Abstract
This review concerns the effects on vision and the eye of medications prescribed at three phases of treatment for women with early-stage breast cancer (BC): (1) adjuvant cytotoxic chemotherapy, (2) adjuvant endocrine therapy, and (3) symptomatic relief. The most common side effects of cytotoxic chemotherapy are epiphora and ocular surface irritation, which can be caused by any of several different regimens. Most notably, the taxane docetaxel can lead to epiphora by inducing canalicular stenosis. The selective-estrogen-receptor-modulator (SERM) tamoxifen, long the gold-standard adjuvant-endocrine-therapy for women with hormone-receptor-positive BC, increases the risk of posterior subcapsular cataract. Tamoxifen also affects the optic nerve head more often than previously thought, apparently by causing subclinical swelling within the first 2 years of use for women older than ∼50 years. Tamoxifen retinopathy is rare, but it can cause foveal cystoid spaces that are revealed with spectral-domain optical coherence tomography (OCT) and that may increase the risk for macular holes. Tamoxifen often alters the perceived color of flashed lights detected via short-wavelength-sensitive (SWS) cone response isolated psychophysically; these altered perceptions may reflect a neural-response sluggishness that becomes evident at ∼2 years of use. The aromatase inhibitor (AI) anastrozole affects perception similarly, but in an age-dependent manner suggesting that the change of estrogen activity towards lower levels is more important than the low estrogen activity itself. Based on analysis of OCT retinal thickness data, it is likely that anastrozole increases the tractional force between the vitreous and retina. Consequently, AI users, myopic AI users particularly, might be at increased risk for traction-related vision loss. Because bisphosphonates are sometimes prescribed to redress AI-induced bone loss, clinicians should be aware of their potential to cause scleritis and uveitis occasionally. We conclude by suggesting some avenues for future research into the visual and ocular effects of AIs, particularly as relates to assessment of cognitive function.
Keywords: Aromatase inhibitor, Dry eye, Estrogen, Retina, Short-wavelength-sensitive cones, Tamoxifen
INTRODUCTION
This review will discuss the effects on vision and the eye of medications commonly prescribed for women diagnosed with early-stage breast cancer (BC). After this introduction, the text will be divided into three main sections, each corresponding to a different treatment phase: (1) adjuvant chemotherapy administered for months shortly after a BC diagnosis and surgery, (2) adjuvant endocrine therapy used thereafter for years in order to reduce the risk of BC recurrence, and (3) supplemental medication used as needed to alleviate or counteract the side effects of the BC medications. A final concluding section suggests directions for future research. This review will focus on medications that appear to affect the visual system in more than rare and isolated cases and are part of the standard-of-care armamentarium for early-stage BC. The term “adjuvant” denotes treatments used for reducing the risk of disease recurrence.
Because cancer medications interfere with cell growth or proliferation, they tend to be especially toxic to normal tissues: (a) that have a high rate of cellular turnover, (b) that have a high biochemical and/or anatomical vulnerability to a given medication, and (c) that are sufficiently exposed to the medication. This combination of factors probably helps explain why excessive tearing (i.e., epiphora, which involves the interplay between tear production, the ocular surface, and tear drainage)1–3 occurs as a side effect of several BC chemotherapeutic medications.4 Although all side effects are manifestations of chemical toxicities, some side effects - most notably those resulting from the virtual abolition of estrogen synthesis caused by inhibition of the enzyme aromatase5, 6 —might be viewed also as consequences of accelerated aging.7 This view is important because patients’ symptoms or complaints (e.g., increased floaters) might too often be misattributed to natural aging rather than to treatment effects.
As detailed later, two distinct classes of adjuvant endocrine medications are now widely prescribed expressly for women whose BC was identified as hormone-receptor-positive.8 Selective estrogen receptor modulators (SERMs) act against BC by occupying estrogen receptors (ERs), while aromatase inhibitors (AIs) act against BC by interfering with estrogen synthesis.9 We will feature the endocrine-therapy portion of this review for a variety of reasons, the most central being that millions of healthy BC survivors in the foreseeable future are projected to use such medications on an adjuvant basis for years at a time.8, 10–13 Four additional sets of reasons are listed next; each reflects the rapid evolution of basic science and clinical care.
First, overwhelming evidence is accumulating to show that estrogenic activity directly impacts a vast array of physiologic functions beyond those involving reproductive and sexual function.6, 14 Estrogen receptors (ERs) are present throughout the body, including the anterior15–18 and posterior19, 20 portions of the human eye and also in the lacrimal18, 21 and meibomian glands18,22, 23 responsible for protecting the surface of the eye. In fact, ERs are present throughout the central nervous system (CNS),24–27 so changes in estrogenic activity have the potential to affect central visual processing, in addition to ocular visual processing. Moreover, because estrogens are synthesized locally throughout the body, much estrogenic activity is autocrine or paracrine, rather than endocrine.28 These autocrine and paracrine estrogenic actions take on increased importance for post-menopausal women, whose estrogen supplies have decreased markedly29
Second, the longstanding standard-of-care for hormone-receptor-positive early-stage BC is changing. That is, use of the SERM tamoxifen (Nolvadex®) as adjuvant endocrine therapy is being supplanted by the use of AIs such as anastrozole (Arimidex®) or letrozole (Femara®) for women who are post-menopausal.30 Because the traditional 5-year period of adjuvant endocrine therapy was based on studies involving only tamoxifen,31 this period may be lengthened in the future, depending on the outcomes of ongoing clinical trials with AIs.31, 32 These changes in adjuvant endocrine therapy raise concerns for long-term eye health, since a prolonged period of sustained estrogen deprivation has the potential to increase the risk or severity of several age-related eye diseases or conditions, including glaucoma33–37 and macular degeneration.38–41 Moreover, because AIs are notorious for reducing bone density42, 43 any visual dysfunction- no matter how subtle- that raises the risk of falling will consequently raise the risk of fracture, even in the short-term.44 This cascade of events may lead to potentially devastating outcomes among at-risk BC survivors.
Third, because adjuvant endocrine medications are self-administered orally on a daily basis, they often are considered by patients to be more elective than the preceding chemotherapy45 which is administered intravenously in a clinical setting. Thus, adherence to adjuvant endocrine therapy often ends prematurely or becomes sub-optimal for any of a variety of reasons,46, 47 including the patient's perceived quality-of-life / side-effect tradeoffs.48, 49 The net consequence is that real-world BC recurrence rates exceed those reported in clinical trials.50 This set of events is especially unfortunate since greater side-effect severity signifies a better chance of BC non-recurrence,5, 51 probably because the severity of AI-induced side effects corresponds to the degree of biologically relevant estrogen suppression.51 Thus, the more reliably side effects can be recognized by clinicians and hence addressed, the better patients’ cancer outcomes are likely to be.
And fourth, the recent wide use of AIs provides a novel means for evaluating the ways by which estrogen may support healthy eyes and normal vision. Many women diagnosed with early-stage BC or with precancerous conditions such as ductal carcinoma in situ (DCIS) or lobular carcinoma in situ (LCIS) will use AIs but never receive cytotoxic chemotherapy,52, 53 so these women comprise a minimally confounded cohort for short- and long-term studies of estrogen deprivation in adults. Parallel studies can be conducted using animal models.
CHEMOTHERAPEUTIC AGENTS USED SHORTLY AFTER BC DIAGNOSIS
Although it would be desirable to specify the frequencies with which ocular side effects such as “dry eye” (also called keratoconjuncrivitis sicca) result from any individual medication, several factors combine to make such estimates uncertain. First, cytotoxic chemotherapeutic agents rarely are administered entirely independently of one another. Second, toxicities typically are cumulative-dose-dependent, and side effects need not manifest immediately following the most recent treatment. Third, the physiologic responses to treatment can differ for individual patients. In fact, the recognition and prospective identification of salient individual differences provides the basis for “personalized medicine", which is being implemented in the clinic at an accelerated pace owing for a variety of scientific and technological advances.54, 55 And fourth, the frequency with which symptoms are reported depends greatly on the expectations and communications among providers, investigators, and patients.56, 57 No studies appear to have been conducted wherein validated survey tools (e.g., the Ocular Surface Disease Index (OSDI)58) have been used for assessing ocular-surface discomfort stemming from chemotherapy, for example. Neither do there appear to be any epidemiologic studies addressing the relation of chemotherapy to dry eye.
Bear in mind that the term dry eye is somewhat of a misnomer in the sense that the eyes of people with dry eye can be quite watery, typically because tears are produced reflexively to counteract the ocular surface discomfort.59 However, occlusion of the tear drainage apparatus may contribute occasionally to ocular irritation,1, 60 and it can allow toxic agents to remain in contact longer with the ocular surface. Dry eye syndrome is diagnosed largely according to the presence of subjective symptoms of discomfort, such as a “gritty” sensation,61 and it occurs most often among post-menopausal women,3,62 who coincidently are the people most likely to develop BC.63
As discussed next, epiphora and ocular surface discomfort may result from several different cytotoxic chemotherapy regimens, and as discussed later, there is reason to hypothesize that AI usage may contribute to dry eye. In addition to being locally irritating or otherwise bothersome,64 epiphora may cause the tear film layer to become asymmetric (thickest at the inferior margin of the pupil), leading to coma-like aberrations and decreased optical quality (vertical “comet tails") after blinking.65
From about 1990 until quite recently, the most common chemotherapeutic regimen for early-stage BC consisted of a 2-drug combination (an anthracycline plus cyclo-phosphamide) administered intravenously four times over a period of ∼2 months.66 Because the anthracycline used most often is doxorubicin (Adriamycin®), this treatment usually is referred to as"AC” chemotherapy. Anthracyclines (also including epirubicin) and cyclo-phosphamide (Cytoxan®) each interfere with DNA replication via multiple mechanisms. A prominent effect of the topoisomerase-poison doxorubicin is to intercalate DNA,67 while the alkylating-agent cyclophosphamide is a prodrug that after hepatic conversion leads to cross-linkages between DNA strands.68,69 The package insert for doxorubicin states that “conjunctivitis, keratitis, and lacrimation occur rarely", and while dry eye apparently due to treatment with cyclophosphamide has been reported for some non-BC patients,70 the package insert makes no mention of ocular or visual effects. Although at least several secondary sources cite articles reporting doxorubicin to cause watery eyes or conjunctivitis in 25% of users, the strongest statement we could locate in these earlier articles was by Blum,71 who commented “… some patients report increased lacrimation…”
The standard of care for early-stage BC is changing, in that taxanes now often are included in the chemotherapy regimen.72 Taxanes act against BC by stabilizing microrubules, thereby inhibiting mitosis.73,74 Two different taxanes- docetaxel (Taxotere®) and paclitaxel (Taxol®)- have been FDA-approved as treatments for early-stage BC, and a 4-cycle Taxotere / Cytoxan ["TC"] regimen with docetaxel has begun to replace the 4-cycle AC regimen. This change follows the 2009 publication of results from a clinical trial directly comparing the two regimens.75 In practice, paciltaxel tends to be used in sequential regimens, e.g., with AC administered first.76, 77 Docetaxel may be administered on either a weekly or, more commonly, a tri-weekly (i.e., once per three weeks) schedule,78 but the tri-weekly schedule with co-administration of cyclophosphamide is the most common regimen for early-stage BC. Docetaxel may also be given in various other regimens.79–81
There is abundant evidence showing that docetaxel often leads to epiphora.64,82 In contrast, there is limited evidence showing that paclitaxel leads to epiphora,83 and this limited evidence is disputed as being artifactual.84 Docetaxel can be present in tears,85 and it leads to epiphora mainly by causing canalicular stenosis in the lacrimal drainage apparatus.86 The severity and frequency of epiphora is less with the tri-weekly dosing schedule,87 but since published studies used the longer treatment regimens for metastaric BC,87–89 reported frequencies of epiphora (as high as ∼40% for tri-weekly treatments vs. ∼65% for weekly treatments)87 would overestimate the corresponding frequencies for early-stage (or shorter)89 treatment regimens. Based on our clinical experience (author SWL), about 1-2% of early-stage BC patients spontaneously report experiencing epiphora by the end of a 4-cycle TC regimen. We expect the percentage would be higher if patients were queried.
The taxanes, perhaps more so paclitaxel,90, 91 are notorious for causing peripheral sensory neuropathies,92 which arise at least partly from the ability of taxanes to stabilize microrubules in neurons93 not sufficiently protected by the blood / brain barrier.94 If this barrier is compromised, taxanes might affect visual function directly. Paclitaxel has been reported to alter elecrroretinographic and pattern-visual-evoked potentials,95 and possibly optic nerve response.96 There are several case reports of taxane-associated cystoid macular edema occurring in the absence of visible angiographic leakage.97–102
The first standard chemotherapeutic regimen for early-stage BC, still in use today, consists of a “CMF” drug triad of cyclophosphamide, methotrexate, and 5-fluorouracil [5-FU].103 The anti-metabolites methotrexate (an anti-folate)104 and 5-FU105 each interfere with DNA replication and RNA synthesis via multiple mechanisms, with each drug acting independently to inhibit thymidine synthesis. Although the anti-cancer actions of docetaxel and 5-FU are distinct, 5-FU can cause canalicular stenosis at least occasionally,106–110 suggesting an inherent vulnerability of the ocular drainage apparatus. Epiphora occurs in ∼25% or more of patients administered 5-FU,110–112 and several studies have reported systemically administered 5-FU to be present in tears.111,113 There are anecdotal reports of low doses of methotrexate leading to ocular surface inflammation,114 and based on clinical trial results, conjunctivitis occurs for a higher percentage of BC survivors on a CMF regimen than on an FAC regimen115 (i.e., conjunctivitis occurs more often when methotrexate is used in place of doxorubicin).
About 15-25% of early-stage BC patients have tumor cells that test positive for the overexpression of Human Epidermal growth factor Receptor 2 (HER2),116–118 a cell membrane protein that modulates many signaling pathways important for cell growth and proliferation.119,120 Trastuzamab (Herceptin®), a HER2-binding monoclonal antibody with multiple mechanisms of anti-cancer action,121 was FDA-approved in 2006 for early-stage BC, and it is now a mainstay of treatment for early-stage BC patients whose tumors test positive for HER2.122 Trasruzamab may be prescribed for early-stage BC in any of several chemotherapeutic combinations, which may involve periodic administration of trastuzamab for up to a year.123 In addition to the drugs mentioned in the preceding paragraphs, trastuzamab-containing chemotherapeutic regimens may include the platinum-based DNA adduct carboplatin.124 Trastuzamab apparently can cause conjunctivitis in a small percentage of patients.125 We can find no evidence for carboplatin affecting the eye or vision at the doses used for early-stage BC.
We conclude this section by noting: (1) that a large but somewhat controversial literature is emerging regarding the ability of cytotoxic (and endocrine) chemotherapies to induce cognitive changes collectively termed chemobrain (or chemofog),126–130 (2) that all cognitive testing batteries rely on sensory (visual and / or auditory) input to subjects or patients, and (3) that testing batteries typically include tests involving higher-order visual information processing or retrieval capabilities.131, 132 Details are outside the scope of this review.
ADJUVANT ENDOCRINE THERAPY
As mentioned earlier, two classes of drugs- SERMs and AIs- are widely used as adjuvant endocrine therapy for women with hormone-receptor-positive early-stage BC.8, 9 The classical view is that by competitively occupying ERs, SERMs act as (a) ER-agonists or (b) ER-antagonists according to whether the SERM stimulates the ER or instead does not stimulate the ER; in the latter case the ER is prevented from functioning appropriately as it would if stimulated by an estrogen.133 The SERMs used against BC act as ER-antagonists in breast tissue,134,135 at least for the first several years of treatment,136, 137 and the mainstay SERM tamoxifen reduces the risk of BC recurrence for women of all ages,138 and also for men.139 The ability of SERMs to function as ER-antagonists in some tissues but as ER-agonists in other tissues depends upon many factors, including the tissue-dependent distribution of the ERα and ERβ subtypes,137,140 and even on the activity of HER2.141 ERα and ERβ each are present within the neural retina of men and women, with ERα apparently distributed more uniformly but exhibiting greater interpersonal variation.20 ERα and ERα are present also within the pigment epithelium of men and women,19 where tamoxifen has been reported to decrease glutamate uptake, for example.142 Less is known about the presence of ERs within portions of the visual system beyond the eye, and effects of SERMs on ERα vs. ERβ activity are yet to be adequately delineated for any level of the visual system.
AIs act entirely differently. By interfering with the actions of the enzyme aromatase, which catalyzes the conversions of androgens to estrogens,6,28 AIs almost completely abolish estrogen synthesis at its sources for women who are post-menopausal, whether naturally or surgically.143 By themselves, AIs are not effective for use in pre-menopausal women because the initial reduction in ovarian estrogen synthesis triggers feedback loops in the hypothalamic-pituitary-gonadal axis that serve to relieve this reduction.144 However, several clinical trials are underway to determine whether using an AI concomitantly with a gonadotrophin-releasing hormone (GnRH) agonist to disrupt this feedback system might reduce the risk of recurrence for pre-menopausal women with early-stage BC.145 Although male BC patients sometimes are prescribed AIs off-label, circulating estrogen levels may be reduced only incompletely for men with testicular function.146 Overall, the information regarding the use of AIs for male BC is quite limited.147–149
ERs may function genomically or non-genomically according to whether the ERs are present within a cell or on the cell membrane, and they may act over long (e.g., hours to days) or short (e.g., msec to sec) times-cales, respectively.27, 150–152 Thus, ER-dependent effects of SERMS and of AIs on vision may be mediated by either genomic or non-genomic means. However, none of the effects described in the subsequent text can be assigned confidently to one means or the other, and of course, some effects may occur indirectly, e.g. via alterations in blood flow.153 Moreover, several of the ocular effects in particular probably involve collateral or separate drug actions; these are identified in the text.
Before proceeding, we note that the next two sections (on the SERM tamoxifen, and on AIs) each contain some previously unpublished data from human subjects. All these data were obtained using protocols approved by the OHSU Institutional Review Board and the OHSU Cancer Institute, and all protocols were conducted in accordance with the tenets of the Declaration of Helsinki.
The SERM tamoxifen
Three different SERMs- tamoxifen, raloxifene (Evista®), and toremifene (Fareston®)- are used to help prevent or treat BC.135 However, tamoxifen is the only SERM FDA-approved as adjuvant therapy for early-stage BC, and it is the only SERM approved for every BC stage, from the prophylactic through the metastaric settings. A fourth SERM, clomiphene (Clomid®) has long served as the standard-of-care fertility drug for inducing ovulation,154 and tamoxifen was used for this purpose before receiving approval as a BC treatment.155 Clomiphene sometimes leads to perceptible vision changes,156,157 especially when the dose is increased by a factor of up to four from the customary 50 mg / day starting dose, to achieve pregnancy.158 Known visual disturbances with clomiphene can include palinopsia (a prolonged afterimage, often with a trailer),156,157 photopsia (entoptic flashing lights),158 and scotomas.159 In addition, temporal resolution may be reduced slightly.156 Tamoxifen, clomiphene, and toremifene each have triphenylethylene structures, while raloxifene has a benzothiophene structure.135 The biochemical similarity of tamoxifen with clomiphene coupled with the exceedingly long ER-binding times for clomiphene160 raise the possibility that some of the striking effects of clomiphene may be related to visual or ocular effects of tamoxifen that are more subtle or infrequent.
Tamoxifen is mainly a prodrug in the sense that two of its many metabolites, notably 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) and also 4-hydroxytamoxifen (4-OHT), now are known to have much greater affinity for ERs than does tamoxifen itself.161,162 The serum concentration of 4-OHT normally is even lower than that of endoxifen, so endoxifen is considered the most important metabolite.161 To the best of our knowledge, only one study has related visual-system side effects to assessment of any tamoxfien metabolites. Gallicchio et al.163 found that 13 of 97 tamoxifen users self-reported unspecified vision problems and that these 13 women had significantly higher serum concentrations of tamoxifen and N-desmethyl-tamoxifen (N-DMT) than did the 84 women not reporting vision problems. N-DMT is a precursor metabolite that is hydroxylated to endoxifen via action of the enzyme CYP2D6.161 Gallicchio et al.163 did not measure endoxifen levels. CYP2D6 activity varies greatly among individuals depending upon which CYP2D6 alleles a person has,164 and this factor with other genetic information are now considered in the development of “personalized” treatment regimens.54, 55, 164 HER2 status and hormone-receptor status also are factors.
(CYP2D6 is a member of the cytochrome P450 superfamily of heme-containing membrane proteins responsible for the hepatic metabolism of the large majority of clinically active drugs.165 See also the Redressing Side Effects section for information on CYP2D6 specifically.)
When tamoxifen was first prescribed in the late 1970s as a treatment for advanced BC, the doses were many times higher than they are now, and several case studies were published in the early 1980s concerning tamoxifen retinopathy.166, 167 Tamoxifen retinopathy classically is characterized by the presence of small crystalline deposits that may occur in the nerve fiber and inner plexiform layers near the fovea, sometimes accompanied by edema.168,169 Although tamoxifen retinopathy typically is considered to depend on total cumulative dose,168 spectral-domain optical coherence tomography (OCT) can reveal foveal cystoid spaces within only a year or two of the start of tamoxifen use for some patients on contemporary dosing levels,170 which are 20 mg / day.
The reported prevalence rates of tamoxifen retinopathy for BC survivors using standard doses vary substantially between studies, from less than 1%171,172 to about 6%,173 with estimates from additional studies falling within this range.174–176 This variation may be in part due to the uncertainty and / or subjectivity in detecting tamoxifen retinopathy photographically,169 particularly since only a few isolated crystals typically are found, and the presence of age-related changes at the fundus can make interpretation difficult.168, 175, 176 It is also possible that some yet undetermined factors cause incidence rates to vary across diverse populations. No subjects with tamoxifen retinopathy were identified in the 98 sets of fundus photographs evaluated for author AE's studies (2 eyes per subject, 74 of the subjects were at least 6 months amenorrheic).177 The subjects in Eisner's studies178–184 were selected for excellent visual acuity and normal reading vision, however, which could have contributed to the observed zero prevalence rate.
In our view, the initial findings of tamoxifen retinopathy at an auspicious time in the evolution of BC treatment have led to an overemphasis on this condition in the sense that vision symptoms (e.g., photopsia) due to other intraocular conditions may be too readily misatrributed to tamoxifen retinopathy and / or downplayed. Small numbers of crystals seem not to cause acuity loss, while mild acuity loss accompanying edema can be reversed by withdrawing the tamoxifen.168,173 The development of foveal cystoid spaces might be more serious since it may contribute to the subsequent development of macular holes,185 which are reported to occur in tamoxifen users at a rate about 5 times that of women not using tamoxifen.186 (Most of the tamoxifen users in this study186 were on 20mg / day but a few were on 40mg / day; Robert Bourke, MD, personal communication). Other possible causes of this elevated prevalence rate are discussed in the portion of the next section regarding virreo-retinal traction.181
Numerous researchers have suggested that tamoxifen retinopathy is not caused by the actions of tamoxifen on ERs, but stems instead from tamoxifen's carionic amphiphilic properties, which resemble those of chloroquine.187 If this suggestion is correct, then post-menopausal women who develop tamoxifen retinopathy may simply be switched to an AI for relief. Similarly, because tamoxifen, chloroquine, and other related drugs all can lead to vortex keratopathies,188 post-menopausal tamoxifen users who develop corneal epithelial deposits may likewise be switched to an AI. Corneal deposits have been reported in as few as 0%169,175,189 to as high as 72% of women using a standard dose of tamoxifen, with the presence of these deposits depending on the duration of use.174,190 The wide discrepancy arguably results from a combination of two factors: (1) differences in the sensitivity of the means by which the cornea is assessed, and (2) the subtlety of the deposits.190 However, other hypothetically pertinent factors (e.g., amount of sunlight exposure)191 might also affect the observed frequencies. Regardless, even readily observable corneal deposits are reversible174 and not sight-threatening.168
Tamoxifen can induce cataracts, particularly posterior subcapsular cataracts, in many users.169 In separate clinical trials regarding BC prophylaxis, tamoxifen users were found to have a significantly higher incidence of cataracts and cataract surgeries compared to a placebo group192 and to raloxifene users,193 and an independent survey of BC survivors found that women who used tamoxifen for the standard 5-year term were more likely than non-users to have seen a physician about cataracts.194 With one exception,195 studies not finding statistical significance display corresponding trends in the data nevertheless.196,197 Moreover, several animal model studies have reported that tamoxifen induces cataracts.198–200 The mechanism of cataract induction involves the blockage of swelling-activated chloride channels in the lens,201, 202 and it is at least partly independent of the actions of tamoxifen on ERs.203,204 The increased risk of posterior subcapsular cataracts may be as high a factor of 4,169 which is important because posterior subcapsular cataracts tend to impair function more than other types of cataracts.205 This visual impairment may adversely affect cognitive test performance, even at a time when visual acuity is affected minimally.206
Clinically evident optic neuritis resulting from tamoxifen use has been reported in only isolated cases,174,207–210 but the optic nerve may be affected often at a subclinical level. Eisner et al.,178 using scanning laser ophthalmoscopy, found that the optic cup dimensions of short-term (≤ 2 years) middle-aged (51-69 years) early-stage BC tamoxifen users were appreciably smaller than those of (a) female control subjects without BC histories and not using hormonal medications, and also (b) a corresponding group of early-stage BC anastrozole users. The cup dimensions of longer-term tamoxifen users211 and of younger tamoxifen users178 were indistinguishable from those of control subjects. (One may speculate that this latter result occurred because the younger tamoxifen users were not yet menopausal despite being amenorrheic.212, 213) The dependence of cup size on the duration of tamoxifen use is noteworthy since it is the opposite of what would occur if the effect of tamoxifen on the optic nerve head (ONH) were due to a cumulative dose toxicity. The smaller cup sizes-which were within the range of normal and thus would be regarded as unremarkable if observed on an individual basis without baseline data for comparison- were consistent with astrocytic swelling.178
The first study to assess the visual function of a population of tamoxifen users using measures other than visual acuity was that of Gorin et al. in 1998.169 They found that tamoxifen users’ color discrimination tended to be slightly compromised as assessed with the Farnsworth Desaturated Panel D-15 test. No axis of loss (e.g., tritanopic) was specified. Ritter et al.214 used this same test to reveal color discrimination deficits in five of six visually symptomatic tamoxifen users, and Salomao et al.215 reported that two of 19 visually asymptomatic tamoxifen users had diffuse color vision defects as assessed with the FM-100 hue test. Gorin et al.169 also reported that neither visual-field macular sensitivities (pattern 10-2) nor high- nor low-contrast visual acuities appeared to be altered. In unpublished results, we (author AE) found the contrast sensitivities of tamoxifen users (assessed with the Pelli-Robson chart) to be indistinguishable from those of female control subjects. All the tamoxifen users whom we tested met a set of strict eligibility criteria that included excellent acuity and passing a conventional color vision screening (a standard D-15 test), and no subjects showed signs of tamoxifen rerinopathy.
Even with our strict eye-health eligibility criteria in place, however, visual responses mediated via short-wavelength-sensitive (SWS) cones were found to be affected often, both in the visual-field periphery180 and at the fovea.184 In particular, visual thresholds assessed with Short Wavelength Automated Perimetry (SWAP) were related systematically to the duration of tamoxifen use, with the peripheral SWAP sensitivities of long-term users (> 2 years) lower than those of short-term users (≥ 4 months, ≤ 2 years).180 There was no suggestion of any corresponding effect for Frequency Doubling Perimetry (FDP),180 implying that a duration-of-use effect does not occur for all types of visual-field tests and suggesting that it may occur preferentially or selectively for vision mediated via SWS cones. The strong dependence of SWAP sensitivities on retinal eccentricity coupled with the strict eye-health eligibility criteria and normal contrast sensitivities make it unlikely that the observed effects on SWAP resulted from lenticular change.
Although the magnitude of the SWAP visual-field effect decreased towards the center of the visual field,180 SWS-cone-mediated response evidently was affected at the fovea also.184 That is, tamoxifen users as a group were more likely than non-BC control subjects to perceive a short-wavelength incremental test stimulus on a yellow adapting background as “white” rather than as colored. Moreover, long-term users were significantly more likely than short-term users to perceive this stimulus as “white” despite not having a selective reduction of SWS-cone-mediated sensitivity at the fovea,184 The absence of a foveal sensitivity loss implied that the perception of white did not result from an acquired tritanopic defect, as might otherwise have been assumed, and estimates of lens density obtained psychophysically183 were unrelated to the choice of color name (unpublished results). Instead, the perception of white more likely resulted from an acquired temporal-response sluggishness that caused the color from the short-wavelength test stimulus to combine, rather than to contrast, with the color from the yellow background stimulus.182 Given the stimulus parameters used for SWAP, a prolongation of the temporal integration (i.e., neural summation) periods for SWS-cone-mediated response would be expected to account for at least part of the long- vs. short-term differences observed in the periphery of the SWAP visual field.179 Related results from various human subject studies involving gender216, 217 or hormonal change218–222 collectively suggest that the ability of tamoxifen to alter SWS-cone-mediated responses involves the actions of tamoxifen on ERs.
The striking change in color perception at about 2 years duration-of-use (see Figure 1) in this cross-sectional study184 presumably resulted from changes in the body's response to sustained tamoxifen exposure, perhaps as an acquired resistance began to develop.223 In any event, the data provided evidence for the ability of tamoxifen to materially affect at least one CNS neural substrate, possibly by altering its timing in response to lights flashed for several hundred msec. This may have pertinence for studies of cognitive function in BC survivors using tamoxifen or other adjuvant endocrine therapies,224 especially if the color perception change is proven to be a consequence of reduced neural processing speed. While there appear to be no studies of clomiphene and SWS-cone-mediated response corresponding to those described in the preceding two paragraphs, the ability of clomiphene to cause palinopsia may be regarded as an extreme example of a prolonged visual response.
FIGURE 1.
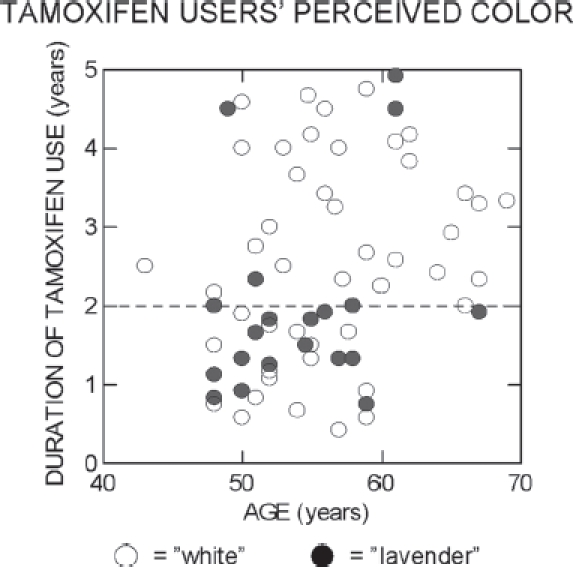
Duration of tamoxifen use versus age for the amenorrheic tamoxifen users tested with the forced-response color-naming paradigm detailed in Eisner & Incognito (2006).184 Open symbols represent subjects who called the threshold-level incremental test stimulus “white", and filled symbols represent subjects who called this stimulus “lavender". The test stimulus was a 3° diameter short-wavelength (440 nm) disc that was square-wave modulated at a rate of 1.5 Hz and was centered within an adapting background stimulus. The background was a moderately bright (3.6 log troland) 11° diameter, yellow (580 nm) disc viewed for at least 5 minutes. The horizontal dashed line signifies 2 years of tamoxifen use. (Data from three tamoxifen users who called the stimulus “blue” are omitted. For one of these subjects, the sensitivity was grossly reduced;184 the other two subjects were ages 56.8 and 59.5 years, and used tamoxifen for 4.0 and 4.9 years, respectively.) This graph has not been published previously.
Little is known regarding the specific physiologic means by which tamoxifen affects SWS-cone-mediated vision or any other visual response. ERs are present in all layers of the neural retina,20 but how these ERs are affected individually or concertedly by tamoxifen remains undetermined. Nor is it known what roles ERs may have for supporting vision other than to contribute to neuroprotection.225–227 Interpreting the effects of tamoxifen on SWS-cone-mediated response is further complicated by the potential for cortical effects and also by the possible salience of effects not depending exclusively on the actions of ERs. The electrorerinograms (ERGs) of clinically asymptomatic tamoxifen users are affected little or not at all,215,228 but because conventional full-field and mulrifocal white-light ERGs each are insensitive to alterations of SWS-cone mediated response, functional alterations to retinal SWS cone pathways would not be evident if they existed. To understand how ER activity affects vision, it probably is more straightforward to investigate the effects of AIs rather than SERMs.
Aromatase Inhibitors: Anastrozole (Arimidex®), Letrozole (Femara®), and Exemestane (Aromasin®)
Three third-generation AIs have received FDA-approval for use as adjuvant endocrine therapy in early-stage BC. Anastrozole (1mg / day) and letrozole (2.5mg / day) were approved in 2002 and 2005, respectively, as first-line monotherapy, while exemestane (25mg / day) currently is approved for use after 2-3 years of tamoxifen use, for a total of 5 continuous years of adjuvant endocrine therapy. In practice, however, oncologists exercise substantial discretion in deciding which adjuvant endocrine medications to prescribe to individual patients. Clinical trials are well underway to determine whether switching regimens, with tamoxifen used for the first 2-3 years and then an AI for the balance of the conventional 5-year adjuvant endocrine period, are more effective than a comparable period of monotherapy.229 Trials also are well on their way regarding the efficacy and safety of extending the duration of adjuvant endocrine therapy beyond 5 years.31,230,231
There are two broad classes of AIs: non-steroidal and steroidal. Anastrozole and letrozole each are non-steroidal AIs that by reversibly binding to the aromatase enzyme interfere with steroidal hydroxylation, thereby inhibiting estrogen synthesis.232 Exemestane is a steroidal AI that structurally resembles the androgen androstenedione and interacts with the substrate binding site of the aromatase enzyme, ultimately leading to the enzyme's irreversible inactivation (meaning that the subsequent production of estrogen requires aromatase to be synthesized anew).232, 233 Each AI reduces aromatization by more than 97%, with letrozole being slightly more effective in this regard than anastrozole.230 The effects of exemestane at this high level of suppression are more difficult to compare, for technical reasons involving the steroidal properties of exemestane.230
Many, but not all, of the extensively documented side effects of AIs resemble those of tamoxifen,5, 31 but relatively little is known about the effects of AIs on the visual system. The resemblance is expected to be imperfect because tamoxifen (despite often being referred to as an “anti-estrogen") is a SERM, whereas the effects of AIs on human physiology are more strictly anti-estrogenic. This difference makes AIs more useful for modeling effects of female aging on vision and the eye. One must nevertheless exercise caution when interpreting the observed effects of an individual AI such as anastrozole as an AI class effect or as an estrogen-deprivation effect. Corroborating evidence is needed, since second-order effects on testosterone levels may differ between AIs, for example.234,235
Two studies by Eisner et al. (2008)177 and (2009)181 researched the ability of anastrozole to induce retinal changes detectable ophthalmologically (A third study, Eisner et al. (2007),178 found that anastrozole did not affect the ONH, whereas tamoxifen did; this study was described in the preceding section.) These three studies all employed a cross-sectional methodology to evaluate data from early-stage BC survivors using anastrozole monotherapy. The test subjects all had excellent visual acuity, no history of eye disease or diabetes, and no high myopia; and they were younger than 70 years. Control subjects were age-matched amenorrheic women without BC histories and not using any hormonal medication. Amenorrheic BC survivors using tamoxifen mono-therapy were also tested
For the first of the two retinal studies, four of 35 (11.4%) of the anastrozole users were found to have a small retinal hemorrhage in the posterior pole of one eye, based on masked assessment of fundus photographs by an ophthalmologist.177 This 11.4% proportion was significantly greater than the corresponding proportions for control subjects and for tamoxifen users.177 Two of the four anastrozole users had a flame hemorrhage (in the nerve fiber layer), two had a blot hemorrhage (deeper in the retina), and all four apparently were asymptomatic. However, a small hemorrhage nearer to the fovea could be symptomatic. Indeed, a 47-year-old anastrozole patient who noticed a grey spot while reading was referred to an ophthalmologist who detected a small juxtafoveal hemorrhage (AE, unpublished case report). This relatively young patient, who was not a subject in our studies, was also taking a GnRH agonist.
Because small hemorrhages can resorb within months,236 the incidence rate of anastrozole-induced retinal hemorrhages over a 5-year period is expected to be higher than the corresponding prevalence rate based on a cross-sectional snapshot, which provided the basis for the 11.4% rate reported by Eisner et al. (2008)177 for their small but select sample. On the other hand, the hemorrhages observed by Eisner et al. (2008)177 might have not have resulted solely or directly from the AI itself. Instead, they might have arisen at least partly from the actions of additional drugs used to relieve muscle or joint pain (aspirin) or to preserve bone density (bisphosphonates).177 This possibility is discussed in greater detail and in a more general context in the next section. Because the retinal hemorrhages were subtle, comparable hemorrhages might be missed in a cursory eye exam, or in an eye exam by someone other than an eye care specialist. The hemorrhages observed by Eisner et al. (2008) did not result from high blood pressure,177 but excessive traction pulling on the retina may have been a factor,181 as menopause appears to increase the risk for several vitreo-retinal fractional conditions.237–241
To observe and quantify effects of traction on retinal anatomy, Eisner et al. (2009)181 transformed retinal thickness data obtained using time-domain OCT into foveal shape profiles corresponding to a slice along the horizontal (i.e., nasal / temporal) meridian. The effects of traction between the vitreous and retina then could be measured and compared across subject groups. As predicted, the foveal shapes of anastrozole users were distorted in a manner consistent with a heightened degree of vitreo-retinal traction, with the upper portion of the temporal side of the foveal slope displaced towards the ONH, an asymmetrically located major anchor for the vitreous.242 The results181 are shown graphically in Figure 2. The nasal-temporal asymmetries differed significantly between anastrozole users and control subjects, but the corresponding asymmetries for tamoxifen users appeared to be intermediate to those of the other two groups and the statistical comparisons were inconclusive.181
FIGURE 2.
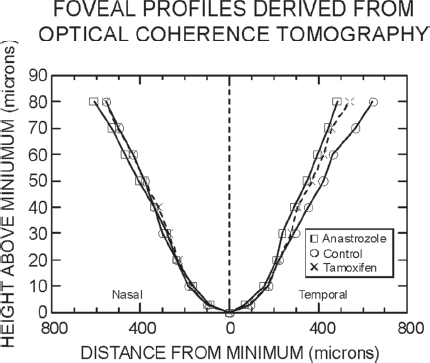
Foveal shape profiles normalized to the locus of minimal retinal thickness; nasal direction is to the left, and temporal to the right. Squares (□) represent median data of anastrozole users, circles (O) represent median data of control subjects, and crosses (x) represent median data of tamoxifen users. Connecting lines are unbroken for the anastrozole users and control subjects, and dashed lines are for the tamoxifen users. All units are microns (μn). Note that the scales on the two axes differ. The locus of minimal thickness defines a height of 0 μm on the ordinate. Data are derived from subjects without detectable PVDs. This figure was previously published as Figure 1 in (Eisner, Thielman, Falardeau, Vetto Vitreo-retinal traction and anastrozole use. Breast Cancer Res Treat. 2009;117:9-16),181 but with a different legend and title. The graph is reproduced with kind permission from Springer Science+Business Media B.V.
The data for Figure 2 were analyzed for subjects without discernible posterior vitreous detachments (PVDs), and in fact, the tamoxifen users without PVDs were observed to have used tamoxifen for a significantly shorter duration than the tamoxifen users with PVDs.181 (Most subjects’ PVDs evidently were partial rather than complete.181) Among anastrozole users, it is likely that the heightened degree of traction resulted from estrogen depletion, since PVDs237 and macular holes238, 239 each are traction-related conditions that occur more often in women than in men, and moreover, may be precipitated by the menopausal transition.240, 241 Estrogen supplementation might even protect against the development of macular holes.238 Thus, AIs may increase the risk of macular holes or other relatively acute traction-related conditions such as rhegmatogenous retinal detachments.243 Any patient reporting worsening floaters or photopsia should be promptly referred by their oncologist to an ophthalmologist. In our clinical experience (author SWL), about 1 in 30 AI users mention that these entoptic phenomena develop or worsen with AI use.
Eisner et al.181 noted in retrospect that their anastrozole subjects were, on average, less myopic than were their subjects in the tamoxifen and control groups. This observation suggested a possible recruitment bias wherein disproportionately many myopic anastrozole users did not enroll in the study, possibly because they felt their vision was no longer normal. Epstein244 subsequently published a case report on the deterioration of visual acuity in two myopic BC survivors who switched from a non-steroidal AI to exemestane; one developed a macular hole after the switch. Myopia is a known risk factor for PVDs237 and macular holes,245 and when combined with excessive vitreo-retinal traction, a shearing force can be created at the fovea.181 In 2004, the Intergroup Exemestane Study (IES) had reported that early-stage BC survivors who had switched from tamoxifen to exemestane after 2-3 years were significantly more likely to develop visual disturbances than were women who remained on tamoxifen, but the types of disturbances were not specified.246 It would be desirable and feasible to test BC survivors before
and after they switch from tamoxifen to an AI in order to determine whether the switch leads to measurable intraocular change, particularly as assessed with spectral-domain OCT.
The eye is unique among all the organs of the body in that its interior can be viewed and measured with an extraordinarily high degree of precision. Thus, changes in intraocular anatomy are potentially useful for marking and tracking changes elsewhere in the body that result from AI use. Of all AI side effects, the one most often leading to medication non-compliance is arthralgia (joint pain).247 The strong degree of biochemical similarity between joints and vitreous242,248–250 raises the possibility that measured changes in vitreo-retinal traction might be useful in helping to mark changes in joints that accompany the development of AI-induced arthralgia,251, 252 and that are likely to result from a decrease in estrogen synthesis.253 This is a matter for future study.
The same types of color perception changes experienced by tamoxifen users184 also were experienced by anastrozole users,182 but with an important difference. That is, whereas the effects of tamoxifen depended on the duration of use,184 the effects of anastrozole depended on age, with the anastrozole users who perceived the test stimulus as white being significantly younger (by about 6 years on average) than the anastrozole users who perceived it as colored.182 Because estrogen production normally is greater for younger post-menopausal women than for older post-menopausal women,254, 255 this result suggests that the anastrozole-induced change in estrogen exposure, rather than just the low estrogen levels themselves, led to the perception of white.182 Bear in mind that because the perception of white was elicited for a very specific set of laboratory conditions (a short-wavelength test stimulus flashed on a larger, moderately bright, yellow background), this perception need not reflect either a more generalized or real-word color vision deficit wherein hues appear desarurated. Instead, the perception of white more likely manifests a reduction of processing speed within the functionally isolated neural substrate responsible for SWS-cone-mediated vision.182 Future studies should directly test the hypothesis that AIs prolong the temporal integration (neural summation) periods for SWS-cone-mediated response, since if AIs cause SWS-cone-mediated response to become more sluggish, the likelihood is strong that the temporal response properties of some other CNS neural substrates are altered similarly. Cognitive processing speed, for example, may be slowed by anastrozole use,256, 257 and senses such as hearing that depend exquisitely on response timing might also be vulnerable to reductions of estrogenic activity.258,259
In unpublished results (author AE), the monocular contrast sensitivities of anastrozole users were found to be significantly lower (average difference = 0.08 log units, corresponding to ∼½ line of loss on the Pelli-Robson chart) than those of female control subjects and tamoxifen users, respectively. Thus, the spatial vision of anastrozole users was affected despite their excellent acuity. Contrast sensitivity should be assessed for AI users reporting unexplained vision difficulties.
One final note: Although chronic dry eye can appreciably impact quality of life,260 and there are physiological and epidemiological reasons for supposing that AI usage can lead to dry eye, only one study appears to have expressly raised this possibility.261 ERs are present in the cornea16 and tear film glands,16, 18, 22, 23 conditions such as premature ovarian failure apparently can lead to dry eye,262 and dry eye is most prevalent among post-menopausal women.3,62 While these considerations suggest that the abrupt reduction of estrogen synthesis can lead to dry eye, the relation of hormonal activity to dry eye evidently is more complex, as it is now seems likely that androgen / estrogen balances are more important than estrogen exposure alone for maintaining the ocular surface and for minimizing ocular surface discomfort.3, 263,264 Either view justifies conducting studies to determine whether AIs may contribute to the development or exacerbation of dry eye. An objective and practical measure of tear film osmolarity has been reported recently to correlate well with dry-eye severity as assessed subjectively,265 so we propose that studies relating tear film osmolarity to BC treatment be conducted along with administration of a validated survey tool. Osmolarity measurements can be performed quickly and easily, and hence can be appended to clinical trials or other studies.
MEDICATIONS USED FOR REDRESSING SIDE EFFECTS OF BC TREATMENTS
Hot flashes are a relatively common side effect of tamoxifen and of all the AIs.5,31 Because supplemental estrogen is contraindicated for most BC survivors, they are instead often prescribed serotonin specific reuptake inhibitors (SSRIs) for symptomatic relief.266 SSRIs also are prescribed as anti-depressants.267 However, at least several different SSRIs, notably paroxerine (Paxil®) and fluoxeteine (Prozac®) but not citalopram (Celexa®), strongly interfere with the ability of CYP2D6 to convert tamoxifen to its active metabolites, particularly endoxifen.162,268 Consequently, women using tamoxifen are no longer routinely prescribed SSRIs that are potent CYP2D6 inhibitors. SSRI usage remains common among tamoxifen and AI users, and several percent of SSRI users overall have been reported to complain of “blurry vision,” possibly due to a mydriatic effect.269,270 Interestingly, metabolism of the intraocular pressure drug timolol depends on the activity of the same CYP2D6 enzyme as tamoxifen, so patients with low CYP2D6 activity may be at increased risk for beta-blocker induced brachycardia following systemic absorption of ophthalmically-administered rimolol.271,273 Oncologists should be aware that the glucocorticoid dexamethasone, one of several types of drugs used for anti-emesis while patients are receiving chemotherapy,274 can acutely raise intraocular pressure in susceptible people.275,276
Because AIs reduce bone density, women who are prescribed an AI are sometimes also prescribed a bisphosphonate,277 a type of medication that decreases bone resorption by disrupting osteoclast function.278 However, bisphosphonates can have serious side effects,279 so they are not prescribed merely as a matter of course.280 It has become clear that bisphosphonates, especially the ones administered intravenously, occasionally lead to clinically significant uveiris and scleritis, generally of the anterior portion of the eye.281–283 The intravenously administered bispshosphonate zolen-dronate (Zometa®) may have anti-cancer properties in its own right,284 so several clinical trials are underway to determine whether and how zolendronate should be added to existing AI regimens.285 The preliminary results regarding upfront use of zolendronate (administered at 6-month intervals) combined with daily AI use indicate that BC recurrence rates are reduced.286 Thus, the use of zolendronate is likely to increase in the future, in which case, the rates of uveitis and scleritis among BC survivors will also likely increase.
The small retinal hemorrhages detected in a significant proportion of anastrozole users177 (see the previous section) might also have depended in some cases on the use of supplemental medications for alleviating AI-induced side effects. Of the four out of 35 anastrozole users with retinal hemorrhages, the two with blot hemorrhages were the only anastrozole users also using both a bisphosphonate (taken orally) and aspirin.177 These limited data suggest that blot hemorrhages can result from a drug combination that includes an AI and a bisphophonate acting synergistically on the posterior portion of the eye. Bisphosphonates may affect the posterior uvea at a greater rate than is recognized,287 and estrogen depletion might increase retinal vascular permeability.288
DIRECTIONS FOR FUTURE RESEARCH
Although treatments for BC are continually evolving, it seems certain that millions of BC survivors will receive adjuvant endocrine therapy involving years of AI use. What remains unknown is the scheduling of this treatment. Will AIs be prescribed after 2-3 years of tamoxifen use, or will AIs be used from the outset? Will patients receive adjuvant endocrine therapy for more than 5 years? Which AIs will be used, and how might their visual and ocular effects differ? How might prior use of tamoxifen interact with the ability of an AI to affect vision and the eye? This last question is important because many post-menopausal women may be using tamoxifen for the first several years at the start of a tamoxifen / AI switching regimen, while many other women will have taken tamoxifen for varying periods of time before reaching the menopause. Tamoxifen will continue to serve as an alternative medication for post-menopausal women whose AI side effects are unacceptable, and it is expected to remain a mainstay for men with BC.
Because iatrogenic (treatment-induced) changes to vision or the eye may impact BC survivors’ quality of life and hence reduce adherence to treatment, such changes may indirectly cause BC recurrence rates to increase. Iatrogenic changes may also sometimes be misattributed to natural aging. Thus, studies are needed to determine whether AIs contribute to the development or exacerbation of dry eye, and if they do contribute, then which AIs contribute most and in which regimens. These dry-eye studies should include measures of tear-film osmolarity. We also suggest that studies be conducted to evaluate the ability of AIs to produce traction capable of altering the existing relation between the vitreous and retina. These studies should include quantitative assessments of foveal shape indices derived from retinal thickness measurements made with spectral-domain OCT devices. Assessing virreo-retinal traction is important because excessive traction (a) may produce entopric symptoms that disturb or concern patients in the short-term, and (b) may raise the risk for subsequent sight-threatening conditions such as macular holes. Perhaps even more importantly, evidence is accumulating to suggest that increased traction may raise the long-term risk for blinding conditions such as macular degeneration.38, 289–291 Thus, we recommend that oncologists routinely query AI users about the development of traction-related symptoms such as floaters or photopsia. We also recommend that AI users be queried about changes in ocular surface discomfort and that contrast sensitivities be routinely assessed, which is feasible in an oncology setting. Worsening of any these factors may warrant referral to an ophthalmologist.
Because drugs that drastically reduce ER activity can be administered to people only with compelling clinical justification, the widespread use of AIs provides a unique chance to investigate the means by which estrogen deprivation (or conversely, estrogen exposure) affects the adult human visual system, particularly for women at and beyond menopause. These investigations should include studies of the etiology of traction-related intraocular conditions such as PVDs and macular holes. Moreover, because the eye is so accessible and visual response is so quantifiable, measures of the eye and vision might also be assessed for their ability to mark related iatrogenic changes occurring in parts of the body that are more difficult to view and / or quantify. For example, the use of OCT to quantify AI-induced anatomical changes at the vitreo-retinal interface might be investigated as a means for marking coincident changes occurring in joints247 (and which have long been postulated to share a common etiology with eye changes292, 293). Because commercial OCT devices are widely distributed and are not intimidating to patients, OCT is well-suited
for use in longitudinal studies, whether conservative or more speculative.
Similarly, future investigations should include assessments of visual functions susceptible to changes in estrogenic activity. Because SWS-cone-mediated visual response is important only for color vision and not, for instance, for fine spatial resolution,294 AI-induced changes in SWS-cone-mediated response182 are not expected to compromise a person's ability to function in her environment. By the same token, however, SWS-cone-mediated response sensitivities can be measured using established procedures that provide an exceptionally high degree of functional isolation,216, 295, 296 thereby allowing the response dynamics of an operationally well-defined neural substrate to be measured behaviorally with little or no intrusion from other neural substrates182 (which do not share the same pre-corrical anatomic pathways dedicated to SWS-cone-mediated vision297, 298). If the temporal integration periods of SWS-cone-mediated response are prolonged by AI use, as we hypothesize, there is only a remote chance that no other neural substrate is made more sluggish in this way. We therefore suggest that psychophysical studies be conducted to determine whether AIs cause the temporal integration periods for SWS-cone-mediated response to become prolonged. (Corresponding fMRI studies may be performed,299, 300 but because of practical constraints, fMRI studies are limited to small numbers of subjects.) If our hypothesis is supported, a next step is to determine whether response sluggishness measured for SWS-cone-mediated vision can be used to mark deficits in cognitive processing that may result from one or more adjuvant endocrine therapies.224 For the present, we recommend that studies of BC survivors’ cognitive function begin to record or screen for eye health and also add tests of spatial vision so that sensory deficits are not misconstrued as cognitive deficits.206
Acknowledgments
Portions of this work were supported by NIH grant EY014594 (to A. Eisner). We would like to thank Alison Conlin, MD, MPH, and David Sullivan, PhD, for critiquing the manuscript; and Linda Simmons, MLS, for help locating references.
Notice of correction
The Early Online version of this article published online ahead of print on 5 August 2011 contained errors.
The start of the Abstract on page 867 has been changed from “This review concerns the visual and ocular effects…” to “This review concerns the effects on vision and the eye…”
The original receipt date has been added at the bottom of page 867.
Capitalization has been corrected in the section heading on page 873.
These errors have been corrected for the current version.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Mathers WD. Why the eye becomes dry: A cornea and lacrimal gland feedback model. CLAO J. 2000;26:159–165. [PubMed] [Google Scholar]
- 2.Mainville N, Jordan DR. Etiology of tearing: A retrospective analysis of referrals to a tertiary care oculoplastics practice. Ophthal Plast Reconstr Surg. 2011;27:155–157. doi: 10.1097/IOP.0b013e3181ef728d. [DOI] [PubMed] [Google Scholar]
- 3.Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. 2005;20:289–298. doi: 10.1080/09513590400027257. [DOI] [PubMed] [Google Scholar]
- 4.Lasudry J. [The pathology of ocular syndromes caused by toxicity] Bull Soc Beige Ophtalmol. 2007:155–178. [PubMed] [Google Scholar]
- 5.Mortimer JE. Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol. 2010;22:56–60. doi: 10.1097/GCO.0b013e328334e44e. [DOI] [PubMed] [Google Scholar]
- 6.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: Saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- 8.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu KC, Anderson WF, Fritz A, Ries LA, Brawley OW. Frequency distributions of breast cancer characteristics classified by estrogen receptor and progesterone receptor status for eight racial / ethnic groups. Cancer. 2001;92:37–45. doi: 10.1002/1097-0142(20010701)92:1<37::aid-cncr1289>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85:892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 12.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 13.Karellas A, Vedantham S. Breast cancer imaging: A perspective for the next decade. Med Phys. 2008;35:4878–4897. doi: 10.1118/1.2986144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson S, Gustafsson JA. Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol. 2002;37:l–28. doi: 10.1080/10409230290771438. [DOI] [PubMed] [Google Scholar]
- 15.Cammarata PR, Chu S, Moor A, Wang Z, Yang SH, Simpkins JW. Subcellular distribution of native estrogen receptor alpha and beta subtypes in cultured human lens epithelial cells. Exp Eye Res. 2004;78:861–871. doi: 10.1016/j.exer.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W, Sotozono C, Kinoshita S. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res. 2001;22:28–33. doi: 10.1076/ceyr.22.1.28.6980. [DOI] [PubMed] [Google Scholar]
- 17.Spelsberg H, Klueppel M, Reinhard T, Glaeser M, Niederacher D, Beckmann MW, Sundmacher R. Detection of oestrogen receptors (ER) alpha and beta in conjunctiva, lacrimal gland, and tarsal plates. Eye. 2004;18:729–733. doi: 10.1038/sj.eye.6701314. [DOI] [PubMed] [Google Scholar]
- 18.Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Castano ME, Elliot SJ, Potier M, Karl M, Striker LJ, Striker GE, Csaky KG, Cousins SW. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:50–59. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- 20.Munaut C, Lambert V, Noel A, Frankenne F, Deprez M, Foidart JM, Rakic JM. Presence of oestrogen receptor type beta inhuman retina. Br J Ophthalmol. 2001;85:877–882. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan DA. Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf. 2004;2:92–123. doi: 10.1016/s1542-0124(12)70147-7. [DOI] [PubMed] [Google Scholar]
- 22.Auw-Haedrich C, Feltgen N. Estrogen receptor expression in meibomian glands and its correlation with age and dry-eye parameters. Graefes Arch Clin Exp Ophthalmol. 2003;241:705–709. doi: 10.1007/s00417-003-0699-4. [DOI] [PubMed] [Google Scholar]
- 23.Esmaeli B, Harvey JT, Hewlett B. Immunohistochemical evidence for estrogen receptors in meibomian glands. Ophthalmology. 2000;107:180–184. doi: 10.1016/s0161-6420(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Segura LM. Aromatase in the brain: Not just for reproduction anymore. J Neuroendocrinol. 2008;20:705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH, Whiteside GT, Brandon NJ. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol. 2009;2:215–236. doi: 10.2174/1874467210902030215. [DOI] [PubMed] [Google Scholar]
- 26.Morissette M, Le Saux M, D'Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. l81:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 29.Speroff L, Fritz M. In Clinical Gynecologic Endocrinology and Infertility, 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Menopause and the perimenopausal transition; pp. 621–688. [Google Scholar]
- 30.Hiscox S, Davies EL, Barrett-Lee P. Aromatase inhibitors in breast cancer. Maturitas. 2009;63:275–279. doi: 10.1016/j.maturitas.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Burstein HJ, Griggs JJ. Adjuvant hormonal therapy for early-stage breast cancer. Surg Oncol Clin N Am. 2010;19:639–647. doi: 10.1016/j.soc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Lin NU, Winer EP. Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol. 2008;26:798–805. doi: 10.1200/JCO.2007.15.0946. [DOI] [PubMed] [Google Scholar]
- 33.Mabuchi F, Sakurada Y, Kashiwagi K, Yamagata Z, Iijima H, Tsukahara S. Estrogen receptor beta gene polymorphism and intraocular pressure elevation in female patients with primary open-angle glaucoma. Am J Ophthalmol. 2010;149:826–830. e821–e822. doi: 10.1016/j.ajo.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: What we know and what we need to know. Curr Opin Ophthalmol. 2010;21:91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: Interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–979. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tint NL, Alexander P, Tint KM, Vasileiadis GT, Yeung AM, Azuara-Blanco A. Hormone therapy and intraocular pressure in nonglaucomatous eyes. Menopause. 2010;17:157–160. doi: 10.1097/gme.0b013e3181b82fb4. [DOI] [PubMed] [Google Scholar]
- 37.Deschenes MC, Descovich D, Moreau M, Granger L, Kuchel GA, Mikkola TS, Fick GH, Chemtob S, Vaucher E, Lesk MR. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2010;51:2587–2600. doi: 10.1167/iovs.09-3710. [DOI] [PubMed] [Google Scholar]
- 38.Gawecki M, Doroszkiewicz M, Rydzewski J. Age related macular degeneration and presence of posterior vitreous detachment. Klin Oczna. 2010;112:210–212. [PubMed] [Google Scholar]
- 39.Boekhoorn SS, Vingerling JR, Uitterlinden AG, Van Meurs JB, van Duijn CM, Pols HA, Hofman A, de Jong PT. Estrogen receptor alpha gene polymorphisms associated with incident aging macula disorder. Invest Ophthalmol Vis Sci. 2007;48:1012–1017. doi: 10.1167/iovs.06-0577. [DOI] [PubMed] [Google Scholar]
- 40.Edwards DR, Gallins P, Polk M, Ayala-Haedo J, Schwartz SG, Kovach JL, Spencer K, Wang G, Agarwal A, Postel EA, Haines JL, Pericak-Vance M, Scott WK. Inverse association of female hormone replacement therapy with age-related macular degeneration and interactions with ARMS2 polymorphisms. Invest Ophthalmol Vis Sci. 2010;51:1873–1879. doi: 10.1167/iovs.09-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feskanich D, Cho E, Schaumberg DA, Colditz GA, Hankinson SE. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Ophthalmol. 2008;126:519–524. doi: 10.1001/archopht.126.4.519. [DOI] [PubMed] [Google Scholar]
- 42.Geisler J, Lonning PE. Impact of aromatase inhibitors on bone health in breast cancer patients. J Steroid Biochem Mol Biol. 2010;118:294–299. doi: 10.1016/j.jsbmb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Valachis A, Polyzos NP, Georgoulias V, Mavroudis D, Mauri D. Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: A meta-analysis. Gynecol Oncol. 2010;117:139–145. doi: 10.1016/j.ygyno.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Winters-Stone KM, Togrimson BM, Horak FB, Eisner A, Leo MC, Nail LM, Chui SY, Luoh SW. Identifying factors associated with falls in postmenopausal breast cancer survivors: A multi-disciplinary approach. Arch Phys Med Rehab. 2011;92:646–652. doi: 10.1016/j.apmr.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 46.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 47.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 48.Fallowfield L. Quality of life issues in relation to the aromatase inhibitor. J Steroid Biochem Mol Biol. 2007;106:168–172. doi: 10.1016/j.jsbmb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Miaskowski C, Shockney L, Chlebowski RT. Adherence to oral endocrine therapy for breast cancer: A nursing perspective. Clin J Oncol Nurs. 2008;12:213–-221. doi: 10.1188/08.CJON.213-221. [DOI] [PubMed] [Google Scholar]
- 50.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: A retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 52.Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100:238–244. doi: 10.1002/cncr.11883. [DOI] [PubMed] [Google Scholar]
- 53.Silverstein MJ, Recht A, Lagios MD, Bleiweiss IJ, Blumencranz PW, Gizienski T, Harms SE, Harness J, Jackman RJ, Klimberg VS, Kuske R, Levine GM, Linver MN, Rafferty EA, Rugo H, Schilling K, Tripathy D, Vicini FA, Whitworth PW, Willey SC. Special report: Consensus conference III. Image-detected breast cancer: State-of-the-art diagnosis and treatment. J Am Coll Surg. 2009;209:504–520. doi: 10.1016/j.jamcollsurg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008;14:8019–8026. doi: 10.1158/1078-0432.CCR-08-0974. [DOI] [PubMed] [Google Scholar]
- 55.Oakman C, Bessi S, Zafarana E, Galardi F, Biganzoli L, Di Leo A. Recent advances in systemic therapy: New diagnostics and biological predictors of outcome in early breast cancer. Breast Cancer Res. 2009;ll:205. doi: 10.1186/bcr2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Estevez L, Tusquets I, Alvarez I, Rodriguez C, Fernandez Y, Segui MA, Garcia-Mata J, Lluch A. Supportive care for patients with early breast cancer. Clin Transl Oncol. 2010;12:32–42. doi: 10.1007/s12094-010-0464-1. [DOI] [PubMed] [Google Scholar]
- 57.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 58.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 59.Redmond N, While A. Dry eye syndrome (DES) and watering eyes. Br J Community Nurs. 2008;13:471–479. doi: 10.12968/bjcn.2008.13.10.31184. [DOI] [PubMed] [Google Scholar]
- 60.Ervin AM, Wojciechowski R, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006775.pub2. CD006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 62.Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85:668–674. doi: 10.1097/OPX.0b013e318181a947. [DOI] [PubMed] [Google Scholar]
- 63.Bouchardy C, Usel M, Verkooijen HM, Fioretta G, Benhamou S, Neyroud-Caspar I, Schaffar R, Vlastos G, Wespi Y, Schafer P, Rapiti E. Changing pattern of age-specific breast cancer incidence in the Swiss canton of Geneva. Breast Cancer Res Treat. 2010;120:519–523. doi: 10.1007/s10549-009-0478-y. [DOI] [PubMed] [Google Scholar]
- 64.Kintzel PE, Michaud LB, Lange MK. Docetaxel-associated epiphora. Pharmacotherapy. 2006;26:853–867. doi: 10.1592/phco.26.6.853. [DOI] [PubMed] [Google Scholar]
- 65.Koh S, Maeda N, Ninomiya S, Watanabe H, Fujikado T, Tano Y, Hirohara Y, Mihashi T. Paradoxical increase of visual impairment with punctal occlusion in a patient with mild dry eye. J Cataract Refract Surg. 2006;32:689–691. doi: 10.1016/j.jcrs.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, Bowman D, Wolmark N, Wickerham DL, Kardinal CG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–1496. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 67.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Tian Q, Zhu YZ, Xu AL, Zhou SF. Reversal of resistance to oxazaphosphorines. Curr Cancer Drug Targets. 2006;6:385–407. doi: 10.2174/156800906777723967. [DOI] [PubMed] [Google Scholar]
- 69.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: Golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 70.Fraunfelder FT, Meyer SM. Ocular toxicity of antineoplastic agents. Ophthalmology. 1983;90:l–3. doi: 10.1016/s0161-6420(83)34600-5. [DOI] [PubMed] [Google Scholar]
- 71.Blum RH. An overview of studies with adriamycin (NSC-123127) in the United States. Cancer Chemotherapy Reports Part 3. 1975;6:247–251. [Google Scholar]
- 72.Saurel CA, Patel TA, Perez EA. Changes to adjuvant systemic therapy in breast cancer: A decade in review. Clin Breast Cancer. 2010;10:196–208. doi: 10.3816/CBC.2010.n.027. [DOI] [PubMed] [Google Scholar]
- 73.Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat Rev. 2009;35:255–261. doi: 10.1016/j.ctrv.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 75.Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, Mclntyre KJ, Pippen JE, Bordelon JH, Kirby RL, Sandbach J, Hyman WJ, Richards DA, Mennel RG, Boehm KA, Meyer WG, Asmar L, Mackey D, Riedel S, Muss H, Savin MA. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 76.Tang SC. Strategies to decrease taxanes toxicities in the adjuvant treatment of early breast cancer. Cancer Invest. 2009;27:206–214. doi: 10.1080/07357900802178520. [DOI] [PubMed] [Google Scholar]
- 77.Nabholtz JM, Gligorov J. Taxane therapy for early stage breast cancer. Womens Health (Lond Engl) 2006;2:99–114. doi: 10.2217/17455057.2.1.99. [DOI] [PubMed] [Google Scholar]
- 78.Bedard PL, Di Leo A, Piccart-Gebhart MJ. Taxanes: Optimizing adjuvant chemotherapy for early-stage breast cancer. Nat Rev Clin Oncol. 2010;7:22–36. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 79.Bear HD, Anderson S, Smith RE, Geyer CE, Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, Paik S, Soran A, Wickerham DL, Wolmark N. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 80.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, Aranda I, Rodriguez-Lescure A, Grosse R, Calvo L, Barnadas A, Isla D, Martinez del Prado P, Ruiz Borrego M, Zaluski J, Arcusa A, Munoz M, Lopez Vega JM, Mel JR, Munarriz B, Llorca C, Jara C, Alba E, Florian J, Li J, Lopez Garcia-Asenjo JA, Saez A, Rios MJ, Almenar S, Peiro G, Lluch A. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 81.Saibil S, Fitzgerald B, Freedman OC, Amir E, Napolskikh J, Salvo N, Dranitsaris G, Clemons M. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: A retrospective, outcomes-based survey. Curr Oncol. 2010;17:42–47. doi: 10.3747/co.v17i4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esmaeli B. Management of excessive tearing as a side effect of docetaxel. Clin Breast Cancer. 2005;5:455–457. doi: 10.3816/cbc.2005.n.004. [DOI] [PubMed] [Google Scholar]
- 83.McCartney E, Valluri S, Rushing D, Burgett R. Upper and lower system nasolacrimal duct stenosis secondary to paclitaxel. Ophthal Plast Reconstr Surg. 2007;23:170–171. doi: 10.1097/IOP.0b013e318032e908. [DOI] [PubMed] [Google Scholar]
- 84.Savar A, Esmaeli B. Re: “Upper and lower system nasolacrimal duct stenosis secondary to paclitaxel". Ophthal Plast Reconstr Surg. 2009;25:418–419. doi: 10.1097/IOP.0b013e3181b398b6. author reply 419. [DOI] [PubMed] [Google Scholar]
- 85.Esmaeli B, Ahmadi MA, Rivera E, Valero V, Hutto T, Jackson DM, Newman RA. Docetaxel secretion in tears: Association with lacrimal drainage obstruction. Arch Ophthalmol. 2002;120:1180–1182. [PubMed] [Google Scholar]
- 86.Esmaeli B, Hidaji L, Admin RB, Faustina M, Coats C, Arbuckle R, Rivera E, Valero V, Tu SM, Ahmadi MA. Blockage of the lacrimal drainage apparatus as a side effect of docetaxel therapy. Cancer. 2003;98:504–507. doi: 10.1002/cncr.11527. [DOI] [PubMed] [Google Scholar]
- 87.Esmaeli B, Amin S, Valero V, Adinin R, Arbuckle R, Banay R, Do KA, Rivera E. Prospective study of incidence and severity of epiphora and canalicular stenosis in patients with metastatic breast cancer receiving docetaxel. J Clin Oncol. 2006;24:3619–3622. doi: 10.1200/JCO.2005.04.4453. [DOI] [PubMed] [Google Scholar]
- 88.Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, Matulonis UA, Garber JE, Clarke KD, Shulman LN, Winer EP. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–1219. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 89.Tsalic M, Gilboa M, Visel B, Miller B, Haim N. Epiphora (excessive tearing) and other ocular manifestations related to weekly docetaxel: Underestimated dose-limiting toxicity. Med Oncol. 2006;23:57–61. doi: 10.1385/MO:23:1:57. [DOI] [PubMed] [Google Scholar]
- 90.Lee JJ, Swain SM. Peripheral neuropathy induced by micro-tubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 91.Kuroi K, Shimozuma K. Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer. 2004;ll:92–99. doi: 10.1007/BF02968010. [DOI] [PubMed] [Google Scholar]
- 92.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–774. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 93.Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15:3081–3094. doi: 10.2174/092986708786848569. [DOI] [PubMed] [Google Scholar]
- 94.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Scaioli V, Caraceni A, Martini C, Curzi S, Capri G, Luca G. Electrophysiological evaluation of visual pathways in paclitaxel-treated patients. J Neurooncol. 2006;77:79–87. doi: 10.1007/s11060-005-9008-x. [DOI] [PubMed] [Google Scholar]
- 96.Capri G, Munzone E, Tarenzi E, Fulfaro F, Gianni L, Caraceni A, Martini C, Scaioli V. Optic nerve disturbances: A new form of paclitaxel neurotoxicity. J Natl Cancer Inst. 1994;86:1099–1101. doi: 10.1093/jnci/86.14.1099. [DOI] [PubMed] [Google Scholar]
- 97.Murphy CG, Walsh JB, Hudis CA, Lake D, Theodoulou M. Cystoid macular edema secondary to nab-paclitaxel therapy. J Clin Oncol. 2010;28:e684–e687. doi: 10.1200/JCO.2010.30.3750. [DOI] [PubMed] [Google Scholar]
- 98.Teitelbaum BA, Tresley DJ. Cystic maculopathy with normal capillary permeability secondary to docetaxel. Optom Vis Sci. 2003;80:277–279. doi: 10.1097/00006324-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 99.Telander DG, Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22:151–153. doi: 10.1080/08820530701457373. [DOI] [PubMed] [Google Scholar]
- 100.Smith SV, Benz MS, Brown DM. Cystoid macular edema secondary to albumin-bound paclitaxel therapy. Arch Ophthalmol. 2008;126:1605–1606. doi: 10.1001/archopht.126.11.1605. [DOI] [PubMed] [Google Scholar]
- 101.Joshi MM, Garretson BR. Paclitaxel maculopathy. Arch Ophthalmol. 2007;125:709–710. doi: 10.1001/archopht.125.5.709. [DOI] [PubMed] [Google Scholar]
- 102.Ito S, Okuda M. [A case of cystic maculopathy during paclitaxel therapy] Nippon Ganka Gakkai Zasshi. 2010;114:23–27. [PubMed] [Google Scholar]
- 103.Verrill M. Chemotherapy for early-stage breast cancer: A brief history. Br J Cancer. 2009;101 Suppl 1:S2–S5. doi: 10.1038/sj.bjc.6605268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Purcell WT, Ettinger DS. Novel antifolate drugs. Curr Oncol Rep. 2003;5:114–125. doi: 10.1007/s11912-003-0098-3. [DOI] [PubMed] [Google Scholar]
- 105.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 106.Prasad S, Kamath GG, Phillips RP. Lacrimal canalicular stenosis associated with systemic 5-fluorouacil therapy. Acta Ophthalmol Scand. 2000;78:110–113. doi: 10.1034/j.1600-0420.2000.078001110.x. [DOI] [PubMed] [Google Scholar]
- 107.Fezza JP, Wesley RE, Klippenstein KA. The treatment of punctal and canalicular stenosis in patients on systemic 5-FU. Ophthalmic Surg Lasers. 1999;30:105–108. [PubMed] [Google Scholar]
- 108.Lee V, Bentley CR, Olver JM. Sclerosing canaliculitis after 5-fluorouracil breast cancer chemotherapy. Eye (Lond) 1998;12(Pt 3a):343–349. doi: 10.1038/eye.1998.83. [DOI] [PubMed] [Google Scholar]
- 109.Brink HM, Beex LV. Punctal and canalicular stenosis associated with systemic fluorouracil therapy. Report of five cases and review of the literature. Doc Ophthalmol. 1995;90:l–6. doi: 10.1007/BF01203288. [DOI] [PubMed] [Google Scholar]
- 110.Eiseman AS, Flanagan JC, Brooks AB, Mitchell EP, Pemberton CH. Ocular surface, ocular adnexal, and lacrimal complications associated with the use of systemic 5-fluorouracil. Ophthal Plast Reconstr Surg. 2003;19:216–224. doi: 10.1097/01.iop.0000066648.33513.3d. [DOI] [PubMed] [Google Scholar]
- 111.Loprinzi CL, Love RR, Garrity JA, Ames MM. Cyclophosphamide, methotrexate, and 5-fluorouracil (CMF)-induced ocular toxicity. Cancer Invest. 1990;8:459–465. doi: 10.3109/07357909009012068. [DOI] [PubMed] [Google Scholar]
- 112.Griffin JD, Garnick MB. Eye toxicity of cancer chemotherapy: A review of the literature. Cancer. 1981;48:1539–1549. doi: 10.1002/1097-0142(19811001)48:7<1539::aid-cncr2820480713>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 113.Christophidis N, Vajda FJ, Lucas I, Louis WJ. Ocular side effects with 5-fluorouracil. Aust N Z J Med. 1979;9:143–144. doi: 10.1111/j.1445-5994.1979.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 114.Hussain MI. Ocular irritation from low-dose methotrexate. Cancer. 1982;50:605. [PubMed] [Google Scholar]
- 115.Martin M, Villar A, Sole-Calvo A, Gonzalez R, Massuti B, Lizon J, Camps C, Carrato A, Casado A, Candel MT, Albanell J, Aranda J, Munarriz B, Campbell J, Diaz-Rubio E. Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: A study by the GEICAM group. Ann Oncol. 2003;14:833–842. doi: 10.1093/annonc/mdg260. [DOI] [PubMed] [Google Scholar]
- 116.Bauer K, Parise C, Caggiano V. Use of ER / PR / HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer. 2010;10:228. doi: 10.1186/1471-2407-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG, McLean CA, Callagy G, Green AR, Ellis I, Gelmon K, Turashvili G, Leung S, Aparicio S, Huntsman D, Caldas C, Pharoah P. BCL2 in breast cancer: A favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–675. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 119.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park JW, Neve RM, Szollosi J, Benz CC. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer. 2008;8:392–401. doi: 10.3816/CBC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 121.Bedard PL, de Azambuja E, Cardoso F. Beyond trastuzumab: Overcoming resistance to targeted HER-2 therapy in breast cancer. Curr Cancer Drug Targets. 2009;9:148–162. doi: 10.2174/156800909787581024. [DOI] [PubMed] [Google Scholar]
- 122.Verma S, Lavasani S, Mackey J, Pritchard K, Clemons M, Dent S, Latreille J, Lemieux J, Provencher L, Verma S, Chia S, Wang B, Rayson D. Optimizing the management of HER2-positive early breast cancer: The clinical reality. Curr Oncol. 2010;17:20–33. doi: 10.3747/co.v17i4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Costa RB, Kurra G, Greenberg L, Geyer CE. Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol. 2010;21:2153–2160. doi: 10.1093/annonc/mdq096. [DOI] [PubMed] [Google Scholar]
- 124.Blommaert FA, van Dijk-Knijnenburg HC, Dijt FJ, den Engelse L, Baan RA, Berends F, Fichtinger-Schepman AM. Formation of DNA adducts by the anticancer drug carbo-platin: Different nucleotide sequence preferences in vitro and in cells. Biochemistry. 1995;34:8474–8480. doi: 10.1021/bi00026a031. [DOI] [PubMed] [Google Scholar]
- 125.Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kuhn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: Results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 126.Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: Is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- 127.Marin AP, Sanchez AR, Arranz EE, Aunon PZ, Baron MG. Adjuvant chemotherapy for breast cancer and cognitive impairment. South Med J. 2009;102:929–934. doi: 10.1097/SMJ.0b013e3181b23bf5. [DOI] [PubMed] [Google Scholar]
- 128.Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: Mechanisms, findings, and potential interventions. Palliat Support Care. 2007;5:273–280. doi: 10.1017/s1478951507000442. [DOI] [PubMed] [Google Scholar]
- 129.Raffa RB, Tallarida RJ. Effects on the visual system might contribute to some of the cognitive deficits of cancer chemotherapy-induced ‘chemo-fog’. J Clin Pharm Ther. 2010;35:249–255. doi: 10.1111/j.1365-2710.2009.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weiss B. Evaluation of multiple neurotoxic outcomes in cancer chemotherapy. Adv Exp Med Biol. 2010;678:96–112. doi: 10.1007/978-1-4419-6306-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Myers JS. Neuropsychologic testing for chemotherapy-related cognitive impairment. Adv Exp Med Biol. 2010;678:55–69. doi: 10.1007/978-1-4419-6306-2_9. [DOI] [PubMed] [Google Scholar]
- 132.Jansen CE, Miaskowski CA, Dodd MJ, Dowling GA. A meta-analysis of the sensitivity of various neuropsychological tests used to detect chemotherapy-induced cognitive impairment in patients with breast cancer. Oncol Nurs Forum. 2007;34:997–1005. doi: 10.1188/07.ONF.997-1005. [DOI] [PubMed] [Google Scholar]
- 133.McDonnell DP, Wijayaratne A, Chang CY, Norris JD. Elucidation of the molecular mechanism of action of selective estrogen receptor modulators. Am J Cardiol. 2002;90:35F–43F. doi: 10.1016/s0002-9149(01)02221-4. [DOI] [PubMed] [Google Scholar]
- 134.Musa MA, Khan MO, Cooperwood JS. Medicinal chemistry and emerging strategies applied to the development of selective estrogen receptor modulators (SERMs) Curr Med Chem. 2007;14:1249–1261. doi: 10.2174/092986707780598023. [DOI] [PubMed] [Google Scholar]
- 135.Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: An update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163–181. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 136.McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: Implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10:620–628. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sengupta S, Jordan VC. Selective estrogen modulators as an anticancer tool: Mechanisms of efficiency and resistance. Adv Exp Med Biol. 2008;630:206–219. doi: 10.1007/978-0-387-78818-0_13. [DOI] [PubMed] [Google Scholar]
- 138.Briest S, Wolff AC. Insights on adjuvant endocrine therapy for premenopausal and postmenopausal breast cancer. Expert Rev Anticancer Ther. 2007;7:1243–1253. doi: 10.1586/14737140.7.9.1243. [DOI] [PubMed] [Google Scholar]
- 139.Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, Hortobagyi GN. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104:2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 140.Agatonovic-Kustrin S, Turner JV. Molecular structural characteristics of estrogen receptor modulators as determinants of estrogen receptor selectivity. Mini Rev Med Chem. 2008;8:943–951. doi: 10.2174/138955708785132747. [DOI] [PubMed] [Google Scholar]
- 141.Johnston SR. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9 Suppl 1:S28–S36. doi: 10.3816/CBC.2009.s.003. [DOI] [PubMed] [Google Scholar]
- 142.Maenpaa H, Saransaari P, Tahti H. Kinetics of inhibition of glutamate uptake by antioestrogens. Pharmacol Toxicol. 2003;93:174–179. doi: 10.1034/j.1600-0773.2003.930404.x. [DOI] [PubMed] [Google Scholar]
- 143.Geisler J, Lonning PE. Endocrine effects of aromatase inhibitors and inactivators in vivo: Review of data and method limitations. J Steroid Biochem Mol Biol. 2005;95:75–81. doi: 10.1016/j.jsbmb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 144.Messinis IE. Ovarian feedback, mechanism of action and possible clinical implications. Hum Reprod Update. 2006;12:557–571. doi: 10.1093/humupd/dml020. [DOI] [PubMed] [Google Scholar]
- 145.Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9 Suppl 1:S6–S17. doi: 10.3816/CBC.2009.s.001. [DOI] [PubMed] [Google Scholar]
- 146.Nordman IC, Dalley DN. Breast cancer in men: Should aromatase inhibitors become first-line hormonal treatment? Breast J. 2008;14:562–569. doi: 10.1111/j.1524-4741.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 147.Harlan LC, Zujewski JA, Goodman MT, Stevens JL. Breast cancer in men in the United States: A population-based study of diagnosis, treatment, and survival. Cancer. 2010;116:3558–3568. doi: 10.1002/cncr.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh J, Cutuli B, Pruneri G, McCaskill-Stevens W, Gralow J, Hortobagyi G, Cardoso F. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J Clin Oncol. 2010;28:2114–2122. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Onami S, Ozaki M, Mortimer JE, Pal SK. Male breast cancer: An update in diagnosis, treatment and molecular profiling. Maturitas. 2010;65:308–314. doi: 10.1016/j.maturitas.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moss RL, Gu Q, Wong M. Estrogen: Nontranscriptional signaling pathway. Recent Prog Horm Res. 1997;52:33–68. discussion 68-39. [PubMed] [Google Scholar]
- 151.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 152.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 153.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2006;86:S187–S193. doi: 10.1016/j.fertnstert.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 155.Jordan VC. Tamoxifen: A most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 156.Racette L, Casson PR, Claman P, Zackon DH, Casson EJ. An investigation of the visual disturbances experienced by patients on clomiphene citrate. Fertil Steril. 2010;93:1169–1172. doi: 10.1016/j.fertnstert.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 157.Purvin VA. Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995;113:482–484. doi: 10.1001/archopht.1995.01100040102034. [DOI] [PubMed] [Google Scholar]
- 158.Bernstein HN. Some iatrogenic ocular diseases from systemically administered drugs. Int Ophthalmol Clin. 1970;10:553–619. [PubMed] [Google Scholar]
- 159.Roch LM, 2nd, Gordon DL, Barr AB, Paulsen CA. Visual changes associated with clomiphene citrate therapy. Arch Ophthalmol. 1967;77:14–17. doi: 10.1001/archopht.1967.00980020016004. [DOI] [PubMed] [Google Scholar]
- 160.Casper RF, Mitwally MF. Review: Aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760–771. doi: 10.1210/jc.2005-1923. [DOI] [PubMed] [Google Scholar]
- 161.Higgins MJ, Stearns V. CYP2D6 polymorphisms and tamoxifen metabolism: Clinical relevance. Curr Oncol Rep. 2010;12:7–15. doi: 10.1007/s11912-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 162.Brauch H, Murdter TE, Eichelbaum M, Schwab M. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55:1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 163.Gallicchio L, Lord G, Tkaczuk K, Danton M, Lewis LM, Lim CK, Flaws JA. Association of tamoxifen (TAM) and TAM metabolite concentrations with self-reported side effects of TAM in women with breast cancer. Breast Cancer Res Treat. 2004;85:89–97. doi: 10.1023/B:BREA.0000021050.92539.b0. [DOI] [PubMed] [Google Scholar]
- 164.Brauch H, Jordan VC. Targeting of tamoxifen to enhance antitumour action for the treatment and prevention of breast cancer: The ‘personalised’ approach? Eur J Cancer. 2009;45:2274–2283. doi: 10.1016/j.ejca.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 165.Meyer RP, Gehlhaus M, Knoth R, Volk B. Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metab. 2007;8:297–306. doi: 10.2174/138920007780655478. [DOI] [PubMed] [Google Scholar]
- 166.McKeown CA, Swartz M, Blom J, Maggiano JM. Tamoxifen retinopathy. Br J Ophthalmol. 1981;65:177–179. doi: 10.1136/bjo.65.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kaiser-Kupfer MI, Kupfer C, Rodrigues MM. Tamoxifen retinopathy. A clinicopathologic report. Ophthalmology. 1981;88:89–93. doi: 10.1016/s0161-6420(81)35071-4. [DOI] [PubMed] [Google Scholar]
- 168.Nayfield SG, Gorin MB. Tamoxifen-associated eye disease. A review. J Clin Oncol. 1996;14:1018–1026. doi: 10.1200/JCO.1996.14.3.1018. [DOI] [PubMed] [Google Scholar]
- 169.Gorin MB, Day R, Costantino JP, Fisher B, Redmond CK, Wickerham L, Gomolin JE, Margolese RG, Mathen MK, Bowman DM, Kaufman DI, Dimitrov NV, Singerman LJ, Bornstein R, Wolmark N, Kaufmann D. Long-term tamoxifen citrate use and potential ocular toxicity. Am J Ophthalmol. 1998;125:493–501. doi: 10.1016/s0002-9394(99)80190-1. [DOI] [PubMed] [Google Scholar]
- 170.Chung H, Kim D, Ahn SH, Kim JG, Lee JY, Lim JY, Yoon YH. Early detection of tamoxifen-induced maculopathy in patients with low cumulative doses of tamoxifen. Ophthalmic Surg Lasers Imaging. 2010;41:el–e5. doi: 10.3928/15428877-20100215-06. [DOI] [PubMed] [Google Scholar]
- 171.Longstaff S, Sigurdsson H, O'Keeffe M, Ogston S, Preece P. A controlled study of the ocular effects of tamoxifen in conventional dosage in the treatment of breast carcinoma. Eur J Cancer Clin Oncol. 1989;25:1805–1808. doi: 10.1016/0277-5379(89)90351-9. [DOI] [PubMed] [Google Scholar]
- 172.Tang R, Shields J, Schiffman J, Li H, Locher D, Hampton J, Prager T, Pardo G. Retinal changes associated with tamoxifen treatment for breast cancer. Eye. 1997;11(Pt 3):295–297. doi: 10.1038/eye.1997.64. [DOI] [PubMed] [Google Scholar]
- 173.Pavlidis NA, Petris C, Briassoulis E, Klouvas G, Psilas C, Rempapis J, Petroutsos G. Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity. A prospective study of 63 patients. Cancer. 1992;69:2961–2964. doi: 10.1002/1097-0142(19920615)69:12<2961::aid-cncr2820691215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 174.Noureddin BN, Seoud M, Bashshur Z, Salem Z, Shamseddin A, Khalil A. Ocular toxicity in low-dose tamoxifen: A prospective study. Eye. 1999;13(Pt 6):729–733. doi: 10.1038/eye.1999.217. [DOI] [PubMed] [Google Scholar]
- 175.Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994;117:772–775. doi: 10.1016/s0002-9394(14)70321-6. [DOI] [PubMed] [Google Scholar]
- 176.Lazzaroni F, Scorolli L, Pizzoleo CF, Savini G, De Nigris A, Giosa F, Meduri RA. Tamoxifen retinopathy: Does it really exist? Graefes Arch Clin Exp Ophthalmol. 1998;236:669–673. doi: 10.1007/s004170050139. [DOI] [PubMed] [Google Scholar]
- 177.Eisner A, Falardeau J, Toomey MD, Vetto JT. Retinal hemorrhages in anastrozole users. Optom Vis Sci. 2008;85:301–308. doi: 10.1097/OPX.0b013e31816bea3b. [DOI] [PubMed] [Google Scholar]
- 178.Eisner A, Toomey MD, Falardeau J, Samples JR, Vetto JT. Differential effects of tamoxifen and anastrozole on optic cup size in breast cancer survivors. Breast Cancer Res Treat. 2007;106:161–170. doi: 10.1007/s10549-006-9486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eisner A, Demirel S. Variability in short-wavelength automated perimetry among peri- or postmenopausal women: A dependence on phyto-oestrogen consumption? Acta Ophthalmol. 2011;89:e217–e224. doi: 10.1111/j.1755-3768.2009.01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Eisner A, Austin DF, Samples JR. Short wavelength automated perimetry and tamoxifen use. Br J Ophthalmol. 2004;88:125–130. doi: 10.1136/bjo.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eisner A, Thielman EJ, Falardeau J, Vetto JT. Vitreo-retinal traction and anastrozole use. Breast Cancer Res Treat. 2009;117:9–16. doi: 10.1007/s10549-008-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eisner A, Toomey MD. The color appearance of stimuli detected via short-wavelength-sensitive cones: Comparisons with visual adaptation and visual field data for peri- or post-menopausal women under 70 years of age. Vision Res. 2008;48:2663–2672. doi: 10.1016/j.visres.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eisner A, Toomey MD, Incognito LJ, O'Malley JP, Samples JR. Contrasting blue-on-yellow with white-on-white visual fields: Roles of visual adaptation for healthy peri- or postmenopausal women younger than 70 years of age. Invest Ophthalmol Vis Sci. 2006;47:5605–5614. doi: 10.1167/iovs.05-1612. [DOI] [PubMed] [Google Scholar]
- 184.Eisner A, Incognito LJ. The color appearance of stimuli detected via short-wavelength-sensitive cones for breast cancer survivors using tamoxifen. Vision Res. 2006;46:1816–1822. doi: 10.1016/j.visres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 185.Chung SE, Kim SW, Chung HW, Kang SW. Estrogen antagonist and development of macular hole. Korean J Ophthalmol. 2010;24:306–309. doi: 10.3341/kjo.2010.24.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cronin BG, Lekich CK, Bourke RD. Tamoxifen therapy conveys increased risk of developing a macular hole. Int Ophthalmol. 2005;26:101–105. doi: 10.1007/s10792-005-5424-3. [DOI] [PubMed] [Google Scholar]
- 187.Reasor MJ, Kacew S. Drug-induced phospholipidosis: Are there functional consequences? Exp Biol Med (Maywood) 2001;226:825–830. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- 188.Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004;15:541–548. doi: 10.1097/01.icu.0000143688.45232.15. [DOI] [PubMed] [Google Scholar]
- 189.Parkkari M, Paakkala AM, Salminen L, Holli K. Ocular side-effects in breast cancer patients treated with tamoxifen and toremifene: A randomized follow-up study. Acta Ophthalmol Scand. 2003;81:495–99. doi: 10.1034/j.1600-0420.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 190.Muftuoglu O, Ucakhan OO, Kanpolat A. Clinical and in vivo confocal microscopy findings in patients receiving tamoxifen citrate. Eye Contact Lens. 2006;32:228–232. doi: 10.1097/01.icl.0000201396.74294.85. [DOI] [PubMed] [Google Scholar]
- 191.Wang L, Wang S, Yin JJ, Fu PP, Yu H. Light-induced toxic effects of tamoxifen: A chemotherapeutic and chemo-preventive agent. J Photochem Photobiol A Chem. 2009;201:50–56. doi: 10.1016/j.jphotochem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-l Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 193.Vogel VG. The NSABP study of tamoxifen and raloxifene (STAR) trial. Expert Rev Anticancer Ther. 2009;9:51–60. doi: 10.1586/14737140.9.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paganini-Hill A, Clark LJ. Eye problems in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2000;60:167–172. doi: 10.1023/a:1006342300291. [DOI] [PubMed] [Google Scholar]
- 195.Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen and cataracts: A null association. Breast Cancer Res Treat. 2004;87:189–196. doi: 10.1023/B:BREA.0000041626.76694.85. [DOI] [PubMed] [Google Scholar]
- 196.Holli K, Valavaara R, Blanco G, Kataja V, Hietanen P, Flander M, Pukkala E, Joensuu H. Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer. Finnish Breast Cancer Group. J Clin Oncol. 2000;18:3487–3494. doi: 10.1200/JCO.2000.18.20.3487. [DOI] [PubMed] [Google Scholar]
- 197.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 198.Zhang JJ, Jacob TJ, Hardy SP, Higgins CF, Valverde MA. Lens opacification by antioestrogens: Tamoxifen vs ICI 182,780. Br J Pharmacol. 1995;115:1347–1348. doi: 10.1111/j.1476-5381.1995.tb16622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 200.Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T. Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res. 1993;53:3919–3924. [PubMed] [Google Scholar]
- 201.Young MA, Tunstall MJ, Kistler J, Donaldson PJ. Blocking chloride channels in the rat lens: Localized changes in tissue hydration support the existence of a circulating chloride flux. Invest Ophthalmol Vis Sci. 2000;41:3049–3055. [PubMed] [Google Scholar]
- 202.Zhang JJ, Jacob TJ. Volume regulation in the bovine lens and cataract. The involvement of chloride channels. J Clin Invest. 1996;97:971–978. doi: 10.1172/JCI118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Borg JJ, Yuill KH, Hancox JC, Spencer IC, Kozlowski RZ. Inhibitory effects of the antiestrogen agent clomiphene on cardiac sarcolemmal anionic and cationic currents. J Pharmacol Exp Ther. 2002;303:282–292. doi: 10.1124/jpet.102.038901. [DOI] [PubMed] [Google Scholar]
- 204.Zhang JJ, Jacob TJ, Valverde MA, Hardy SP, Mintenig GM, Sepulveda FV, Gill DR, Hyde SC, Trezise AE, Higgins CF. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J Clin Invest. 1994;94:1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Stifter E, Sacu S, Weghaupt H. Functional vision with cataracts of different morphologies: Comparative study. J Cataract Refract Surg. 2004;30:1883–1891. doi: 10.1016/j.jcrs.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 206.Wood J, Chaparro A, Anstey K, Lacherez P, Chidgey A, Eisemann J, Gaynor A, La P. Simulated visual impairment leads to cognitive slowing in older adults. Optom Vis Sci. 2010;87:1037–1043. doi: 10.1097/OPX.0b013e3181fe64d7. [DOI] [PubMed] [Google Scholar]
- 207.Ashford AR, Donev I, Tiwari RP, Garrett TJ. Reversible ocular toxicity related to tamoxifen therapy. Cancer. 1988;61:33–35. doi: 10.1002/1097-0142(19880101)61:1<33::aid-cncr2820610107>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 208.Colley SM, Elston JS. Tamoxifen optic neuropathy. Clin Experiment Ophthalmol. 2004;32:105–106. doi: 10.1046/j.1442-9071.2004.00769.x. [DOI] [PubMed] [Google Scholar]
- 209.Pugesgaard T, Von Eyben FE. Bilateral optic neuritis evolved during tamoxifen treatment. Cancer. 1986;58:383–386. doi: 10.1002/1097-0142(19860715)58:2<383::aid-cncr2820580232>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 210.Therssen R, Jansen E, Leys A, Rutten J, Meyskens J. Screening for tamoxifen ocular toxicity: A prospective study. Eur J Ophthalmol. 1995;5:230–234. doi: 10.1177/112067219500500406. [DOI] [PubMed] [Google Scholar]
- 211.Eisner A, O'Malley JP, Incognito LJ, Toomey MD, Samples JR. Small optic cup sizes among women using tamoxifen: Assessment with scanning laser ophthalmoscopy. Curr Eye Res. 2006;31:367–379. doi: 10.1080/02713680600602547. [DOI] [PubMed] [Google Scholar]
- 212.Mourits MJ, de Vries EG, ten Hoor KA, van der Zee AG, Willemse PH. Beware of amenorrhea during tamoxifen: It may be a wolf in sheep's clothing. J Clin Oncol. 2007;25:3787–3788. doi: 10.1200/JCO.2007.11.1633. author reply 3788-3789. [DOI] [PubMed] [Google Scholar]
- 213.Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, Rodriguez G, Simon J, Pincus S. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–791. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 214.Ritter C, Renner AB, Wachtlin J, Bechrakis NE, Krause L. [Tamoxifen retinopathy: A case series of clinical and functional data] Ophthalmologe. 2008;105:544–549. doi: 10.1007/s00347-007-1677-8. [DOI] [PubMed] [Google Scholar]
- 215.Salomao SR, Watanabe SE, Berezovsky A, Motono M. Multifocal electroretinography, color discrimination and ocular toxicity in tamoxifen use. Curr Eye Res. 2007;32:345–352. doi: 10.1080/02713680701229638. [DOI] [PubMed] [Google Scholar]
- 216.Eisner A, Samples JR, Campbell HM, Cioffi GA. Foveal adaptation abnormalities in early glaucoma. J Opt Soc Am A Opt Image Sci Vis. 1995;12:2318–2328. doi: 10.1364/josaa.12.002318. [DOI] [PubMed] [Google Scholar]
- 217.Eisner A. Longitudinal changes of visual function over 18 months: Evaluation of eyes with high-and low-risk macular degeneration characteristics. Doc Ophthalmol Proc Ser. 1993;56:175–187. [Google Scholar]
- 218.Eisner A, Burke SN, Toomey MD. Visual sensitivity across the menstrual cycle. Vis Neurosci. 2004;21:513–531. doi: 10.1017/S0952523804214031. [DOI] [PubMed] [Google Scholar]
- 219.Akar Y, Yucel I, Akar ME, Taskin O, Ozer HO. Menstrual cycle-dependent changes in visual field analysis of healthy women. Ophthalmologica. 2005;219:30–35. doi: 10.1159/000081780. [DOI] [PubMed] [Google Scholar]
- 220.Aydin E, Demyr Denyz H, Demyrturk F, Calpkan A, Aytan H. Effect of menopause on central retinal sensitivity. J Retina-Vitreous. 2007;15:197–201. [Google Scholar]
- 221.Verit FF, Oguz H, Ozkul Y, Bozkurt O. Long-term effects of tibolone on ocular functions in postmenopausal women. Arch Gynecol Obstet. 2007;275:255–261. doi: 10.1007/s00404-006-0251-y. [DOI] [PubMed] [Google Scholar]
- 222.Lakowski R, Morton A. Acquired colour losses and oral contraceptives. Mod Probl Ophthalmol. 1978;19:314–318. [PubMed] [Google Scholar]
- 223.Milano A, Dal Lago L, Sotiriou C, Piccart M, Cardoso F. What clinicians need to know about antioestrogen resistance in breast cancer therapy. Eur J Cancer. 2006;42:2692–2705. doi: 10.1016/j.ejca.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 224.Agrawal K, Onami S, Mortimer JE, Pal SK. Cognitive changes associated with endocrine therapy for breast cancer. Maturitas. 2010;67:209–214. doi: 10.1016/j.maturitas.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Elliot SJ, Catanuto P, Espinosa-Heidmann DG, Fernandez P, Hernandez E, Saloupis P, Korach K, Karl M, Cousins SW. Estrogen receptor beta protects against in vivo injury in RPE cells. Exp Eye Res. 2010;90:10–16. doi: 10.1016/j.exer.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L / Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci. 2003;44:3155–3162. doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- 227.Hayashi Y, Kitaoka Y, Munemasa Y, Ohtani-Kaneko R, Kikusui T, Uematsu A, Takeda H, Hirata K, Mori Y, Ueno S. Neuroprotective effect of 17beta-estradiol against N-methyl-D-aspartate-induced retinal neurotoxicity via p-ERK induction. J Neurosci Res. 2007;85:386–394. doi: 10.1002/jnr.21127. [DOI] [PubMed] [Google Scholar]
- 228.Watanabe SE, Berezovsky A, Motono M, Sacai PY, Pereira JM, Sallum JM, Gebrim LH, Salomao SR. Retinal function in patients treated with tamoxifen. Doc Ophthalmol. 2010;120:137–143. doi: 10.1007/s10633-009-9203-8. [DOI] [PubMed] [Google Scholar]
- 229.van de Velde CJ, Verma S, van Nes JG, Masterman C, Pritchard KI. Switching from tamoxifen to aromatase inhibitors for adjuvant endocrine therapy in postmenopausal patients with early breast cancer. Cancer Treat Rev. 2010;36:54–62. doi: 10.1016/j.ctrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 230.Lonning PE. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Ann Oncol. 2011;22:503–514. doi: 10.1093/annonc/mdq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Goss PE, Muss HB, Ingle JN, Whelan TJ, Wu M. Extended adjuvant endocrine therapy in breast cancer: Current status and future directions. Clin Breast Cancer. 2008;8:411–417. doi: 10.3816/CBC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 232.Petkov PI, Temelkov S, Villeneuve DL, Ankley GT, Mekenyan OG. Mechanism-based categorization of aromatase inhibitors: A potential discovery and screening tool. SAR QSAR Environ Res. 2009;20:657–678. doi: 10.1080/10629360903438347. [DOI] [PubMed] [Google Scholar]
- 233.Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457:219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ariazi EA, Leitao A, Oprea TI, Chen B, Louis T, Bertucci AM, Sharma CG, Gill SD, Kim HR, Shupp HA, Pyle JR, Madrack A, Donato AL, Cheng D, Paige JR, Jordan VC. Exemestane's 17-hydroxylated metabolite exerts biological effects as an androgen. Mol Cancer Ther. 2007;6:2817–2827. doi: 10.1158/1535-7163.MCT-07-0312. [DOI] [PubMed] [Google Scholar]
- 235.Rossi E, Morabito A, Di Rella F, Esposito G, Gravina A, Labonia V, Landi G, Nuzzo F, Pacilio C, De Maio E, Di Maio M, Piccirillo MC, De Feo G, D'Aiuto G, Botti G, Chiodini P, Gallo C, Perrone F, de Matteis A. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: The HOBOE trial. J Clin Oncol. 2009;27:3192–3197. doi: 10.1200/JCO.2008.18.6213. [DOI] [PubMed] [Google Scholar]
- 236.McCabe CM, Flynn HW, Jr, McLean WC, Brod RD, McDonald HR, Johnson MW, Williams GA, Mieler WF. Nonsurgical management of macular hemorrhage secondary to retinal artery macroaneurysms. Arch Ophthalmol. 2000;118:780–785. doi: 10.1001/archopht.118.6.780. [DOI] [PubMed] [Google Scholar]
- 237.Hayreh SS, Jonas JB. Posterior vitreous detachment: Clinical correlations. Ophthalmologica. 2004;218:333–343. doi: 10.1159/000079476. [DOI] [PubMed] [Google Scholar]
- 238.Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol. 1994;118:754–761. [PubMed] [Google Scholar]
- 239.Bainbridge J, Herbert E, Gregor Z. Macular holes: Vitreoretinal relationships and surgical approaches. Eye (Lond) 2008;22:1301–1309. doi: 10.1038/eye.2008.23. [DOI] [PubMed] [Google Scholar]
- 240.Chuo JY, Lee TY, Hollands H, Morris AH, Reyes RC, Rossiter JD, Meredith SP, Maberley DA. Risk factors for posterior vitreous detachment: A case-control study. Am J Ophthalmol. 2006;142:931–937. doi: 10.1016/j.ajo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 241.Evans JR, Schwartz SD, McHugh JD, Thamby-Rajah Y, Hodgson SA, Wormald RP, Gregor ZJ. Systemic risk factors for idiopathic macular holes: A case-control study. Eye. 1998;12(Pt 2):256–259. doi: 10.1038/eye.1998.60. [DOI] [PubMed] [Google Scholar]
- 242.Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- 243.Mitry D, Fleck BW, Wright AF, Campbell H, Charteris DG. Pathogenesis of rhegmatogenous retinal detachment: Predisposing anatomy and cell biology. Retina. 2010;30:1561–1572. doi: 10.1097/IAE.0b013e3181f669e6. [DOI] [PubMed] [Google Scholar]
- 244.Epstein RJ. Visual impairment in myopic patients with breast cancer receiving adjuvant therapy with aromatase inhibitors. Clin Breast Cancer. 2009;9:184–186. doi: 10.3816/CBC.2009.n.030. [DOI] [PubMed] [Google Scholar]
- 245.Kobayashi H, Kobayashi K, Okinami S. Macular hole and myopic refraction. Br J Ophthalmol. 2002;86:1269–1273. doi: 10.1136/bjo.86.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 247.Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: What do we know and how can we find out more? Breast Cancer Res Treat. 2010;120:525–538. doi: 10.1007/s10549-010-0757-7. [DOI] [PubMed] [Google Scholar]
- 248.Madea B. Is there recent progress in the estimation of the postmortem interval by means of thanatochemistry? Forensic Sci Int. 2005;151:139–149. doi: 10.1016/j.forsciint.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 249.Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 250.Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 251.Dizdar O, Ozcakar L, Malas FU, Harputluoglu H, Bulut N, Aksoy S, Ozisik Y, Altundag K. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia. J Clin Oncol. 2009;27:4955–960. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]
- 252.Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–3152. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 253.Magliano M. Menopausal arthralgia: Fact or fiction. Maturitas. 2010;67:29–33. doi: 10.1016/j.maturitas.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 254.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 255.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 256.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: A prospective study. Psychooncology. 2009;18:811–821. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 257.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004;13:61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 258.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jenkins V, Low R, Mitra S. Hearing sensitivity in women following chemotherapy treatment for breast cancer: Results from a pilot study. Breast. 2009;18:279–283. doi: 10.1016/j.breast.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 260.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Laroche M, Borg S, Lassoued S, De Lafontan B, Roche H. Joint pain with aromatase inhibitors: Abnormal frequency of Sjogren's syndrome. J Rheumatol. 2007;34:2259–2263. [PubMed] [Google Scholar]
- 262.Smith J A, Vitale S, Reed GF, Grieshaber SA, Goodman LA, Vanderhoof VH, Calis KA, Nelson LM. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:151–156. doi: 10.1001/archopht.122.2.151. [DOI] [PubMed] [Google Scholar]
- 263.Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and /or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009;15:1553–1572. [PMC free article] [PubMed] [Google Scholar]
- 264.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 265.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, Pepose JS, Kosheleff V, Porreco A, Lemp MA. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 266.Loprinzi CL, Sloan J, Stearns V, Slack R, Iyengar M, Diekmann B, Kimmick G, Lovato J, Gordon P, Pandya K, Guttuso T, Jr, Barton D, Novotny P. Newer antidepres-sants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fann JR, Thomas-Rich AM, Katon WJ, Cowley D, Pepping M, McGregor BA, Gralow J. Major depression after breast cancer: A review of epidemiology and treatment. Gen Hosp Psychiatry. 2008;30:112–126. doi: 10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 268.Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome p-450 mediated drug-drug interactions: An update. Curr Drug Metab. 2002;3:13–37. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]
- 269.Richa S, Yazbek JC. Ocular adverse effects of common psy-chotropic agents: A review. CNS Drugs. 2010;24:501–526. doi: 10.2165/11533180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 270.Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, Markson LE. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J Clin Psychiatry. 2004;65:959–965. doi: 10.4088/jcp.v65n0712. [DOI] [PubMed] [Google Scholar]
- 271.Yang Y, Wu K, Yuan H, Yu M. Cytochrome oxidase 2D6 gene polymorphism in primary open-angle glaucoma with various effects to ophthalmic timolol. J Ocul Pharmacol Ther. 2009;25:163–171. doi: 10.1089/jop.2008.0028. [DOI] [PubMed] [Google Scholar]
- 272.Nieminen T, Lehtimaki T, Maenpaa J, Ropo A, Uusitalo H, Kahonen M. Ophthalmic timolol: Plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67:237–245. doi: 10.1080/00365510601034736. [DOI] [PubMed] [Google Scholar]
- 273.Yuan H, Yu M, Yang Y, Wu K, Lin X, Li J. Association of CYP2D6 single-nucleotide polymorphism with response to ophthalmic timolol in primary open-angle glaucoma- a pilot study. J Ocul Pharmacol Ther. 2010;26:497–501. doi: 10.1089/jop.2010.0013. [DOI] [PubMed] [Google Scholar]
- 274.Wickham R. Best practice management of CINV in oncology patients: II. Antiemetic guidelines and rationale for use. J Support Oncol. 2010;8:10–15. [PubMed] [Google Scholar]
- 275.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: A review of the literature. Eye (Lond) 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 276.Weinreb RN, Polansky JR, Kramer SG, Baxter JD. Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci. 1985;26:170–175. [PubMed] [Google Scholar]
- 277.Tang SC. Women and bone health: Maximizing the benefits of aromatase inhibitor therapy. Oncology. 79:13–26. doi: 10.1159/000314997. [DOI] [PubMed] [Google Scholar]
- 278.Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 279.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 280.Santen RJ. Effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96:308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 281.Dasanu CA, Alexandrescu DT. Acute retinal pigment epithelial detachment secondary to pamidronate administration. J Oncol Pharm Pract. 2009;15:119–121. doi: 10.1177/1078155208097632. [DOI] [PubMed] [Google Scholar]
- 282.Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol. 2003;135:219–222. doi: 10.1016/s0002-9394(02)01840-8. [DOI] [PubMed] [Google Scholar]
- 283.Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95:4380–387. doi: 10.1210/jc.2010-0597. [DOI] [PubMed] [Google Scholar]
- 284.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008;34:453–475. doi: 10.1016/j.ctrv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 285.Coleman RE. Adjuvant bisphosphonates in breast cancer: Are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009;45:1909–1915. doi: 10.1016/j.ejca.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 286.Brufsky A, Bundred N, Coleman R, Lambert-Falls R, Mena R, Hadji P, Jin L, Schenk N, Ericson S, Perez EA. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 287.Haverbeke G, Pertile G, Claes C, Zeyen T. Posterior uveitis: An under-recognized adverse effect of pamidronate: 2 case reports. Bull Soc Beige Ophtalmol. 2003:71–76. [PubMed] [Google Scholar]
- 288.Zhang H, Zhang JJ, Lin MA, et al. Effect of estrogen on the permeability of retinal blood vessel. Chinese Journal of Ocular Fundus Diseases. 2005;21:174–176. [Google Scholar]
- 289.Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior vitreomacular adhesion: A potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144:741–746. doi: 10.1016/j.ajo.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 290.Mojana F, Cheng L, Bartsch DU, Silva GA, Kozak I, Nigam N, Freeman WR. The role of abnormal vitreomacular adhesion in age-related macular degeneration: Spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008;146:218–227. doi: 10.1016/j.ajo.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Schulze S, Hoerle S, Mennel S, Kroll P. Vitreomacular traction and exudative age-related macular degeneration. Acta Ophthalmol. 2008;86:470–481. doi: 10.1111/j.1755-3768.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- 292.Nguyen BQ, Fife RS. Vitreous contains a cartilage-related protein. Exp Eye Res. 1986;43:375–382. doi: 10.1016/s0014-4835(86)80074-4. [DOI] [PubMed] [Google Scholar]
- 293.Maumenee IH. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol. 1979;88:432–449. doi: 10.1016/0002-9394(79)90645-7. [DOI] [PubMed] [Google Scholar]
- 294.Lee BB. Paths to colour in the retina. Clin Exp Optom. 2004;87:239–248. doi: 10.1111/j.1444-0938.2004.tb05054.x. [DOI] [PubMed] [Google Scholar]
- 295.Sample PA, Johnson CA, Haegerstrom-Portnoy G, Adams AJ. Optimum parameters for short-wavelength automated perimetry. J Glaucoma. 1996;5:375–383. [PubMed] [Google Scholar]
- 296.Pugh EN, Jr, Mollon JD. A theory of the pi1 and pi3 color mechanisms of Stiles. Vision Res. 1979;19:293–312. doi: 10.1016/0042-6989(79)90175-5. [DOI] [PubMed] [Google Scholar]
- 297.Tailby C, Szmajda BA, Buzas P, Lee BB, Martin PR. Transmission of blue (S) cone signals through the primate lateral geniculate nucleus. J Physiol. 2008;586:5947–5967. doi: 10.1113/jphysiol.2008.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Roy S, Jayakumar J, Martin PR, Dreher B, Saalmann YB, Hu D, Vidyasagar TR. Segregation of short-wavelength-sensitive (S) cone signals in the macaque dorsal lateral geniculate nucleus. Eur J Neurosci. 2009;30:1517–1526. doi: 10.1111/j.1460-9568.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wade A, Augath M, Logothetis N, Wandell B. fMRI measurements of color in macaque and human. J Vis. 2008;8:l–19. doi: 10.1167/8.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu J, Wandell BA. Specializations for chromatic and temporal signals in human visual cortex. J Neurosci. 2005;25:3459–3468. doi: 10.1523/JNEUROSCI.4206-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


