Repeated measures analyses of the vocal tic categories showed statistically significant changes from baseline across to week 6 ratings in interference (f = 2.53, df = 6, p = 0.03) and a trend toward significance in vocal tic severity scores (f = 1.963, df = 6, p = 0.085).
Total tic categories were calculated by summing motor and vocal tic scores. Significant drops in total tic frequency (f = 2.93, df = 6, p = 0.014), intensity (f = 2.29, df = 6, p = 0.047), interference (f = 3.36, df = 6, p = 0.006), impairment (f = 6.45, df = 7, p = 0.001), and total tic severity (f = 3.12, df = 6, p = 0.01) were observed. Effect sizes were calculated for each of the motor, vocal, and total tic variables. The effect sizes indicate a small-to-moderate effect size for all categories, the smallest at 0.19 for vocal tic complexity and the largest at 0.52 for global severity.
Secondary outcome measure 1: Effectiveness of olanzapine on associated symptoms of overactivity, aggression, and other disruptive behaviors
To assess the effectiveness of olanzapine on overactivity, aggression, and other disruptive behaviors, categories from the parent version of the SNAP IV Rating Scale were used. The SNAP IV Scale categories included: ADHD Inattention, ADHD Hyperactivity/Impulsivity, ODD, Inattention/Over-activity, Aggression/Defiance, and Conner's Index. General linear model repeated measures analyses identified statistically significant within-subject decreases for all parent SNAP Rating Scale categories: ADHD Inattention (f = 7.19, df = 4, p < 0.001), ADHD Hyperactivity/ Impulsivity (f = 10.69, df = 4, p < 0.001), ODD (f = 5.65, df = 4, p = 0.001), Inattention/Overactivity (f = 8.97, df = 4, p = 0.002), Aggression/Defiance (f = 4.17, df = 4, p = 0.009), and Conner's Index (f = 8.62, df = 4, p < 0.001).
Analysis of total OAS score indicated that no significant within subject differences existed for overall OAS score. When analyzing the number of episodes, as defined by the OAS Scale, statistically significant between-subject (f = 10.19, df = 1, p < 0.02) decreases existed.
Both child and parent reported anxiety scores were measured using the MASC. The four scales evaluated were the Physical Symptoms, Harm Avoidance, Social Anxiety, and Separation Anxiety/Panic. Repeated measure analyses of these scales showed a significantly lower score in child reported Physical Symptoms across treatment weeks (f = 2.69, df = 4, p< 0.05), however all other scores did not differ.
Secondary outcome measure 2: Short-term safety of olanzapine in children and adolescents
A repeated measures analysis of side effect data indicated that all 12 subjects receiving olanzapine experienced an increase in weight over the 6-week trial period (f = 27.32, df = 6, p < 0.001). The mean percent change from baseline to week 6 was 8.4 ± 4.4 (t = 6.55, p < 0.001). In addition, a paired t-test showed a significant increase between initial and final body mass index (BMI) measurements (t = –2.885, df = 5, p = 0.03). Other common side effects included drowsiness (see Table 4), increased appetite, and sedation. From baseline to week 6, a significant increase was observed in heart rate (73 vs. 86, respectively, t = 3.16, p = 0.009). Systolic blood pressure showed an increase that was not significant (110 vs. 117, respectively, t = 2.07, p = 0.063). Diastolic pressure showed no change. Paired t-tests of pre- and week-6 nonfasting lab results showed several statistically significant changes from baseline (see Table 5), but none was deemed clinically significant (all values within normal ranges). No significant changes in nonfasting glucose, low-density lipoprotein (LDL), high-density lipoprotein (HDL), or triglycerides concentrations were observed.
Table 4.
Frequency of Reports of Drowsiness/Sedataion during 6-Week Exposure to Olanzapine
| Drowsiness | Mild percentage | Moderate/severe percentage |
|---|---|---|
| Week 1 | 58.3% | 8.3% |
| Week 2 | 58.3% | 25.0% |
| Week 3 | 58.3% | 25.0% |
| Week 4 | 75.0% | 0.0% |
| Week 5 | 75.0% | 0.0% |
| Week 6 | 83.3% | 16.7% |
| Cumulative percentages (all weeks) | 100.0% | 50.0% |
Percentage of patients who reported drowsiness as a side effect, separated by week and severity of symptom. Cumulative percentage refers to all patients from all weeks who reported drowsiness as a side effect (n = 12).
Table 5.
Lab Report Comparison between Initial and Final Visit
| Lab test | Initial value | Final value | Mean difference (± SD) | p value |
|---|---|---|---|---|
| Albumin | 4.23 | 4.03 | 0.20 | 0.001 |
| ALT | 20.33 | 30.42 | 10.09 | <0.001 |
| AST | 27.42 | 30.75 | 3.33 | 0.028 |
| Cholesterol | 150.1 | 163 | 12.9 | 0.011 |
| Creatinine | 0.59 | 0.525 | –0.07 | 0.005 |
| Phosphorus | 5.01 | 5.56 | 0.55 | 0.024 |
Paired t-test analysis of lab results at initial and final visits. Only significant differences from lab results are shown. None of the changes in measurements were deemed clinically significant (all within lab normal ranges). Uncorrected p values are represented (n = 12).
ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Table 3.
Symptom Measures at Baseline and Week 6 for 12 Subjects Treated with Olanzapine
| Baseline (mean SD) | End of trial (mean SD) | Within subjects p value | Effect size (baseline to end) of trial) | |
|---|---|---|---|---|
| YGTSS–Motor Tic Category | ||||
| Number | 4.33 ± 0.49 | 3.08 ± 1.73 | 0.015** | 0.44 |
| Frequency | 4.75 ± 0.62 | 3.58 ± 1.83 | 0.003** | 0.39 |
| Intensity | 3.75 ± 0.75 | 2.58 ± 1.51 | 0.029** | 0.44 |
| Complexity | 3.33 ± 0.78 | 2.42 ± 1.24 | 0.037** | 0.40 |
| Interference | 3.17 ± 1.11 | 2.08 ± 1.44 | 0.021** | 0.39 |
| Total Motor Tic Severity Score | 19.33 ± 2.61 | 13.75 ± 7.06 | 0.006** | 1.04 |
| YGTSS–Vocal Tic Category | ||||
| Number | 2.17 ± 1.19 | 1.58 ± 1.00 | 0.222 | 0.26 |
| Frequency | 3.5 ± 1.34 | 2.75 ± 1.42 | 0.223 | 0.26 |
| Intensity | 2.83 ± 1.33 | 2.08 ± 1.24 | 0.389 | 0.40 |
| Complexity | 1.83 ± 1.59 | 1.25 ± 1.48 | 0.368 | 0.19 |
| Interference | 2.25 ± 1.48 | 1.08 ± 1.16 | 0.030** | 0.40 |
| Total Tic Severity Score | 12.58 ± 6.35 | 8.75 ± 5.10 | 0.085* | 0.67 |
| YGTSS–Total Tic Category | ||||
| Number | 6.5 ± 1.51 | 4.67 ± 2.19 | 0.121 | 0.44 |
| Frequency | 8.25 ± 1.54 | 6.33 ± 2.77 | 0.014** | 0.39 |
| Intensity | 6.58 ± 1.68 | 4.67 ± 1.92 | 0.047** | 0.47 |
| Complexity | 5.17 ± 1.90 | 3.67 ± 1.67 | 0.135 | 0.39 |
| Interference | 5.42 ± 1.83 | 3.17 ± 2.17 | 0.006** | 0.49 |
| Total Tic Severity Score | 31.92 ± 7.39 | 22.50 ± 9.37 | 0.010** | 0.49 |
| YGTSS–Overall Functioning Category | ||||
| Overall Impairment Rating | 33.33 ± 10.73 | 20.50 ± 13.73 | <0.001*** | 0.46 |
| Global Severity | 65.25 ± 14.12 | 43.00 ± 21.47 | <0.001*** | 0.52 |
| SNAP IV Rating Scale Category–Parent | ||||
| ADHD Inattention | 1.92 ± .67 | 1.29 ± 0.62 | <.001*** | 0.44 |
| ADHD Hyperactivity/Impulsivity | 1.39 ± .87 | 0.69 ± 0.59 | <0.001*** | 0.43 |
| ODD | 1.27 ± .88 | 0.61 ± 0.39 | 0.001*** | 0.43 |
| Inattention/Overactivity | 1.93 ± .73 | 1.22 ± 0.71 | 0.002*** | 0.45 |
| Aggression/Defiance | 1.25 ± .65 | 0.67 ± 0.42 | 0.009*** | 0.47 |
| Conners' Index | 1.46 ± .56 | 0.90 ± 0.51 | <0.001*** | 0.46 |
| OAS score | ||||
| Total | 15.82 ± 24.31 | 7.58 ± 11.71 | 0.199 | 0.43 |
| Number of episodes | 2.91 ± 3.42 | 1.33 ± 1.5 | 0.011** | 0.59 |
| MASC score–Child | ||||
| Physical Symptoms | 52.75 ± 11.07 | 48.58 ± 11.23 | 0.047** | 0.37 |
| Harm Avoidance | 51.92 ± 10.05 | 47.83 ± 10.6 | 0.681 | 0.50 |
| Social Anxiety | 58.50 ± 11.18 | 57.83 ± 14.31 | 0.822 | 0.05 |
| Separation Anxiety/Panic | 56.75 ± 11.55 | 57.50 ± 10.15 | 0.110 | 0.07 |
| Total Score | 56.67 ± 11.71 | 53.50 ± 11.34 | 0.331 | 0.28 |
| MASC score–Parent | ||||
| Physical Symptoms | 47.73 ± 7.30 | 48.42 ± 8.44 | 0.251 | 0.09 |
| Harm Avoidance | 48.27 ± 10.01 | 46.83 ± 12.94 | 0.551 | 0.12 |
| Social Anxiety | 60.64 ± 10.98 | 59.92 ± 9.36 | 0.134 | 0.07 |
| Separation Anxiety/Panic | 56.27 ± 9.87 | 53.08 ± 10.17 | 0.236 | 0.32 |
| Total Score | 53.91 ± 10.06 | 53.67 ± 9.55 | 0.316 | 0.02 |
Means, standard deviations, p values, and effect size. Weekly comparison of motor tic, vocal tic, total tic, impairment, global severity, parent SNAP-IV subcategory scores, and OAS Scores using repeated measures analyses. Indicated p values are post-Bonferroni correction. N = 12. SNAP scores are presented as average score per individual item.
SD = standard deviation; YGTSS = Yale Global Tic Severity Scale; SNAP IV = Swanson, Nolan and Pelham Questionnaire revision; OAS = Overt Aggression Scale; MASC = Multidimensional Anxiety Scale for Children.
less than .05; ***less than .001.
Secondary outcome measure 3: Dosage effects on both tic and associated behavioral symptoms
Two of the 12 subjects (16.7%) decreased olanzapine dosage at some time between first and last visit, one for enuresis and the other for excessive sedation. Mean final daily dosage was 11.3 ± 5.6 mg/day (range 2.5–20 mg). Absolute daily dosage was not significantly correlated with pre-post treatment changes on any of the tic measures. A mixed-model regression using week, dosage, and week by dosage interaction indicated no significant effect on any motor, vocal, or total tic categories.
Discussion
In our open-label, acute treatment trial of children and adolescents with moderate to severe TS, olanzapine administration was associated with noticeable improvement on dimensional measures across a variety of symptomatic areas, including reduced total tic frequency, intensity, interference, impairment, and severity; decreased disruptive behavior; and reduced frequency of aggressive outbursts. Paralleling these findings were the global ratings of significant improvement in over 64% of subjects as rated by both parents and clinicians, indicative of meaningful clinical change during the 6-week study period. In general, these data replicate and extend prior observations from similar reports suggesting that olanzapine can exert beneficial effects on tics in individuals with TS (Budman et al. 2001; Stephens et al. 2004).
The magnitude and significance of effect of olanzapine on measures of tic severity was in line with an array of other medications. Olanzapine was associated with a statistically significant decrease in the YGTSS Total Tic score of 30%, comparable to that reported for ziprasidone (35%; Sallee et al. 2000), risperidone (21–42%; Gaffney et al., 2002; Scahill et al., 2003; Gilbert et al. 2004), and pimozide (21%; Gilbert et al. 2004). Although our sample would not be considered to be “treatment resistant,” by virtue of only a moderate degree of exposure to prior treatments (including 50% without prior treatment), it was notable for displaying a baseline severity of tics greater than that seen in most other child medication treatment of tics observations, as well as more prominent variability. Both of these characteristics may have contributed to the observation of somewhat less robust effect size estimates for olanzapine's impact on tics (d = 0.49) versus some prior reports. Nevertheless, overall clinical improvement rates (67% and 64% much or very much improved as rated by study physician or parent, respectively) were also comparable to those noted with other medications. Taken together with the significant drop in YGTSS Impairment ratings, these data suggest that the overall clinical benefit for tic control per se is meaningful. Although unknown, the mechanism of these effects may be via antagonism of dopamine 2 (D2) receptors, given olanzapine's moderate binding affinity for the receptor (Ki 20 nM) (Duncan et al. 1999).
An important aim of this study was to evaluate the possible broader effectiveness of olanzapine on commonly co-occurring disruptive behavior in TS. Indeed, two thirds of our sample met categorical criteria for ADHD, and average dimensional ratings of ADHD symptoms for the entire sample were at or above usual cutoffs for clinical significance. We observed robust decreases in parent ratings of disruptive behaviors, particularly hyperactive-impulsive behaviors, where we noted a 50% decrease (effect size estimate 0.49) from baseline to week 6 of olanzapine on the SNAP-IV Hyperactive-Impulsive items as rated by parents, comparable to decreases associated with methylphenidate treatment of ADHD in children (MTA Cooperative Group 1999). Though less pronounced, parent ratings of SNAP-IV Inattentive symptoms decreased by 33% from baseline (as compared to a 45% decrease in the MTA Study with methylphenidate), and the number of aggressive episodes as reported on the OAS also declined. Our findings of decreases in several domains of disruptive behavior significantly expand on the observation (Stephens et al. 2004) that scores on the aggression subscale of the Achenbach Child Behavior Checklist (primarily containing oppositional defiant behaviors) was decreased with olanzapine treatment.
Given that disruptive behaviors in TS often form the primary source of impairment and strain, we believe olanzapine's beneficial effects are potentially clinically important. It should be noted that the few other studies that reported on the effects of tic-suppressing agents on disruptive behavior either found no significant change, such as with metoclo-pramide (Nicolson et al. 2005), or more limited improvements, such as guanfacine treatment reducing hyperactive-impulsive symptoms by 27% from baseline in a sample of children with ADHD and less severe tic disorders (Scahill et al. 2001). Thus far, we are unaware of other tic disorder monotherapies demonstrated to display comparable effects on disruptive behaviors as what we have observed for olanzapine. It was of interest that we documented little if any change in various symptoms of anxiety, another common co-morbidity in TS.
In this acute trial, olanzapine was reasonably tolerated, as 11 of 12 (92%) of subjects were able to complete the protocol, and adverse events were generally mild to moderate in severity. However, a significant increase in BMI and an average weight gain of 4 kg (range 1.1–7.7 kg) were noted during the 6-week period, quite consistent with other reports of olanzapine administration in TS and other child clinical populations (Malone et al. 2001; Stephens et al. 2004). It is unclear if the rate of weight increase seen in the 6-week acute trial would continue over a longer treatment period; if so, this would clearly constitute a clinical concern in many children as exceeding the rate of developmentally expected weight gain. Drowsiness as an adverse event was experienced by all subjects, but this was rated mild in the majority of cases. Drowsiness was noted much more commonly in weeks 1–3 versus weeks 4–6 of exposure, suggesting the possibility of habituation to this effect. In addition, we noted a modest but significant increase in pulse (+ 13 beats per minute [bpm]), and a trend for an increase in systolic blood pressure (+ 7.25 mmHg) from baseline to week 6 of olanzapine. Cardiovascular parameters remained in normal ranges. We are unaware of any similar reports of such cardiovascular effects of olanzapine, as no effects were found in prior publications (Malone et al. 2001; Stephens et al. 2004), and can only speculate that olanzapine's affinity for the muscarinic 1 (m1) receptor may relate to such effects (Duncan et al. 1999) if they are replicated. Although no clinically significant laboratory abnormalities were noted during olanzapine exposure, our observations were limited by our not obtaining fasting lab values.
For both clinical effectiveness and adverse effects such as weight gain, variability in response was apparent. At present, there are no established predictors of treatment efficacy or tolerability, so the observed variation of olanzapine effect from individual to individual is unexplained. Additional larger-scale studies hold the promise of identifying moderators and mediators of treatment effects, which could aid in the selection of treatments.
The results from this preliminary study are limited by our small sample size, the absence of a placebo control, the need for more structured adverse event assessments, and a total study observation period of only 6 weeks. Longer treatment duration could better inform on tolerability of the treatment, on the durability of the response, and whether the short-term improvements noted are associated with other important longer-term outcomes, such as reduced need for concomitant medications, improved school performance, and parent or family satisfaction. As awareness of the potential importance of metabolic adverse events has increased since the time our study was conducted, we would concur with the recommendations for more systematic monitoring of extrapyramidal symptoms (such as with the Abnormal Involuntary Movement Scale), adverse events, fasting metabolic parameters, and prolactin (Correll 2008). Although the dosage used in this study was moderate, the design was not sufficient to assess either minimum or optimal dosing given the delimited trial length. Other trial designs using fixed-dose assignments and longer-term treatment periods could assist in better determining the best range of olanzapine doses for application to the treatment of TS. At present, there are few controlled trials of drug treatments for TS, and the modest number of controlled trials themselves are small. Given the possible benefits of olanzapine for this often complicated condition, more research efforts are needed to delineate a fully evidence-based approach to the comprehensive treatment of TS and related disorders.
FIG. 1.
Mean total YGTSS scores and standard deviations at baseline and over the six weekly visits of the clinical trial (n = 12).
Disclosures
Dr. McCracken has served as a consultant, advisor, and/or lecturer for Abbott, Shire, Eli Lilly, Bristol-Myers Squibb, Wyeth, Novartis, UCB, and Janssen. Drs. Suddath, Chang, and Piacentini and Ms. Thakur have no financial ties or conflicts of interest to report. Ms. Thakur performed the statistical analyses. The manuscript was written solely by the listed authors.
References
- American Psychiatric Association. Diagnostic, Statistical Manual of Mental Disorders, 4th edition (DSM-IV) Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Budman CL. Gayer A. Lesser M. Bruun R. An open-label study of the treatment efficacy of olanzapine for Tourette's disorder. J Clin Psychiatry. 2001;62:290–294. doi: 10.4088/jcp.v62n0412. [DOI] [PubMed] [Google Scholar]
- Correll C. Antipsychotic use in children and adolescents: Minimizing adverse events to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:9–20. doi: 10.1097/chi.0b013e31815b5cb1. [DOI] [PubMed] [Google Scholar]
- Duncan GE. Zorn S. Lieberman JA. Mechanisms of typical and atypical antipsychotic drug action in relation to dopamine and NMDA receptor hypofunction hypotheses of schizophrenia. Mol Psychiatry. 1999;4:418–428. doi: 10.1038/sj.mp.4000581. [DOI] [PubMed] [Google Scholar]
- Freeman RD. Fast DK. Burd L. Kerbeshian J. Robertson MM. Sandor P. An international perspective on Tourette syndrome: Selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436–447. doi: 10.1017/s0012162200000839. [DOI] [PubMed] [Google Scholar]
- Gaffney GR. Perry PJ. Lund BC. Bever-Stille KA. Arndt S. Kuperman S. Risperidone versus clonidine in the treatment of children and adolescents with Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 2002;41:330–336. doi: 10.1097/00004583-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Gilbert DL. Batterson JR. Sethuraman G. Sallee FR. Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. J Am Acad Child Adolesc Psychiatry. 2004;43:206–214. doi: 10.1097/00004583-200402000-00017. [DOI] [PubMed] [Google Scholar]
- Kadesjo B. Gillberg C. Tourette's disorder: Epidemiology and comorbidity in primary school children. J Am Acad Child Adolesc Psychiatry. 2000;39:548–555. doi: 10.1097/00004583-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Ryan N. Diagnostic interview: Kiddie-SADS-Present, Lifetime Version (K-SADS-PL) Pittsburgh, PA: University of Pittsburgh; 1996. [Google Scholar]
- Leckman JF. Riddle MA. Hardin MT. Ort SI. Swartz KL. Stevenson J. Cohen DJ. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Bloch MH. Scahill L. King RA. Tourette syndrome: The self under siege. J Child Neurol. 2006;21:642–649. doi: 10.1177/08830738060210081001. [DOI] [PubMed] [Google Scholar]
- Malone RP. Cater J. Sheikh RM. Choudruy M. Delaney M. Olanzapine versus haloperidol in children with autistic disorder: An open pilot study. J Amer Acad Child Adolesc Psychiatry. 2001;40:887–894. doi: 10.1097/00004583-200108000-00009. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- McCracken JT. Tourette, the Tic disorders in Comprehensive. In: Kaplan HI, editor; Sadock B, editor. Textbook of Psychiatry/VII. New York: Williams and Wilkins; 1999. pp. 2711–2719. [Google Scholar]
- Nicolson R. Craven-Thuss B. Smith J. McKinlay BD. Castellanos FX. A randomized, double-blind, plcebo-controlled trial of metoclopramide for the treatment of Tourette's disorder. J Amer Acad Child Adoles Psychiatry. 2005;44:640–646. doi: 10.1097/01.chi.0000163279.39598.44. [DOI] [PubMed] [Google Scholar]
- Sallee FR. Kurlan R. Goetz CG. Singer H. Scahill L. Law G. Dittman VM. Chappell PB. Ziprasidone treatment of children and adolescents with Tourette's syndrome: A pilot study. J Am Acad Child Adoles Psychiatry. 2000;39:292–299. doi: 10.1097/00004583-200003000-00010. [DOI] [PubMed] [Google Scholar]
- Scahill L. Chappell PM. Kim YS. Schultz R. Katsovich L. Shepard E. Arnsten A. Cohen DJ. Leckman JF. A placebo-controlled trial of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Scahill L. Leckman JF. Schultz RT. Katsovich L. Peterson BS. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130–1135. doi: 10.1212/01.wnl.0000055434.39968.67. [DOI] [PubMed] [Google Scholar]
- Silverman WK. Albano AM. Manual for the Anxiety Disorders Interview Schedule for DSM-IV-Child and Parent Versions. Albany (New York): Graywind; 1996. [Google Scholar]
- Silverman WK. Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV Research, Lifetime Version for Children Parents (ADIS-RLV) New York: Columbia University; 2002. [Google Scholar]
- Stephens RJ. Bassel C. Sandor P. Olanzapine in the treatment of aggression and tics in children with Tourette's syndrome–A pilot study. J Child Adolesc Psychopharmacol. 2004;14:255–266. doi: 10.1089/1044546041648959. [DOI] [PubMed] [Google Scholar]
- Wood J. Piacentini J. Bergman RL. McCracken JT. Barrios V. Concurrent validity of the Anxiety Disorders Interview Schedule for Children (ADIS-IV) J Clinl Child Adolesc Psychol. 2002;31:335–342. doi: 10.1207/S15374424JCCP3103_05. [DOI] [PubMed] [Google Scholar]