Abstract
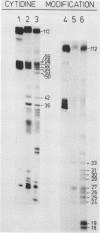
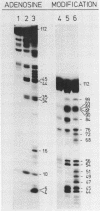
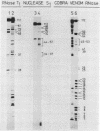
The 3' terminus of TYMV RNA, which possesses tRNA-like properties, has been studied. A 3' terminal fragment of 112 nucleotides was obtained by cleavage with RNase H after hybridization of a synthetic oligodeoxynucleotide to the viral RNA. The accessibility of cytidine and adenosine residues was probed with chemical modification. Enzymatic digestion studies were performed with RNase T1, nuclease S1 and the double-strand specific RNase from the venom of the cobra Naja naja oxiana. A model is proposed for the secondary structure of the 3' terminal region of TYMV RNA comprising 86 nucleotides. The main feature of this secondary structure is the absence of a conventional acceptor stem as present in canonical tRNA. However, the terminal 42 nucleotides can be folded in a tertiary structure which bears strong resemblance with the acceptor arm of canonical tRNA. Comparison of this region of TYMV RNA with that of other RNAs from both the tymovirus group and the tobamovirus group gives support to our proposal for such a three-dimensional arrangement. The consequences for the recognition by TYMV RNA of tRNA-specific enzymes is discussed.
Full text
PDF


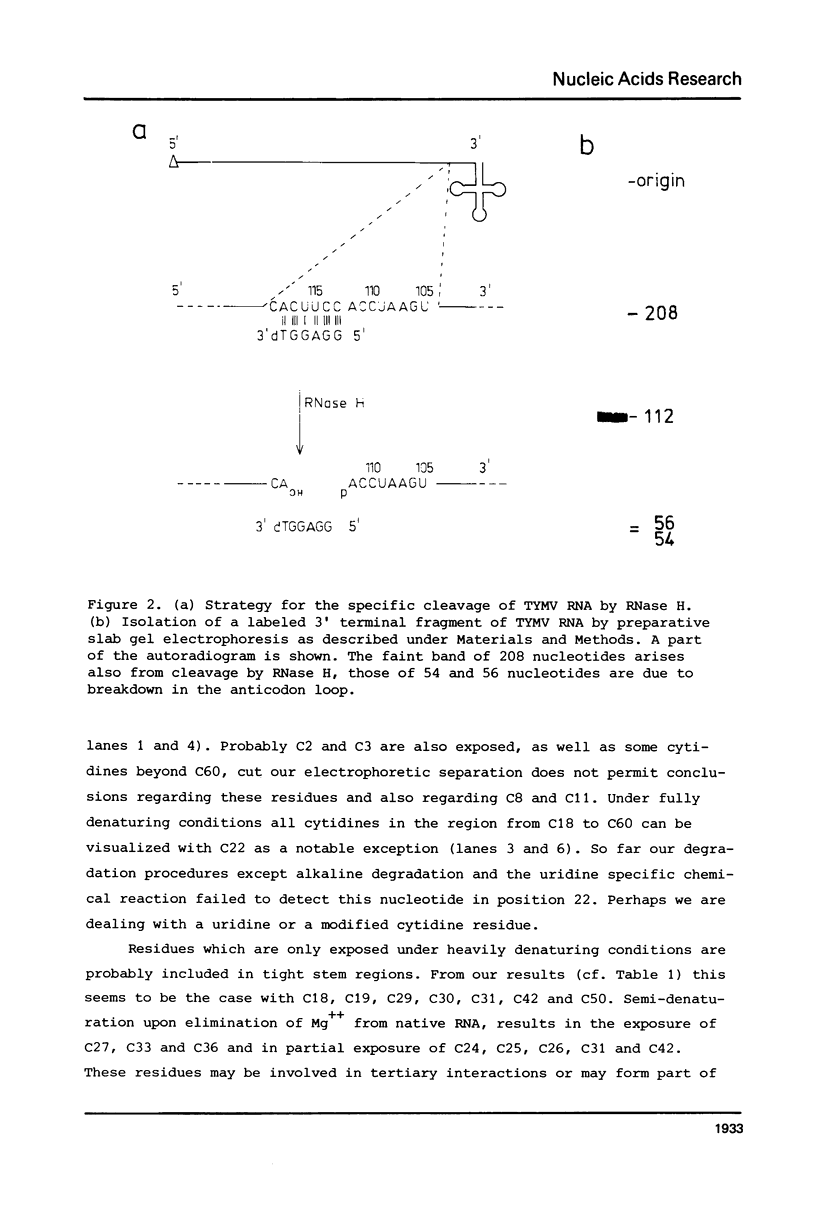
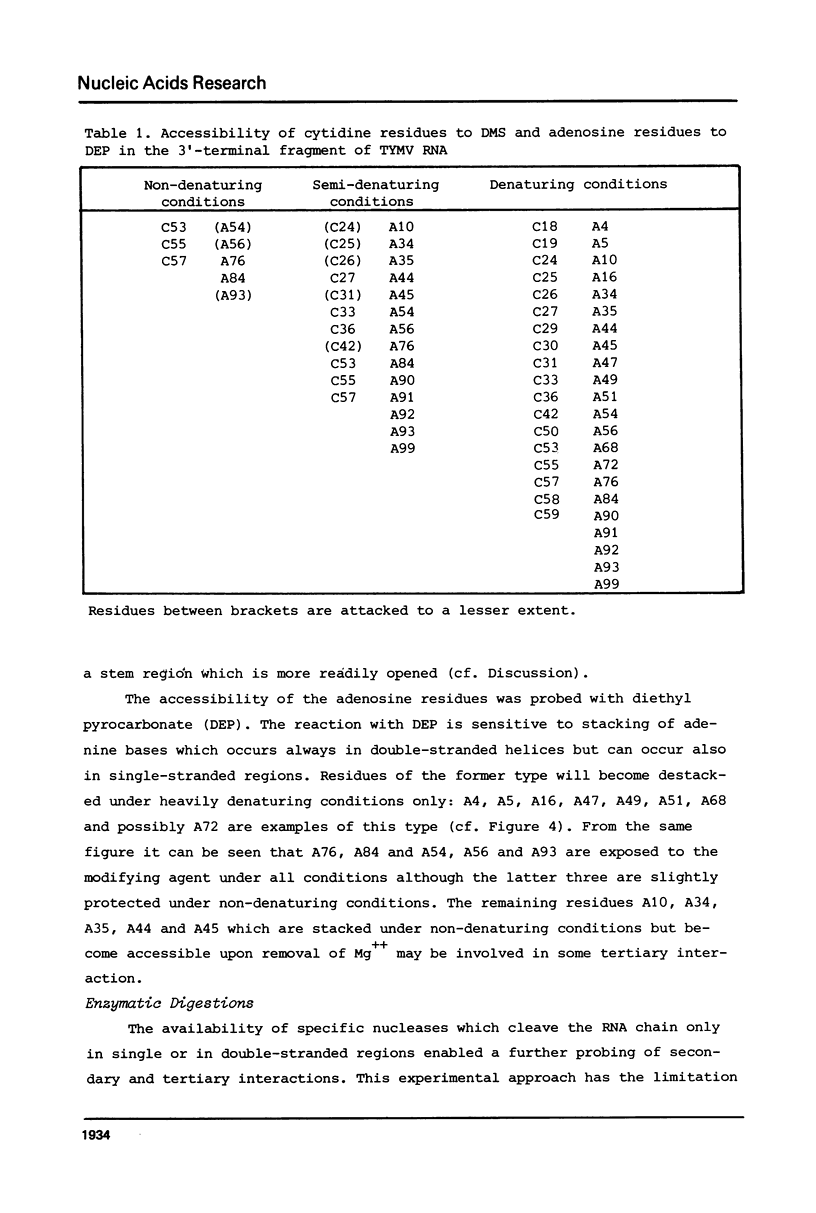
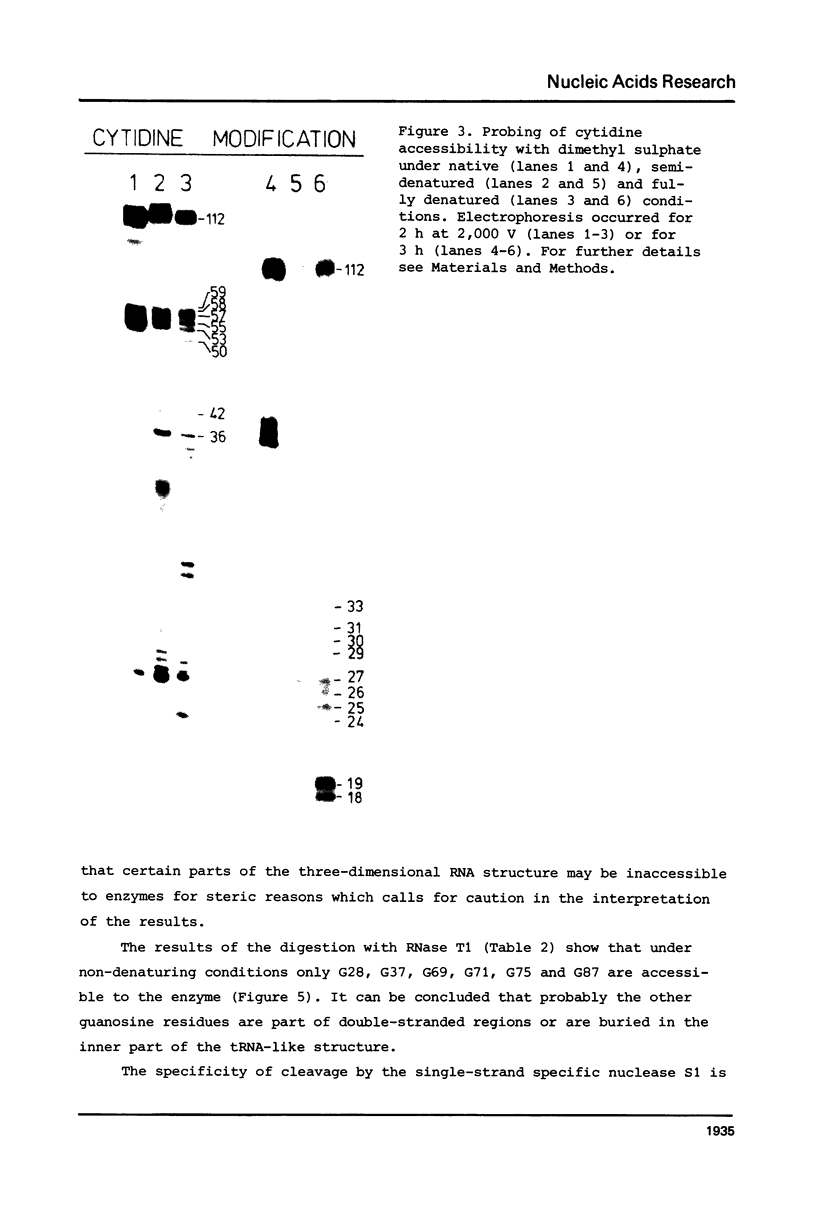
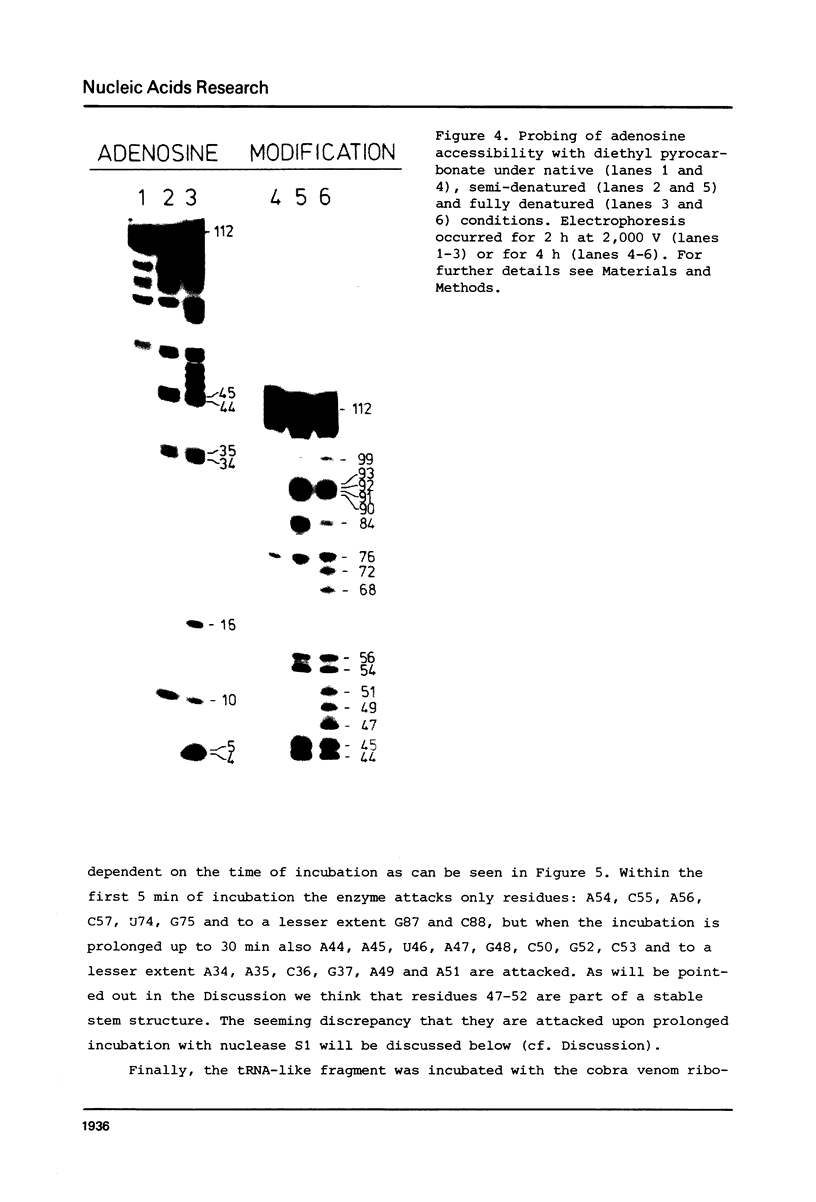







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M., Bruening G., Israel H. W. A genetic map for the cowpea strain on TMV. Virology. 1976 Sep;73(2):498–507. doi: 10.1016/0042-6822(76)90411-6. [DOI] [PubMed] [Google Scholar]
- Bosch L., Bonnet-Smits E. M., van Duin J. In situ breakage of turnip yellow mosaic virus RNA and in situ aggregation of the fragments. Virology. 1967 Mar;31(3):453–460. doi: 10.1016/0042-6822(67)90226-7. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Richards K. E., Bouley J. P., Witz J., Hirth L. Stucture of the amino-acid accepting 3'-end of high-molecular-weight eggplant mosaic virus RNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):737–741. doi: 10.1073/pnas.73.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butorin A. S., Remy P., Ebel J. P., Vassilenko S. K. Comparison of the hydrolysis patterns of several tRNAs by cobra venom ribonuclease in different steps of the aminoacylation reaction. Eur J Biochem. 1982 Jan;121(3):587–595. doi: 10.1111/j.1432-1033.1982.tb05827.x. [DOI] [PubMed] [Google Scholar]
- DUNN D. B., HITCHBORN J. H. THE USE OF BENTONITE IN THE PURIFICATION OF PLANT VIRUSES. Virology. 1965 Feb;25:171–192. doi: 10.1016/0042-6822(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorova O. O., Fasiolo F., Keith G., Vassilenko S. K., Ebel J. P. Partial digestion of tRNA--aminoacyl-tRNA synthetase complexes with cobra venom ribonuclease. Biochemistry. 1981 Feb 17;20(4):1006–1011. doi: 10.1021/bi00507a055. [DOI] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981 Oct 10;9(19):5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Iba H., Okada Y. Nucleotide sequence of a cloned cDNA copy of TMV (cowpea strain) RNA, including the assembly origin, the coat protein cistron, and the 3' non-coding region. Mol Gen Genet. 1981;184(1):20–25. doi: 10.1007/BF00271189. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinck M., Yot P., Chapeville F., Duranton H. M. Enzymatic binding of valine to the 3' end of TYMV-RNA. Nature. 1970 Jun 6;226(5249):954–956. doi: 10.1038/226954a0. [DOI] [PubMed] [Google Scholar]
- Ponomareva R. B., Kavunenko T. N., Kalatcheva T. N., Tikhomirova-Sidorova N. S., Samsonov G. V. [Modification of pancreatic ribonuclease activity in complexes with polyanions]. Biokhimiia. 1975 May-Jun;40(3):468–475. [PubMed] [Google Scholar]
- Renaud M., Bacha H., Dietrich A., Remy P., Ebel J. P. Study of the interaction between yeast tRNAphe and yeast phenylalanyl-tRNA synthetase by monochromatic ultraviolet irradiation at various wavelengths. Advantages and limits of the method. Biochim Biophys Acta. 1981 Apr 27;653(2):145–159. doi: 10.1016/0005-2787(81)90151-9. [DOI] [PubMed] [Google Scholar]
- Renaud M., Dietrich A., Giegé R., Remy P., Ebel J. P. Interaction between yeast tRNAVal and yeast valyl-tRNA synthetase studied by monochromatic-ultraviolet-light-induced cross-linking. Eur J Biochem. 1979 Nov;101(2):475–483. doi: 10.1111/j.1432-1033.1979.tb19742.x. [DOI] [PubMed] [Google Scholar]
- Rether B., Bonnet J., Ebel J. P. Studies on tRNA nucleotidyltransferase from baker's yeast. 1. Purification of the enzyme. Protection against thermal inactivation and inhibition by several substrates. Eur J Biochem. 1974 Dec 16;50(1):281–288. doi: 10.1111/j.1432-1033.1974.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Rosa J. J., Rosa M. D., Sigler P. B. Photocross-linking analysis of the contact surface of tRNA Met in complexes with Escherichia coli methionine:tRNA ligase. Biochemistry. 1979 Feb 20;18(4):637–647. doi: 10.1021/bi00571a014. [DOI] [PubMed] [Google Scholar]
- Stepanova O. B., Metelev V. G., Chichkova N. V., Smirnov V. D., Rodionova N. P., Atabekov J. G., Bogdanov A. A., Shabarova Z. A. Addressed fragmentation of RNA Molecules. FEBS Lett. 1979 Jul 1;103(1):197–199. doi: 10.1016/0014-5793(79)81280-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wrede P., Wurst R., Vournakis J., Rich A. Conformational changes of yeast tRNAPhe and E. coli tRNA2Glu as indicated by different nuclease digestion patterns. J Biol Chem. 1979 Oct 10;254(19):9608–9616. [PubMed] [Google Scholar]
- Yot P., Pinck M., Haenni A. L., Duranton H. M., Chapeville F. Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1345–1352. doi: 10.1073/pnas.67.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]