Abstract
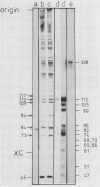
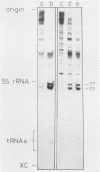
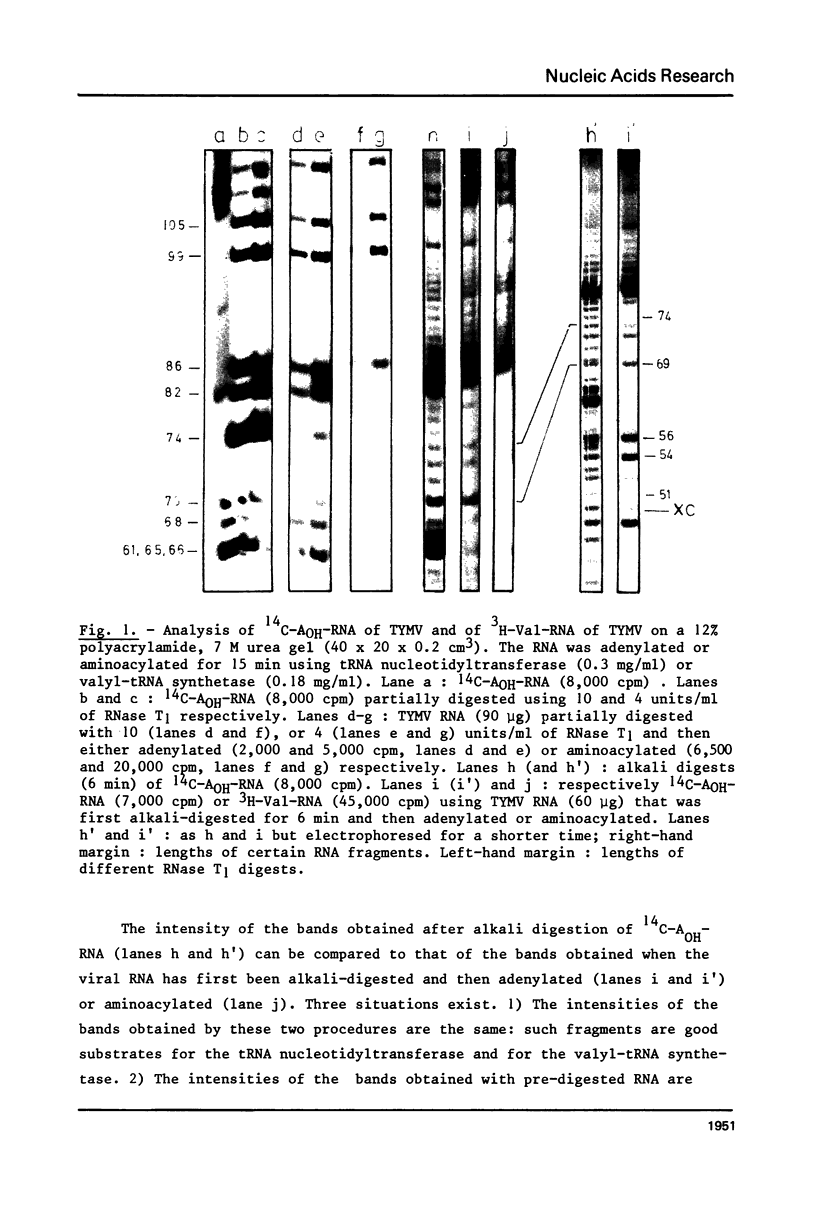
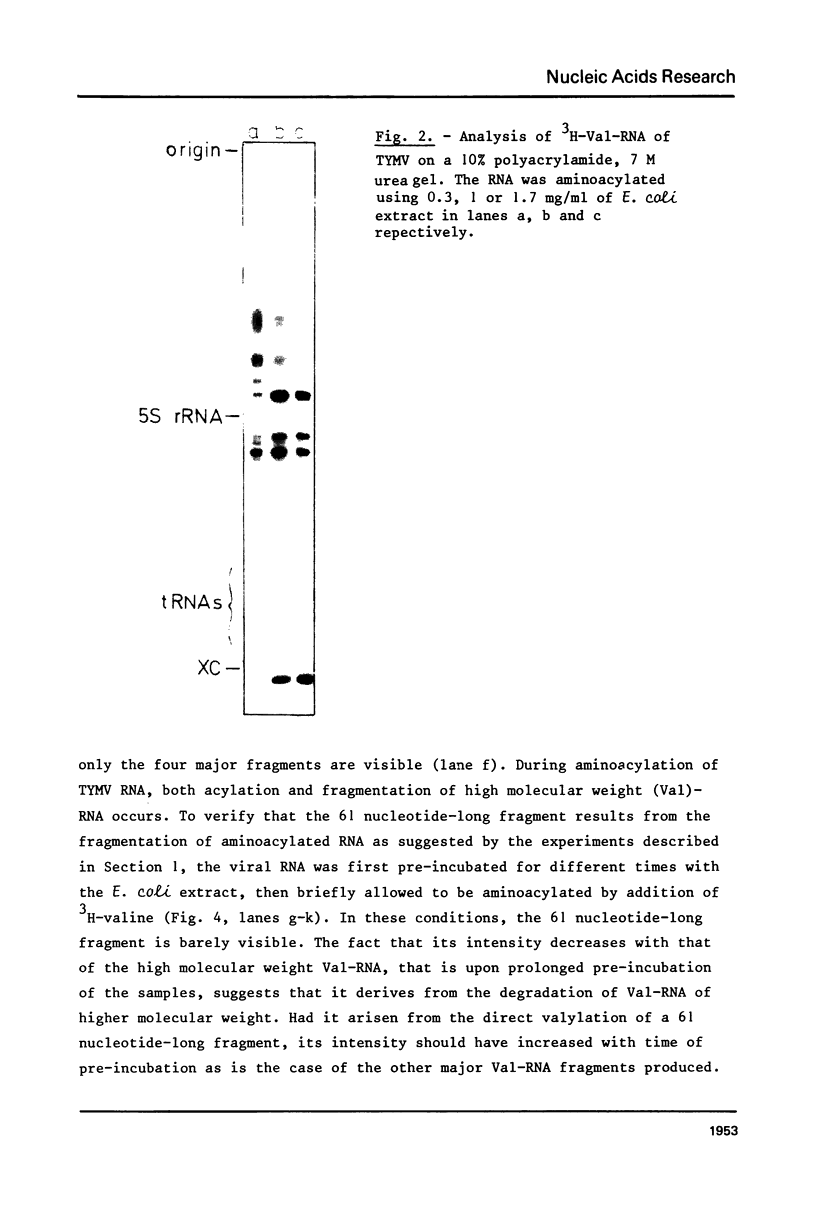
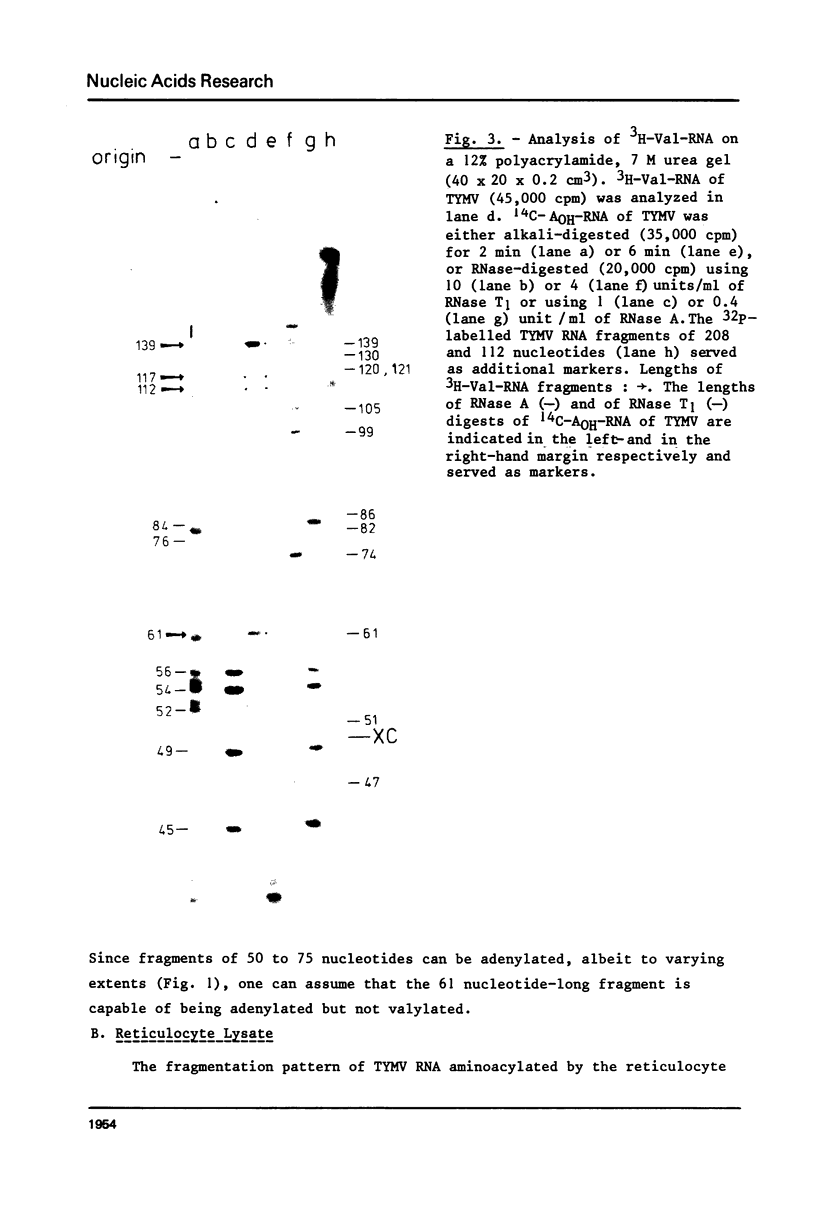
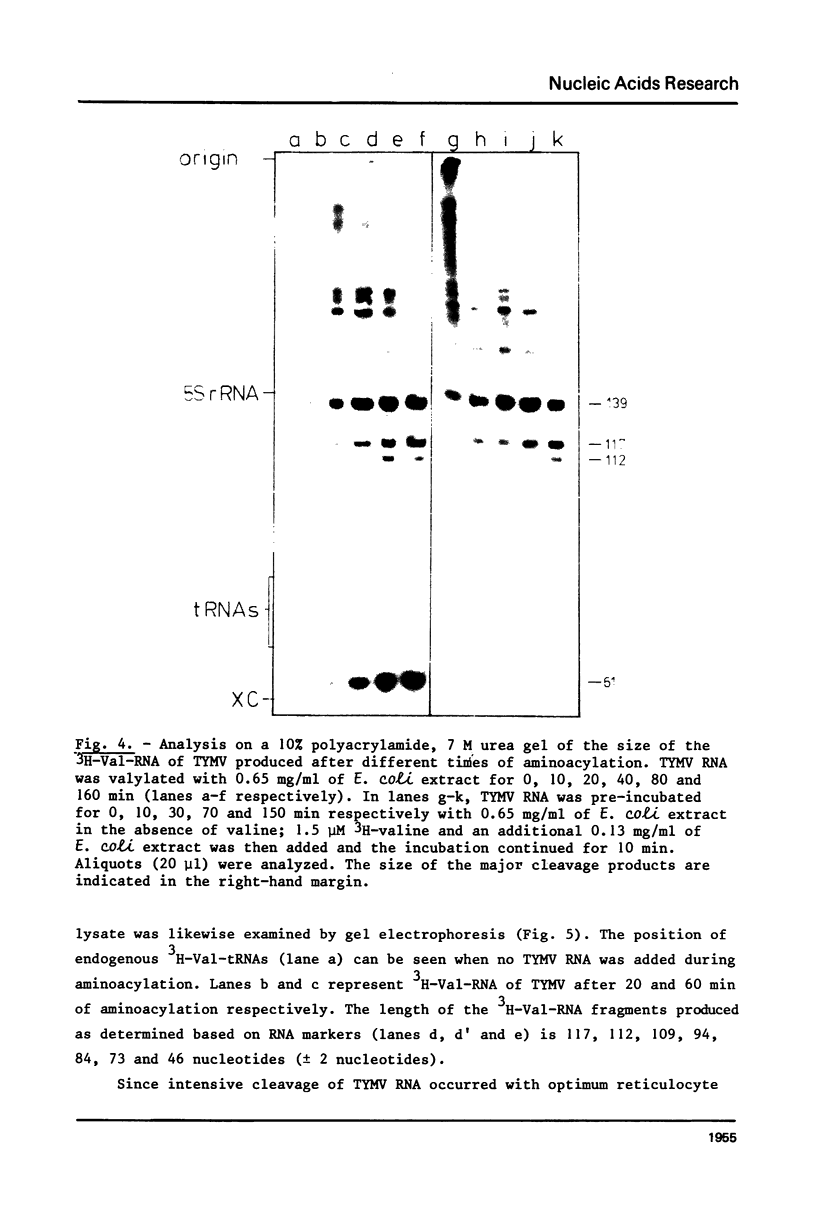
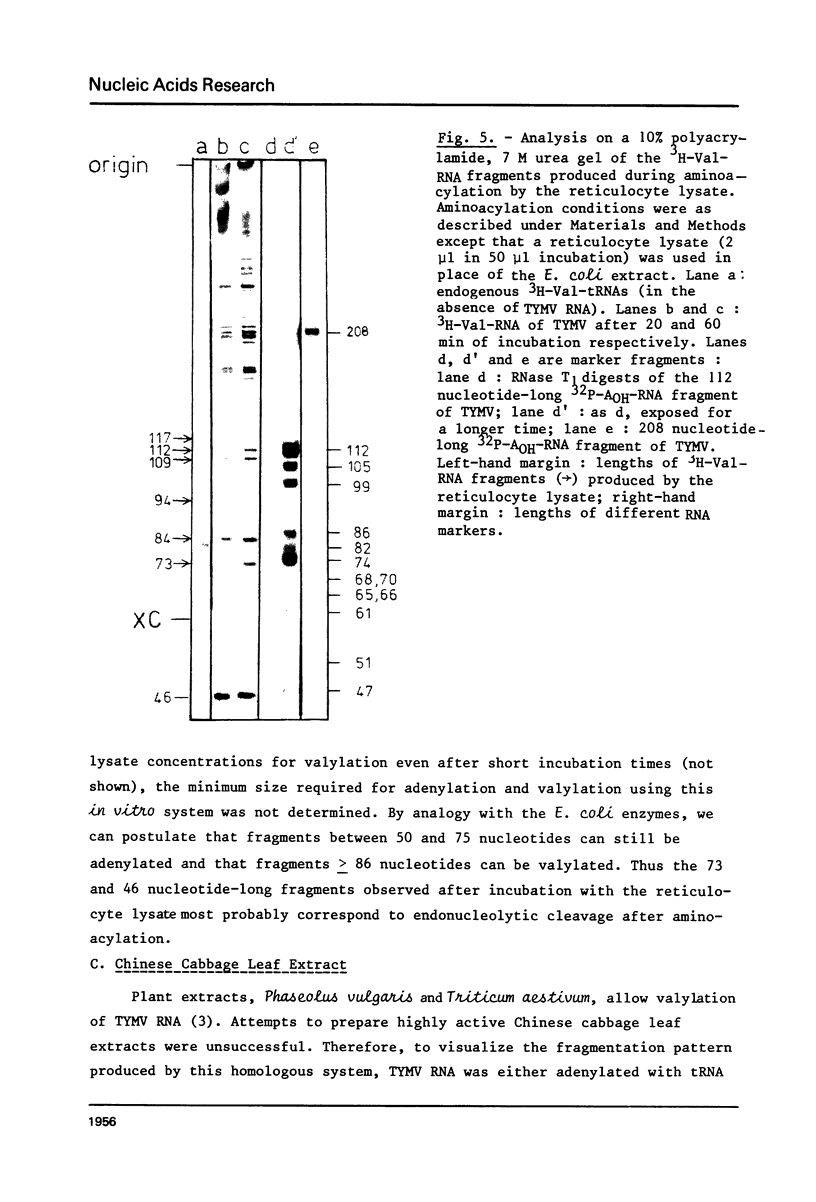
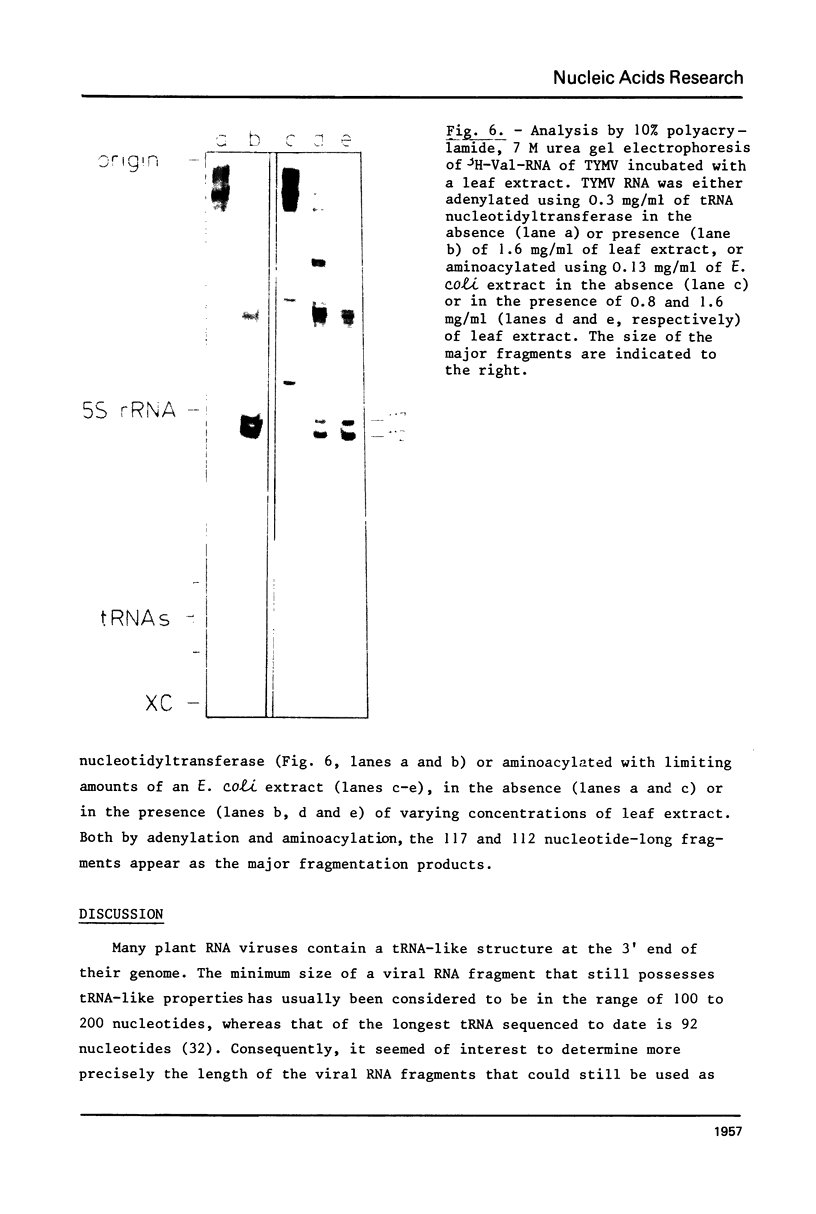
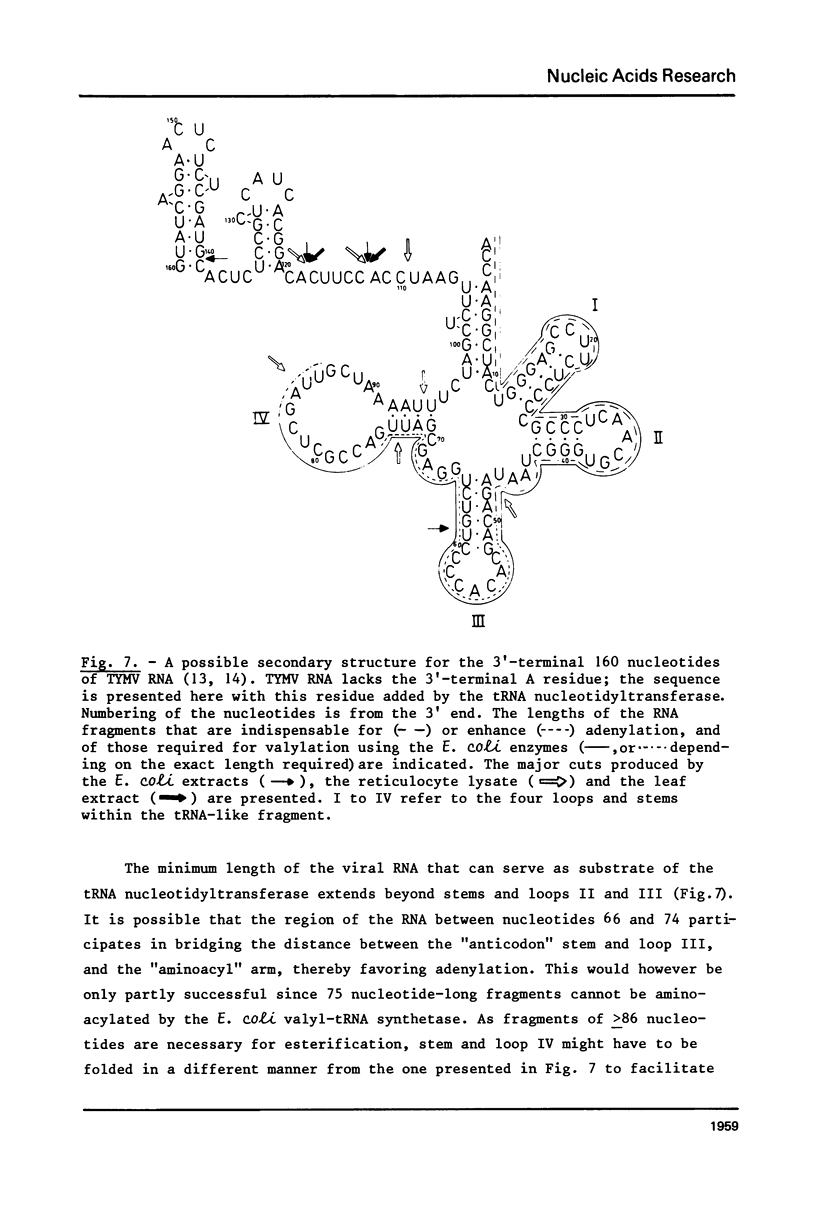
This paper describes the minimum length of the turnip yellow mosaic virus (TYMV) RNA necessary to fulfill the tRNA-like properties of the viral RNA: 50 to 75 nucleotides and 86 nucleotides from the 3' end of TYMV RNA are sufficient for adenylation and valylation respectively by the Escherichia coli system. The size of the tRNA-like fragments obtained in vitro in the presence of an E. coli, a reticulocyte or a chinese cabbage leaf extract has also been determined. Among the major fragments liberated from the 3' end of TYMV RNA by the three systems are fragments of 117 and 112 nucleotides. In addition, the E. coli extract liberates fragments of 139 and 61 nucleotides, and the reticulocyte lysate fragments of 109, 94, 84, 73 and 46 nucleotides. The cleavage of the viral RNA by several systems in vitro to yield RNA fragments encompassing the tRNA-like sequence suggests that such fragments might also be liberated in vivo.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastin M., Dasgupta R., Hall T. C., Kaesberg P. Similarity in structure and function of the 3'-terminal region of the four brome mosaic viral RNAs. J Mol Biol. 1976 Jun 5;103(4):737–745. doi: 10.1016/0022-2836(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M., Bruening G., Israel H. W. A genetic map for the cowpea strain on TMV. Virology. 1976 Sep;73(2):498–507. doi: 10.1016/0042-6822(76)90411-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Ebel J. P., Shershneva L. P., Krutilina A. I., Venkstern T. V., Bayev A. A., Dirheirmer G. The corrected nucleotide sequence of valine tRNA from baker's yeast. Biochimie. 1974;56(9):1211–1213. doi: 10.1016/s0300-9084(74)80013-1. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Carre D. S., Litvak S., Chapeville F. Purification and properties of Escherichia coli CTP (ATP)-tRNA nucleotidyltransferase. Biochim Biophys Acta. 1970 Dec 14;224(2):371–381. doi: 10.1016/0005-2787(70)90570-8. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Roe B. A. Sequence studies on human placenta tRNAVal : comparison with the mouse myeloma tRNAVal. Biochem Biophys Res Commun. 1977 Sep 23;78(2):631–640. doi: 10.1016/0006-291x(77)90226-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Kaesberg P. Sequence of an oligonucleotide derived from the 3' end of each of the four brome mosaic viral RNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4900–4904. doi: 10.1073/pnas.74.11.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1981 Jan 10;9(1):r1–23. [PMC free article] [PubMed] [Google Scholar]
- Giegé R., Briand J. P., Mengual R., Ebel J. P., Hirth L. Valylation of the two RNA components of turnip-yellow mosaic virus and specificity of the tRNA aminoacylation reaction. Eur J Biochem. 1978 Mar;84(1):251–256. doi: 10.1111/j.1432-1033.1978.tb12163.x. [DOI] [PubMed] [Google Scholar]
- Guilley H., Briand J. P. Nucleotide sequence of turnip yellow mosaic virus coat protein mRNA. Cell. 1978 Sep;15(1):113–122. doi: 10.1016/0092-8674(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Joshi S., Haenni A. L. Fluorographic detection of nucleic acids labelled with weak beta-emitters in gels containing high acrylamide concentrations. FEBS Lett. 1980 Aug 25;118(1):43–46. doi: 10.1016/0014-5793(80)81214-2. [DOI] [PubMed] [Google Scholar]
- Joshi S., Haenni A. L., Hubert E., Huez G., Marbaix G. In vivo aminoacylation and 'processing' of turnip yellow mosaic virus RNA in Xenopus laevis oocytes. Nature. 1978 Sep 28;275(5678):339–341. doi: 10.1038/275339a0. [DOI] [PubMed] [Google Scholar]
- Kohl R. J., Hall T. C. Aminoacylation of RNA from several viruses: amino acid specificity and differential activity of plant, yeast and bacterial synthetases. J Gen Virol. 1974 Nov;25(2):257–261. doi: 10.1099/0022-1317-25-2-257. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Litvak S., Carr D. S., Chapeville F. TYMV RNA As a substrate of the tRNA nucleotidyltransferase. FEBS Lett. 1970 Dec 18;11(5):316–319. doi: 10.1016/0014-5793(70)80557-9. [DOI] [PubMed] [Google Scholar]
- Litvak S., Tarragó A., Tarragó-Litvak L., Allende J. E. Elongation factor-viral genome interaction dependent on the aminoacylation of TYMV and TMV RNAs. Nat New Biol. 1973 Jan 17;241(107):88–90. doi: 10.1038/newbio241088a0. [DOI] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Iba H., Okada Y. Nucleotide sequence of a cloned cDNA copy of TMV (cowpea strain) RNA, including the assembly origin, the coat protein cistron, and the 3' non-coding region. Mol Gen Genet. 1981;184(1):20–25. doi: 10.1007/BF00271189. [DOI] [PubMed] [Google Scholar]
- Overath H., Fittler F., Harbers K., Thiebe R., Zachau H. G. Cytidylic and adenylic acid incorporation into fragments of tRNA. FEBS Lett. 1970 Dec 11;11(4):289–294. doi: 10.1016/0014-5793(70)80551-8. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pinck M., Yot P., Chapeville F., Duranton H. M. Enzymatic binding of valine to the 3' end of TYMV-RNA. Nature. 1970 Jun 6;226(5249):954–956. doi: 10.1038/226954a0. [DOI] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., Haenni A. L. TYMV RNA as a substrate of tRNA maturation endonuclease. Nat New Biol. 1973 Feb 7;241(110):168–170. doi: 10.1038/newbio241168a0. [DOI] [PubMed] [Google Scholar]
- Renaud M., Ehrlich R., Bonnet J., Remy P. Lack of correlation between affinity of the tRNA for the aminoacyl-tRNA synthetase and aminoacylation capacity as studied with modified tRNAPhe. Eur J Biochem. 1979 Oct;100(1):157–164. doi: 10.1111/j.1432-1033.1979.tb02044.x. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Wevers W. F., Baguley B. C., Ralph R. K. Comparison of transfer RNA and aminoacyl RNA ligases from calf liver and spleen. Biochim Biophys Acta. 1966 Sep;123(3):503–509. doi: 10.1016/0005-2787(66)90218-8. [DOI] [PubMed] [Google Scholar]
- Yot P., Pinck M., Haenni A. L., Duranton H. M., Chapeville F. Valine-specific tRNA-like structure in turnip yellow mosaic virus RNA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1345–1352. doi: 10.1073/pnas.67.3.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]