Abstract
Background
Social determinants of health (SDOH) may influence the probability of people living with HIV also being infected with hepatitis C virus (HCV). We compared the SDOH of adults co-infected with HCV/HIV with that of HIV mono-infected adults to identify factors independently associated with HCV infection.
Methods
In this cross-sectional study, face-to-face interviews were conducted with 509 HIV-infected adults affiliated with or receiving services from community-based AIDS service organizations (CBAOs). The primary outcome measure was self-reported HCV infection status. Chi-square, Student’s t tests, and Wilcoxon rank-sum tests were performed to compare SDOH of HCV/HIV co-infected participants with that of HIV mono-infected participants. Multivariable hierarchical logistic regression was used to identify factors independently associated with HCV co-infection.
Results
Data on 482 (95 HCV/HIV co-infected and 387 HIV mono-infected) adults were analyzed. Compared with participants infected with HIV only, those who were co-infected with HIV and HCV were more likely to be heterosexual, Aboriginal, less educated and unemployed. They were more likely to have a low income, to not be receiving antiretroviral treatment, to live outside the Greater Toronto Area (GTA), to use/abuse substances, experience significant depression, and utilize addiction counselling and needle-exchange services. They also were more likely to report a history of homelessness and perceived housing-related discrimination and to have moved twice or more in the previous 12 months. Factors independently associated with HCV/HIV co-infection were history of incarceration (odds ratio [OR] 8.81, 95% CI 4.43–17.54), history of homelessness (OR 3.15, 95% CI 1.59–6.26), living outside of the GTA (OR 3.13, 95% CI 1.59–6.15), and using/abusing substances in the past 12 months (OR 2.05, 95% CI 1.07–3.91).
Conclusion
Differences in SDOH exist between HIV/HCV co-infected and HIV mono-infected adults. History of incarceration, history of homelessness, substance use, and living outside the GTA were independently associated with HCV/HIV co-infection. Interventions that reduce homelessness and incarceration may help prevent HCV infection in people living with HIV.
Hepatitis C virus (HCV) infection has become increasingly common among people living with HIV. Approximately 30% of people with HIV infection worldwide and 20% of Canadians living with HIV are also infected with HCV.1-4 In Canada, HCV is transmitted mainly through the sharing of injection drug use equipment and the receipt of blood and blood products.5-6 Other mechanisms of transmission include travelling or living in HCV-endemic countries, sharing equipment for inhalation drug use (such as crack pipes), sexual contact, perinatal or mother-to-child transmission, tattooing or body piercing with contaminated equipment, and the sharing of personal hygiene items such as razors and toothbrushes; these modes of transmission account for a small proportion of HCV cases.1,2,7,8-12
In more than 75% of people living with HIV infection, acute HCV infections develop into chronic infections.13 Among those with chronic HCV infection, HIV infection significantly impairs the cell-mediated responses to HCV antigens,14 leading to more rapid liver fibrosis.15,16 People with chronic HCV infection are at higher risk of severe liver disease,17 liver decompensation,18 progression to an AIDS-defining event,19 lower health-related quality of life,20 and death.18,19 Because of their increased burden of disease, co-infected individuals use more health care services than HIV mono-infected individuals.21 Prevention or early treatment of HCV co-infection would, therefore, benefit individual patients and the health care system as a whole.
Social determinants of health (SDOH) may have a bearing on an individual’s risk of becoming infected with HCV. Raphael22 defines SDOH as the economic and social conditions that determine the extent to which a person possesses the physical, social, and personal resources to identify and achieve health. These determinants include Aboriginal status, education, employment and working conditions, food security, health care services, housing, income and its distribution, the existence of a social safety net, social exclusion, unemployment and employment security.22 Among Canadians living with HIV, for example, injection drug use is a strong predictor of HCV infection,23,24 while injection drug use, in turn, is prevalent among Aboriginal people, street youth, current and former prisoners, and homeless people.25,26 Incarceration and homelessness are increasingly being recognized as determinants of health among injection drug users and those infected with HCV.27-29 There is also evidence linking HCV infection with other determinants of health, including age, gender, ethnicity, education, income, and unstable housing.2,7,30-34
To prevent co-infections and provide effective care for people who are already HCV/HIV co-infected, it is important to understand the complex interplay between demographic and socioeconomic factors, such as incarceration, substance use, HIV, and housing, that may affect the probability of becoming infected with HCV. Housing may play a particularly important role as an intermediate structural factor that links broader societal processes with an individual’s immediate social and physical environment and in turn influences health and well-being.35 Within the context of HIV, housing is powerfully linked with risk factors for exposure and transmission, and with the care and health of persons living with HIV.36-38 However, the potential influence of housing instability on HCV infection among those living with HIV in Ontario and Canada has not been explored. As such, our current study was designed to determine the unique contribution of housing characteristics, particularly housing instability factors, in the context of other social determinants of health for people with HCV/HIV co-infection.
Methods
Participants
Data for this study came from the 1-year follow-up of the Positive Spaces, Healthy Places (PSHP) study, an observational study involving a cohort of 602 people living with HIV infection and affiliated with or receiving services from community-based AIDS service organizations (CBAOs) in Ontario. The purpose of the PSHP study was to examine the impact of housing on health and health-related quality of life among people living with HIV.
To ensure the representativeness of the study sample, participants in the PSHP study were recruited using a wide range of access points, including shelters for homeless people; agencies serving women, families, and youth; Aboriginal organizations; transitional housing providers; and supportive housing agencies. Efforts were made to include harder-to-reach populations such as injection drug users and street-involved people (individuals who live in and out of hostels and homeless shelters).
Recruitment
Posters and flyers with information about the PSHP study were distributed to individuals receiving services from CBAOs across Ontario. Calls for study participants were also posted in CBAO newsletters and in a gay and lesbian news magazine. Interested participants were asked to call a toll-free telephone number.
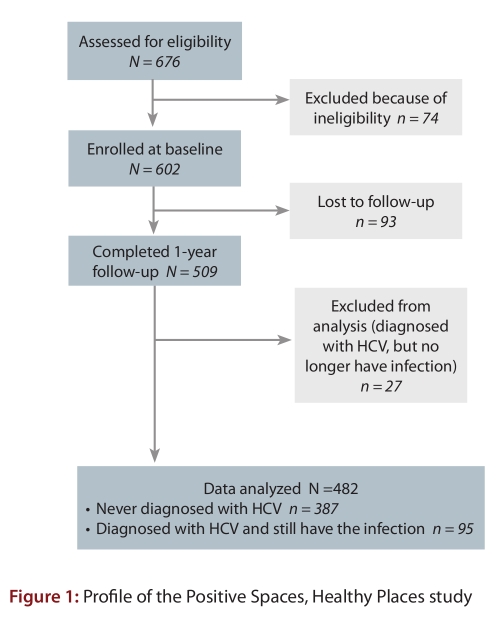
Of the 676 individuals who expressed interest in participating, 602 met the eligibility criteria and were enrolled in the PSHP study (see Fig. 1); thus the study’s targeted recruitment goal of 600 people was met. Participants were eligible if they lived in Ontario and were HIV-positive, were aged 18 years or older, and were able to provide informed consent. To minimize bias, recruitment was guided by predefined targets that reflected the regional, gender, sexual orientation, and ethnic distribution of HIV prevalence in the province. The 602 individuals enrolled were from various communities across Ontario, including Eastern Ontario (Ottawa, Kingston, and Peterborough), Northern Ontario (North Bay, Sudbury, and Thunder Bay), South and South Western Ontario (Niagara Falls, St. Catharines, Hamilton, London, Guelph, and Windsor) and the Greater Toronto Area (Toronto, Mississauga, Brampton, Oakville, Markham, Oshawa, Ajax, and Pickering).
Figure 1.
Profile of the Positive Spaces, Healthy Places study
To reflect the study’s strong commitment to community-based research (CBR)39 and the Greater Involvement of People living with HIV and AIDS (GIPA) principle,40 all consent forms and questionnaires were administered by 7 peer research assistants (PRAs), that is, people living with HIV who received training from the study team on interviewing techniques, principles of CBR, ethical issues in CBR, and CBR in Aboriginal communities. Ethics approval for the PSHP study was obtained from the Research Ethics Board of McMaster University, Hamilton, Ontario.
Data collection
Face-to-face interviews were conducted at baseline (April 2006 to September 2006) and at 1-year follow-up (April 2007 to December 2007) using a quantitative questionnaire that was piloted with 33 participants. It took approximately 75 minutes to complete the questionnaire/survey. Interviews were conducted at a local CBAO or in a public place (e.g., coffee shop), according to the participant’s preference. Study participants were allowed to self-complete questions that were considered confidential (e.g., the Drug Abuse Screening Test). Participants were paid an honorarium of $60 for the baseline interview and $40 for the 1-year follow-up interview. This study, however, uses data from the 1-year follow-up interviews only, as data on HCV infection were not collected at baseline.
Outcome measures
The following outcome measures were used.
HCV infection
The primary outcome in this study is self-reported HCV co-infection status. At the 1-year follow-up interview, participants were asked whether they had ever been tested for HCV and, if they had a history of HCV, whether they still had the infection. Those who reported a history of HCV diagnosis and still had the infection were considered to be HCV/HIV co-infected. Participants with a history of HCV infection who reported being clear of the infection at the time of the interview were excluded to minimize potential misclassification bias.
Sociodemographic characteristics
Data collected on sociodemographic characteristics included age, gender, sexual orientation, Aboriginal status, education, employment status, and personal income. We categorized gender as male, female or transgender; sexual orientation as heterosexual and gay, lesbian, or bisexual; Aboriginal status as Aboriginal and non-Aboriginal; education as less than high school or high school diploma or above; employment status as unemployed or employed; and income as ≤ CAD$1200/month or > CAD$1200/month. Participants were also asked whether they had ever been incarcerated; these responses were dichotomized into “have been incarcerated” or “have never been incarcerated.”
Health status and health care utilization
The study gathered information on selected HIV disease markers (i.e., highest CD4 count in the past 6 months, year of HIV diagnosis, history of diagnosis of AIDS, and receipt of antiretroviral therapy) and utilization of health care services, including addiction counselling and needle exchange program, in the past 12 months.
We categorized recent CD4 counts as ≥ 500 cells/mm3 or < 500 cells/mm3; history of diagnosis of AIDS into “yes, have been diagnosed with at least 1 AIDS-defining condition” or “have never been diagnosed with an AIDS defining condition”; receipt of antiretroviral therapy as “not receiving antiretroviral treatment currently” or “receiving antiretroviral treatment.” Time since HIV diagnosis was computed by subtracting the year of HIV diagnosis from the year the interview was conducted. Utilization of health service was defined as visiting a service provider at least once in the previous 12 months.
Health-related quality of life
Health-related quality of life was assessed using the MOS-HIV survey.41 This survey has 35 items that are used to compute 2 summary scores—Physical Health Summary (PHS) and Mental Health Summary (MHS)—and 10 subscales scores. This instrument has high internal consistency and test-retest reliability.42 All scores were used as continuous variables.
Depression
Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D).43 The CES-D is a self-report scale consisting of 20 items that assess the presence of depressive symptoms in the general population. The CES-D total score ranges from 0 to 60, such that higher scores indicate more severe depressive symptoms. This instrument has high test-retest reliability.44 A 16-point cut-off was used to categorize participants into 2 groups: significantly depressed (i.e., CES-D ≥ 16), or not significantly depressed or not depressed (CES-D < 16).
Alcohol consumption
Alcohol consumption was assessed using the Alcohol Use Disorders Identification Test (AUDIT-10).45 The AUDIT-10 has 10 items and is designed to assess and identify hazardous alcohol consumption, alcohol dependence and alcohol-related harm. This instrument has high internal consistency and test-retest reliability.46,47 In this paper, however, we used responses to items 1 to 3 of the AUDIT survey to dichotomize participants into heavy drinkers (i.e., consumed 6 or more drinks on 1 occasion at least once a month in the previous 12 months) and light drinkers (i.e., consumed 5 or fewer drinks on 1 occasion in the previous 12 months) or non-drinkers (i.e., did not consume any alcohol in the previous 12 months).
Substance use
The presence and degree of substance use/abuse was assessed using the Drug Abuse Screening Test (DAST-20) instrument.48 The DAST-20 is designed to assess and identify the abuse of psychoactive drugs; its validity is reported elsewhere.49-50 In this analysis, the first item of this instrument was used to assess the presence or absence of substance use in the past 12 months.
Housing characteristics
Housing-related data collected includedcity/town of current residence; type of housing (i.e., house, condominium, apartment, a room in a hotel/motel/boarding house, HIV housing facility with shared kitchen and bathroom, outdoors [including parks and streets], couch surfing, and shelter); length of residence in current housing; amount of rent; receipt of rent assistance; difficulty paying housing cost; and number of times moved in the past year. Participants were also asked whether they were currently homeless, had ever been homeless, and had ever experienced housing-related discrimination. Responses for city or town of residence were coded as living in the Greater Toronto Area (GTA) (i.e., Toronto, Mississauga, Brampton, Oakville, Markham, Oshawa, Ajax, and Pickering) and outside of the GTA (i.e., Ottawa, Kingston, Peterborough, North Bay, Sudbury, Thunder Bay, Niagara Falls, St. Catharines, Hamilton, London, Guelph, and Windsor). We dichotomized type of housing into “homeless or inadequately housed” (living outdoors, including parks and streets; living in a room in a hotel, motel, or boarding house; living in a shelter; and couch surfing) or “not homeless or adequately housed” (i.e., living in own residence or renting an apartment, condominium, or house); history of homelessness into “I have been homeless at least once” or “I have never been homeless”; housing-related discrimination into “experienced housing-related discrimination at least once in my lifetime” or “never experienced housing-related discrimination”; and number of times moved in the past 12 months into “moved twice or more” or “moved once or less.”
Several items from another study51 were adapted for our survey to assess participants’ level of satisfaction about various aspects of their dwelling (e.g., layout of rooms), meaningful dimensions of their housing (e.g., “proud of my home”), and their neighbourhood (e.g., satisfied with neighbourhood as a whole). Participants were asked to indicate on a Likert scale their level of satisfaction (ranging from 1 [very satisfied] to 5 [very dissatisfied]), or their agreement with statements describing meaningful dimensions of their housing (ranging from 1 [strongly agree] to 5 [strongly disagree]). For data analysis, items measuring satisfaction with dwelling and neighbourhood features were dichotomized into satisfied (i.e., very satisfied/satisfied) or neutral or dissatisfied (i.e., neutral/dissatisfied/very dissatisfied). Participants’ agreements with statements describing meaningful dimensions of their housing were dichotomized into “strongly agree/agree” or “neutral/disagree/strongly disagree.”
Statistical analysis
Data analyses were conducted in 2 stages. First, means and standard deviations were calculated for continuous variables, and frequencies were calculated for categorical variables. Characteristics of HIV/HCV co-infected and HIV mono-infected groups were compared using Student’s t test and Wilcoxon rank-sum tests for continuous variables and a chi-square test for categorical variables.
A hierarchical multivariable logistic regression model was fitted to examine factors independently associated with HCV co-infection. Hierarchical regression is not constrained by the assumption of independence observations, allows the use of multiple levels of information (thus providing additional statistical benefits), and is suited to public health research involving determinants of health.52,53 Variables that were associated (p < 0.05) with HCV co-infection in bivariable analyses and supported by existing literature were considered candidates for the regression model. We further examined Pearson product-moment correlation coefficients among the candidate variables and allowed only sexual orientation (between gender and sexual orientation) and history of homelessness (among history of homelessness and current homelessness or living in inadequate housing) to enter the final model.
As we were particularly interested in exploring the association between housing and HCV co-infection, selected variables were entered into the regression model in a set of four blocks, with housing variables entered last. First, sociodemographic variables were entered into the model. Health status variables were entered in the second step, followed by incarceration and substance use variables. Finally, housing variables were entered into the model.
Cases with missing data on covariates were omitted from the final regression model. To examine the potential bias that may arise because of the exclusion or inclusion of participants with a history of HCV diagnosis but who were clear of the infection at the time of data collection, sensitivity analysis was conducted by running the hierarchical regression model treating the excluded cases first as HCV/HIV co-infected and then as HIV mono-infected. All analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL) and all reported p values are two-tailed.
Results
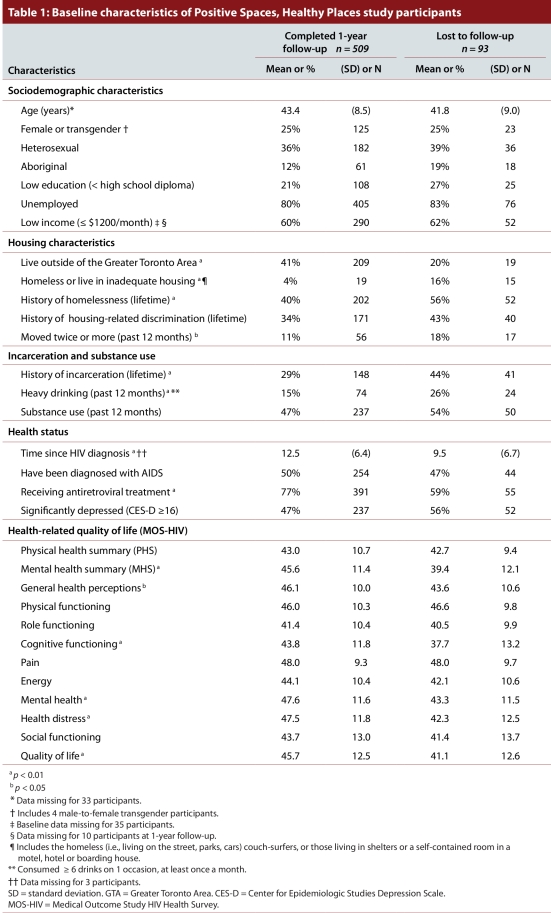
In total, 602 people living with HIV were enrolled in the PSHP study and completed the baseline interview in 2006. Of these, 509 (85%) completed the 1-year follow-up interview in 2007. Table 1 compares the baseline characteristics of the 509 participants who completed the 1-year follow-up with the characteristics of the 93 participants who were lost to follow-up between baseline and 1-year follow-up. Those who were lost to follow-up were more likely to be homeless or to live in adequate housing, to have moved twice or more in the previous 12 months, to have a history of homelessness, to have a history of incarceration, to live in the GTA and to be heavy drinkers than those who completed the 1-year follow-up interview. They were diagnosed with HIV more recently and were less likely to be on antiretroviral treatment. They also had significantly poor functioning and well-being as measured by the mental health summary and the general health perceptions, cognitive functioning, mental health, health distress, and quality of life dimension scores of the MOS-HIV health survey.
Table 1.
Baseline characteristics of Positive Spaces, Healthy Places study participants
Of the 509 participants who completed the 1-year follow-up and were considered for this analysis, 122 (24%) reported a history of diagnosis of HCV infection. Of the 122 with a history of HCV infection, 27 individuals indicated that they were free of the infection and were excluded. The 27 participants excluded from this analysis had sociodemographic, housing, drug use, and health characteristics that were similar to those of the 482 who were included, except for past history of incarceration (43% vs 24%, p < 0.05).
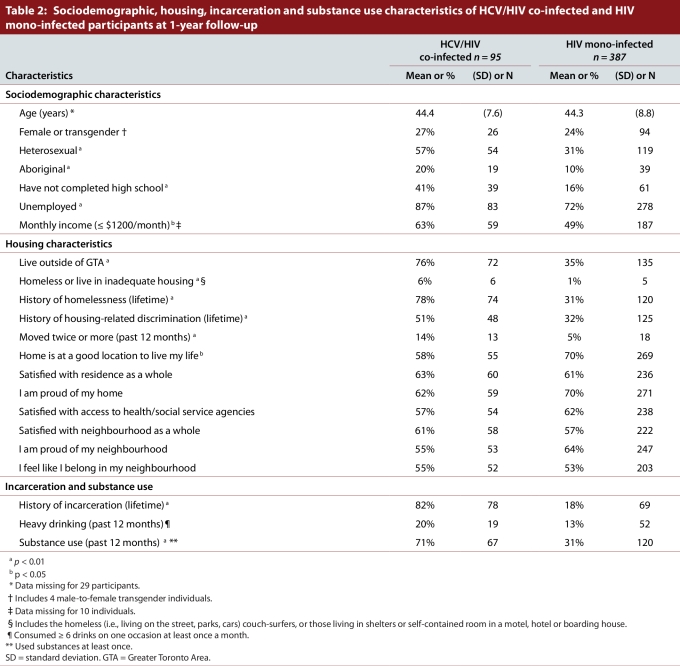
Four hundred and eighty-two (482) individuals were eligible for this analysis, including 95 HCV/HIV co-infected and 387 HIV mono-infected participants. CD4 count was unknown for 81 individuals, and 29 participants did not provide age information. Ten (10) participants refused to provide income information. Table 2 summarizes the sociodemographic, housing, incarceration, and substance use characteristics of HCV/HIV co-infected versus HIV mono-infected participants
Table 2.
Sociodemographic, housing, incarceration and substance use characteristics of HCV/HIV co-infected and HIV mono-infected participants at 1-year follow-up
There were 120 (25%) women or men-to-women transgendered participants, and 362 men (75%); the mean age was 43 years. Co-infected individuals were more likely to be heterosexual (57% vs 31%, p < 0.01), to be Aboriginal (20% vs 10%, p < 0.01), to have less than a high school education (41% vs 16%, p < 0.01), to be unemployed (87% vs 72%, p < 0.01), and to report a monthly income of $1200 or less (63% vs 49%, p < 0.05).
Participants with HCV/HIV co-infection were more likely than participants with HIV mono-infection to live outside of the Greater Toronto Area (76% vs 35%, p < 0.01), to have a history of homelessness (78% vs 31%, p < 0.01), to have experienced housing-related discrimination (51% vs 32%, p < 0.01), to have moved twice or more in the previous 12 months (14% versus 5%, p < 0.01), and to be less satisfied with the location of their home (58% vs 70%, p < 0.05). They were more likely to report a history of incarceration (82% versus 18%, p < 0.01) and substance use (71% versus 31%, p < 0.01) in the previous 12 months.
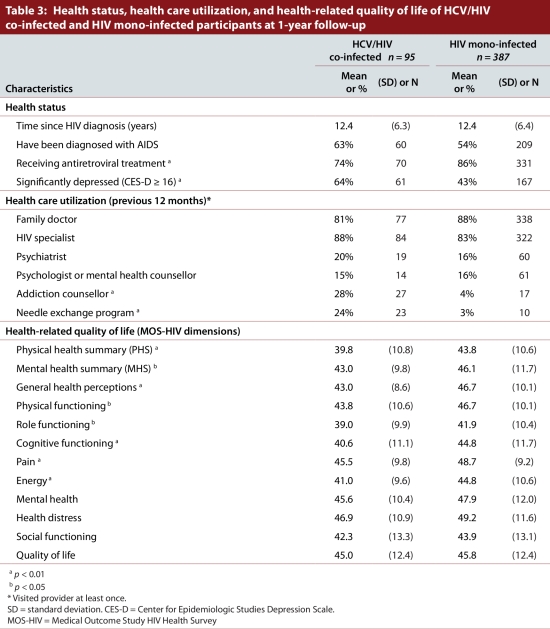
As summarized in Table 3, co-infected individuals were less likely to be on antiretroviral treatment (74% vs 86%, p < 0.01), but were more likely to report a significant level of depressive symptoms (64% vs 43%, p < 0.01). They also reported significantly (p < 0.05) poorer health-related quality of life in both physical and mental health summary measures of the MOS-HIV. HCV/HIV co-infected participants also reported significantly (p < 0.05) lower scores in general health perception, physical functioning, role functioning, cognitive functioning, pain, and energy dimensions of the MOS-HIV health survey. The utilization of services of a family doctor, HIV specialist and mental health providers in the past 12 months did not differ statistically between HCV/HIV co-infected and HIV mono-infected participants. However, co-infected participants reported higher utilization of addictions counselling (28% vs 4%, p < 0.01) and needle exchange (24% vs 3%, p < 0.01) services.
Table 3.
Health status, health care utilization, and health-related quality of life of HCV/HIV co-infected and HIV mono-infected participants at 1-year follow-up
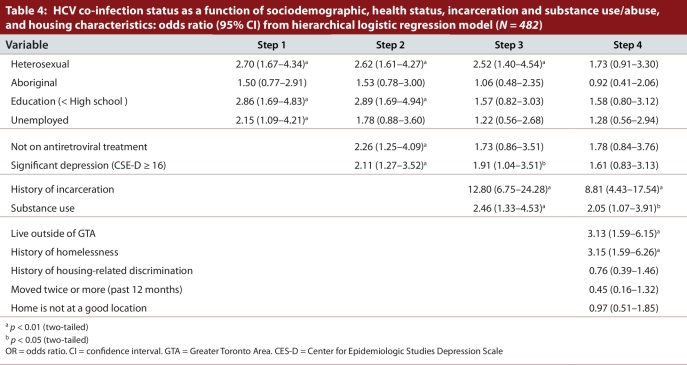
Results of the final hierarchical logistic regression model are presented in Table 4. None of the sociodemographic variables entered in the first block were significant in the final model. Similarly, receipt of antiretroviral treatment and depression were not associated independently with HCV co-infection. Both variables entered in the third block—history of incarceration (OR = 8.81, 95% CI 4.43–17.54, p < 0.001) and substance use (OR = 2.05, 95 % CI 1.07–3.91; p < 0.05)—were independently associated with being infected with HCV. Among the 5 housing-related variables entered in the last block, history of homelessness (OR = 3.15, 95% CI 1.59–6.26, p < 0.01) and living outside of the GTA (OR = 3.13, 95% CI 1.59–6.15, p < 0.01) were significant in the final regression model.
Table 4.
HCV co-infection status as a function of sociodemographic, health status, incarceration and substance use/abuse, and housing characteristics: odds ratio (95% CI) from hierarchical logistic regression model
To examine the potential bias of excluding 27 participants from the analysis, we repeated the regression model first by treating them as HCV/HIV co-infected and then as HIV mono-infected. Results of the regression models (not presented here) showed that history of incarceration, history of homelessness, substance use, and living outside of GTA remained significant in both models. However, the effects of magnitudes were slightly lower than the final model presented in Table 4.
Discussion
Although the prevalence of HCV infection among people living with HIV in Ontario is estimated to be 12%,4 our study found a 20% prevalence in a community sample. Of all variables associated with HCV infection in bivariable analyses, only history of incarceration, history of homelessness, substance use, and living outside of the GTA were independently associated with self-reported HCV infection status in multivariable analysis.
Our finding of a strong association between history of incarceration and HCV co-infection is consistent with evidence showing high infection rates among the prison population.4,54 Prisons may influence the transmission of HCV infection among inmates in several ways. First, a high proportion of those incarcerated use illicit drugs, including injection drugs.55 Second, because a high proportion of incarcerated individuals are infected with HCV,55 the prison setting may facilitate HCV transmission among inmates who engage in injection drug use. In addition to injection drug use, other risk factors for hepatitis C transmission, such as unsafe body piercings and unprotected anal sex with male injection drug users are also common in prisons.56,57 The limited access to or lack of harm-reduction interventions in prisons, such as needle exchange programs or condoms,55 increases the probability that inmates will contract HCV infection.
In our sample, history of homelessness was strongly associated with HCV co-infection. Previous studies have shown a link between homelessness and increased risk factors for HIV and HCV infection among injection drug users, such as needle sharing, going to a shooting gallery, having multiple sexual partners, and having unprotected sex.58,59 Although we did not collect data on current injection drug use, needle sharing, and sexual practices of participants, it is possible that those who were homeless at some point in their lives were exposed to these HCV risk factors. In our sample, 55% of the participants (92% of HCV/HIV co-infected and 32% of HIV mono-infected) who reported a history of homelessness had also been incarcerated in the past.
The higher prevalence of HIV/HCV co-infection among those living outside of the GTA may be attributable in part to the high HCV infection rates among injection drug users in South West, Central West, East, and Northern Ontario, as compared with Central Eastern Ontario, which includes the GTA.60 In our sample, higher proportions of participants from areas outside of the GTA reported a history of incarceration, a history of homelessness, and substance use. Therefore, the higher prevalence of HCV infection outside of the GTA may be a reflection of the higher prevalence of HCV risk factors in those areas of the province. We also hypothesize that the lack or shortage of health care, housing, employment, and social support services outside of the GTA compared with in the GTA may be a factor for the high prevalence of HIV/HIV co-infection. For example, in our sample, participants living outside of the GTA were more likely to report the need for more needle exchange services and addictions counselling than those who lived in the GTA.
There are limitations to our study. First, our study participants are primarily individuals affiliated with or receiving services from CBAOs. Although it is not possible to establish how representative these participants were of people living with HIV in Ontario, comparison of our participants with epidemiological data on people living with HIV in Ontario in 200661 shows some similarity. For example, 76% of our participants were male (vs 82%), 60% were gay or bisexual men (vs 68%), 91% were between the ages of 15 and 60 years (vs 97%). However, we cannot rule out the possibility of other biases, since individuals recruited from organizations serving stigmatized populations may be different from those who do not receive these services,62 and a study63 has found that those who receive services from CBAOs are more likely to have poor health and physical disability. Second, it is possible that, because of their HIV-positive status, the participants may have had increased contact with the health care system and hence may be more likely to be aware of their HCV status and to report a diagnosis of HCV infection than the general population. The use of self-reported HCV status as an outcome measure may also underestimate the true prevalence of HCV infection: studies have reported its low sensitivity in comparison with laboratory diagnosis.34,64 Third, all data are self-reported and were collected through face-to-face interviews and may be subject to recall and social desirability response biases. Finally, given the cross-sectional nature of the study, we are unable to draw conclusions about the cause and effect or direction of association between social determinants of health and HCV co-infection.
Despite these limitations, our findings contribute to existing evidence that certain determinants of health are associated with HCV infection among people living with HIV. Our study suggests that incarceration, homelessness, substance use, and living outside of the GTA are independently associated with HCV infection among people living with HIV in Ontario. Three of these four factors—homelessness, incarceration, and substance use—are highly interconnected and are influenced by socioeconomic and social factors.27,29 Interventions that reduce homelessness may reduce the risk of HCV infection, and housing may represent one potential amenable factor in the prevention of HCV co-infection among those living with HIV. Interventions that reduce the risk of unsafe drug use and tattooing in closed housing environments, such as prisons—including access to clean equipment as well as substitution therapy and other addiction treatment services—would also likely reduce the risk of HCV co-infection in people with HIV.
Acknowledgments
We thank M. Hamilton, D.B. Hintzen, J. Truax and the late P. White, who conducted the interviews. We are indebted to the study participants for their continued participation and to the community-based AIDS service organizations in Ontario for their sustained support to the Positive Spaces, Healthy Places study. We are grateful to the three anonymous reviewers who provided helpful comments on a previous draft of this paper.
Biographies
Sean Rourke is the Scientific and Executive Director of the Ontario HIV Treatment Network, Director of the CIHR Centre for Research Evidence in Action for Community Heath (REACH) in HIV/AIDS, Director of Universities Without Walls (CIHR STIHR), Professor in the Department of Psychiatry at University of Toronto, and Scientist at the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Toronto, Ontario.
Michael Sobota is the former Executive Director of AIDS Thunder Bay, Thunder Bay, Ontario.
Ruthann Tucker is Advisor, the Ontario HIV Treatment Network, Toronto, Ontario.
Tsegaye Bekele is Research Analyst at the Ontario HIV Treatment Network, Toronto, Ontario.
Katherine Gibson, medical student, is at the University of Calgary, Calgary, Alberta.
Saara Greene is Assistant Professor, School of Social Work, McMaster University, Hamilton, Ontario.
Colleen Price is Chair of the Canadian Treatment Action Council (CTAC) HIV and Hepatitis Working Group and board member of the Canadian Treatment Action Council (CTAC), Toronto, Ontario.
J.J. (Jay) Koornstra is Executive Director of Bruce House, Ottawa, Ontario.
LaVerne Monette (deceased) was Executive Director of the Ontario Aboriginal HIV/AIDS Strategy, Toronto, Ontario.
Steve Byers is Executive Director of AIDS Niagara, St. Catharines, Ontario.
James Watson is Coordinator of the Positive Spaces, Healthy Places study at the Ontario HIV Treatment Network, Toronto, Ontario.
Stephen Hwang is Associate Professor, Faculty of Medicine, University of Toronto, and Scientist at the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Toronto, Ontario.
Dale Guenter is Associate Professor, Department of Family Medicine, McMaster University, Hamilton, Ontario.
James Dunn is Associate Professor, Department of Health, Aging & Society, McMaster University, Hamilton, Ontario and Research Scientist at the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute of St. Michael’s Hospital, Toronto, Ontario
Amrita Ahluwalia is Director, Research and Evaluation, Fife House, Toronto, Ontario.
Michael Wilson is Associate Scientist, Knowledge Transfer and Exchange, The Ontario HIV Treatment Network, Toronto, Ontario.
Jean Bacon is Director, Health Policy, the Ontario HIV Treatment Network, Toronto, Ontario.
Footnotes
Competing interests: None declared.
Contributors: Sean Rourke conceived the project, contributed to the design of the study, oversaw data collection, guided the data analysis, and was the principal writer of the manuscript. He is responsible for the integrity of the work as a whole. Michael Sobota conceived the study, oversaw data collection, contributed to interpretation of the data, and revised the manuscript drafts. Ruthann Tucker conceived the study, contributed to the design of the study and interpretation of data, and revised the manuscript. Saara Greene, LaVerne Monette, Jay Koornstra, and Steve Byers conceived the study, contributed to the design of the study, oversaw data collection, and reviewed the manuscript. Tsegaye Bekele conducted the data analysis and participated in all phases of the writing. Katherine Gibson participated in the design of the study, participated in drafting the manuscript, and reviewed the manuscript. Colleen Price participated in the conception of the study, contributed to interpretation of the data, and reviewed the manuscript. James Watson participated in the design of the study and collection of data, contributed to the interpretation of data, and reviewed the manuscript. Stephen Hwang, Dale Guenter, and James Dunn conceived the project, participated in the design of the study, contributed to the data interpretation, and revised the article. Michael Wilson contributed to the interpretation of the data, drafted the article, and reviewed the manuscript. Amrita Ahluwalia oversaw data collection and revised manuscript drafts. Jean Bacon contributed to the design of the study, drafted the manuscript, and reviewed the article. All of the authors approved the final version of the manuscript.
Funding source: The Positive Spaces, Healthy Places study was funded by grants from the Canadian Institutes of Health Research (CBR-75568 and CBR-94036), the Ontario Ministry of Health and Long Term Care, the Ontario AIDS Network, the Wellesley Institute, and the Ontario HIV Treatment Network (CCB115). The sponsors had no role in the collection, analysis or interpretation of the data, in the writing of the report or in the decision to submit the report for publication.
Dedication: This article is dedicated to the memory of LaVerne Monette, co-investigator with the CIHR-funded Positive Spaces, Healthy Places (PSHP) research project, who passed away December 1, 2010. Responsible for the Aboriginal arm of the study, she played a key role in developing the questionnaire, analyzing the data and presenting the findings. She brought to our team her life experiences as an Aboriginal woman and her passion to help Aboriginal people living with and at risk of HIV. She understood the critical role of housing in health and quality of life, and was a strong advocate for research to identify the housing needs of Aboriginal people in Ontario and for policy change that will lead to safe, stable housing for all.
References
- 1.Rauch Andri, Rickenbach Martin, Weber Rainer, Hirschel Bernard, Tarr Philip E, Bucher Heiner C, Vernazza Pietro, Bernasconi Enos, Zinkernagel Annelies S, Evison John, Furrer Hansjakob Swiss HIV Cohort Study. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005 Jun 21;41(3):395–402. doi: 10.1086/431486. http://www.cid.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=16007539. [DOI] [PubMed] [Google Scholar]
- 2.Hershow R C, Kalish L A, Sha B, Till M, Cohen M. Hepatitis C virus infection in Chicago women with or at risk for HIV infection: evidence for sexual transmission. Sex Transm Dis. 1998;25(10):527–32. doi: 10.1097/00007435-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Jones R, Dunning J, Nelson M. HIV and hepatitis C co-infection. Int J Clin Pract. 2005;59(9):1082–1087. doi: 10.1111/j.1742-1241.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 4.Remis R. Final report: Estimating the number of persons co-infected with hepatitis C virus and human immunodeficiency virus in Canada. Ottawa: Population and Public Health Branch, Health Canada; 2001. [accessed 2010 August 23]. http://www.phac-aspc.gc.ca/hepc/pubs/hivhcv-vhcvih/index-eng.php. [Google Scholar]
- 5.Public Health Agency of Canada. Epidemiology of acute hepatitis C infection in Canada: results from the Enhanced Hepatitis Strain Surveillance System (EHSSS) 2009. [accessed 2010 August 23]. http://www.phac-aspc.gc.ca/sti-its-surv-epi/pdf/hcv-epi-eng.pdf.
- 6.Blanchette V, Walker I, Gill P, Adams M, Roberts R, Inwood M. Hepatitis C infection in patients with hemophilia: results of a national survey. Canadian Hemophilia Clinic Directors Group. Transfus Med Rev. 1994;8(3):210–217. doi: 10.1016/s0887-7963(94)70112-7. [DOI] [PubMed] [Google Scholar]
- 7.Frederick Toni, Burian Pamela, Terrault Norah, Cohen Mardge, Augenbraun Michael, Young Mary, Seaberg Eric, Justman Jessica, Levine Alexandra M, Mack Wendy J, Kovacs Andrea. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women's Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23(11):915–923. doi: 10.1089/apc.2009.0111. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19877800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortu Stephanie, McMahon James M, Pouget Enrique R, Hamid Rahul. Sharing of noninjection drug-use implements as a risk factor for hepatitis C. Subst Use Misuse. 2004;39(2):211–224. doi: 10.1081/ja-120028488. [DOI] [PubMed] [Google Scholar]
- 9.Howe Chanelle J, Fuller Crystal M, Ompad Danielle C, Galea Sandro, Koblin Beryl, Thomas David, Vlahov David. Association of sex, hygiene and drug equipment sharing with hepatitis C virus infection among non-injecting drug users in New York City. Drug Alcohol Depend. 2005 Apr 18;79(3):389–395. doi: 10.1016/j.drugalcdep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Roy K M, Goldberg D J, Hutchinson S, Cameron S O, Wilson K, MacDonald L. Hepatitis C virus among self declared non-injecting sexual partners of injecting drug users. J Med Virol. 2004;74(1):62–66. doi: 10.1002/jmv.20146. [DOI] [PubMed] [Google Scholar]
- 11.Fischer Benedikt, Powis Jeff, Firestone Cruz Michelle, Rudzinski Katherine, Rehm Jürgen. Hepatitis C virus transmission among oral crack users: viral detection on crack paraphernalia. Eur J Gastroenterol Hepatol. 2008;20(1):29–32. doi: 10.1097/MEG.0b013e3282f16a8c. [DOI] [PubMed] [Google Scholar]
- 12.Vertical transmission of the hepatitis C virus: Current knowledge and issues. Paediatr Child Health. 2008;13(6):529–541. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19436425. [PMC free article] [PubMed] [Google Scholar]
- 13.Schnuriger Aurélie, Dominguez Stéphanie, Guiguet Marguerite, Harfouch Sawsan, Samri Assia, Ouazene Zineb, Slama Laurence, Simon Anne, Valantin Marc-Antoine, Thibault Vincent, Autran Brigitte ANRS HC EP21 study group. Acute hepatitis C in HIV-infected patients: rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS. 2009;23(16):2079–2089. doi: 10.1097/QAD.0b013e328330ed24. [DOI] [PubMed] [Google Scholar]
- 14.Capa Laura, Soriano Vincent, García-Samaniego Javier, Nuñez Marina, Romero Miriam, Cascajero Almudena, Muñoz Fernando, González-Lahoz Juan, Benito José M. Influence of HCV genotype and co-infection with human immunodeficiency virus on CD4(+) and CD8(+) T-cell responses to hepatitis C virus. J Med Virol. 2007;79(5):503–510. doi: 10.1002/jmv.20856. [DOI] [PubMed] [Google Scholar]
- 15.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 16.Osinusi Anu, Kleiner David, Wood Brad, Polis Michael, Masur Henry, Kottilil Shyam. Rapid development of advanced liver fibrosis after acquisition of hepatitis C infection during primary HIV infection. AIDS Patient Care STDS. 2009;23(6):403–406. doi: 10.1089/apc.2008.0238. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19519227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham C S, Baden L R, Yu E, Mrus J M, Carnie J, Heeren T, Koziel M J. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001 Jul 06;33(4):562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 18.Monga H K, Rodriguez-Barradas M C, Breaux K, Khattak K, Troisi C L, Velez M, Yoffe B. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis. 2001 Jun 15;33(2):240–247. doi: 10.1086/321819. [DOI] [PubMed] [Google Scholar]
- 19.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti J C, Hirschel B, Janin P, Francioli P, Flepp M, Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 20.Tsui Judith I, Bangsberg David R, Ragland Kathleen, Hall Christopher S, Riley Elise D. The impact of chronic hepatitis C on health-related quality of life in homeless and marginally housed individuals with HIV. AIDS Behav. 2007;11(4):603–610. doi: 10.1007/s10461-006-9157-8. [DOI] [PubMed] [Google Scholar]
- 21.Braitstein P, Li K, Kerr T, Montaner J S G, Hogg R S, Wood E. Differences in access to care among injection drug users infected either with HIV and hepatitis C or hepatitis C alone. AIDS Care. 2006;18(7):690–693. doi: 10.1080/09540120500359330. [DOI] [PubMed] [Google Scholar]
- 22.Raphael D. Social determinants of health: Canadian perspectives. Toronto: Canadian Scholars' Press; 2004. [Google Scholar]
- 23.Amin J, Kaye M, Skidmore S, Pillay D, Cooper D A, Dore G J. HIV and hepatitis C coinfection within the CAESAR study. HIV Med. 2004;5(3):174–179. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 24.Remis R. The epidemiology of hepatitis C infection in Ontario, 2004 [presentation]; Ontario Harm Reduction Distribution Program; 4-6 March 2007; Toronto. http://www.phs.utoronto.ca/ohemu/doc/EpiHCVOnt.pdf. [Google Scholar]
- 25.Remis R. Epidemiology of hepatitis C infection in Canada [presentation]; 1st Canadian Conference on Hepatitis C; 1-4 May 2001; Montreal (QC). http://www.phs.utoronto.ca/ohemu/doc/HepCrev4.ppt. [Google Scholar]
- 26.Public Health Agency of Canada. Enhanced surveillance of risk behaviours among injecting drug users in Canada. 2006. [accessed 2010 August 23]. http:// www.phac-aspc.gc.ca/i-track/sr-re-1/index-eng.php.
- 27.Galea Sandro, Vlahov David. Social determinants and the health of drug users: socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(Suppl 1):S135–S145. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/12435837. [PMC free article] [PubMed] [Google Scholar]
- 28.Freudenberg Nicholas. Adverse effects of US jail and prison policies on the health and well-being of women of color. Am J Public Health. 2002;92(12):1895–1899. doi: 10.2105/ajph.92.12.1895. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/12453803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awofeso Niyi. Prisons as social determinants of hepatitis C virus and tuberculosis infections. Public Health Rep. 2010;125(Suppl 4):25–33. doi: 10.1177/00333549101250S406. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/20626190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Operskalski Eva A, Mack Wendy J, Strickler Howard D, French Audrey L, Augenbraun Michael, Tien Phyllis C, Villacres Maria C, Spencer LaShonda Y, Degiacomo Marina, Kovacs Andrea. Factors associated with hepatitis C viremia in a large cohort of HIV-infected and -uninfected women. J Clin Virol. 2008 Feb 20;41(4):255–263. doi: 10.1016/j.jcv.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyamathi Adeline M, Dixon Elizabeth L, Robbins Wendie, Smith Cynthia, Wiley Dorothy, Leake Barbara, Longshore Douglas, Gelberg Lillian. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med. 2002;17(2):134–143. doi: 10.1046/j.1525-1497.2002.10415.x. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/11841529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman Kenneth E, Rouster Susan D, Chung Raymond T, Rajicic Natasa. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002 Feb 06;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 33.Kim Christina, Kerr Thomas, Li Kathy, Zhang Ruth, Tyndall Mark W, Montaner Julio S G, Wood Evan. Unstable housing and hepatitis C incidence among injection drug users in a Canadian setting. BMC Public Health. 2009 Jul 29;9(1):270–279. doi: 10.1186/1471-2458-9-270. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19640297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall Christopher S, Charlebois Edwin D, Hahn Judith A, Moss Andrew R, Bangsberg David R. Hepatitis C virus infection in San Francisco's HIV-infected urban poor. J Gen Intern Med. 2004;19(4):357–365. doi: 10.1111/j.1525-1497.2004.30613.x. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/15061745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irwin A, Scali E. Action on the social determinants of health: learning from previous experiences. Background paper. Geneva: World Health Organization; 2005. [accessed 2010 August 23]. http://www.who.int/social_determinants/resources/action_sd.pdf. [Google Scholar]
- 36.Aidala Angela A, Sumartojo Esther. Why housing? AIDS Behav. 2007 Aug 21;11(6 Suppl):1–6. doi: 10.1007/s10461-007-9302-z. [DOI] [PubMed] [Google Scholar]
- 37.Corneil Trevor A, Kuyper Laura M, Shoveller Jean, Hogg Robert S, Li Kathy, Spittal Patricia M, Schechter Martin T, Wood Evan. Unstable housing, associated risk behaviour, and increased risk for HIV infection among injection drug users. Health Place. 2004 Dec 15;12(1):79–85. doi: 10.1016/j.healthplace.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS. 2000;14(Suppl 1):S3–S10. doi: 10.1097/00002030-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 39.Minkler M, Wallerstein N. In: Community-based participatory research for health. Minkler M, editor. San Francisco: Jossey-Bass; 2003. [Google Scholar]
- 40.Joint United Nations Programme on HIV and AIDS. The Greater Involvement of People Living with HIV (GIPA) 2007. http://whqlibdoc.who.int/unaids/2007/policybrief_gipa_eng.pdf.
- 41.Wu A W, Revicki D A, Jacobson D, Malitz F E. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6(6):481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 42.Revicki D A, Sorensen S, Wu A W. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36(2):126–137. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 44.Devins GM, Orme CM, Costello CG, Binik YM, Frizzell B, Starn HJ. Measuring depressive symptoms in illness populations: psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) scale. Psychol Health. 1988;2(2):139–156. [Google Scholar]
- 45.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test (AUDIT) 2nd ed. Geneva: World Health Organization; 2001. http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6a.pdf. [Google Scholar]
- 46.Fleming M F, Barry K L, MacDonald R. The alcohol use disorders identification test (AUDIT) in a college sample. Int J Addict. 1991;26(11):1173–1185. doi: 10.3109/10826089109062153. [DOI] [PubMed] [Google Scholar]
- 47.Hays RD, Merz JF, Nicholas R. Response burden, reliability, and validity of the CAGE, Short MAST, and AUDIT alcohol screening measures. Behav Res Methods Instrum Comput. 1995;27(2):277–280. [Google Scholar]
- 48.Skinner H A. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 49.Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychol Assess. 1998;10(4):408–414. [Google Scholar]
- 50.Saltstone R, Halliwell S, Hayslip M A. A multivariate evaluation of the Michigan Alcoholism Screening Test and the Drug Abuse Screening Test in a female offender population. Addict Behav. 1994;19(5):455–462. doi: 10.1016/0306-4603(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 51.Dunn J R, Hayes M V. Social inequality, population health, and housing: a study of two Vancouver neighborhoods. Soc Sci Med. 2000;51(4):563–587. doi: 10.1016/s0277-9536(99)00496-7. [DOI] [PubMed] [Google Scholar]
- 52.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000;29(1):158–167. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 53.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21(1):171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 54.Remis RS. Modelling the incidence and prevalence of hepatitis C infection and its sequelae in Canada, 2007. Final report. Ottawa: Public Health Agency of Canada; 2009. [accessed 2011 June 26]. http://www.phac-aspc.gc.ca/sti-its-surv-epi/model/pdf/model07-eng.pdf. [Google Scholar]
- 55.Ford P M, Pearson M, Sankar-Mistry P, Stevenson T, Bell D, Austin J. HIV, hepatitis C and risk behaviour in a Canadian medium-security federal penitentiary. Queen's University HIV Prison Study Group. QJM. 2000;93(2):113–119. doi: 10.1093/qjmed/93.2.113. [DOI] [PubMed] [Google Scholar]
- 56.Holsen D S, Harthug S, Myrmel H. Prevalence of antibodies to hepatitis C virus and association with intravenous drug abuse and tattooing in a national prison in Norway. Eur J Clin Microbiol Infect Dis. 1993;12(9):673–676. doi: 10.1007/BF02009378. [DOI] [PubMed] [Google Scholar]
- 57.Fox Rena K, Currie Sue L, Evans Jennifer, Wright Teresa L, Tobler Leslie, Phelps Bruce, Busch Michael P, Page-Shafer Kimberly A. Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005 Jun 09;41(2):177–186. doi: 10.1086/430913. [DOI] [PubMed] [Google Scholar]
- 58.Wright Nat M J, Tompkins Charlotte N E, Jones Lesley. Exploring risk perception and behaviour of homeless injecting drug users diagnosed with hepatitis C. Health Soc Care Community. 2005;13(1):75–83. doi: 10.1111/j.1365-2524.2005.00552.x. [DOI] [PubMed] [Google Scholar]
- 59.German Danielle, Davey Melissa A, Latkin Carl A. Residential transience and HIV risk behaviors among injection drug users. AIDS Behav. 2007 May 08;11(6 Suppl):21–30. doi: 10.1007/s10461-007-9238-3. [DOI] [PubMed] [Google Scholar]
- 60.Millson Peggy, Myers Ted, Calzavara Liviana, Wallace Evelyn, Major Carol, Degani Naushaba. Regional variation in HIV prevalence and risk behaviours in Ontario injection drug users (IDU) Can J Public Health. 2003;94(6):431–435. doi: 10.1007/BF03405080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remis R, Swantee C, Schiedel L, Liu J. Report on HIV/AIDS in Ontario 2006. Technical report. Toronto: Ontario Ministry of Health and Long-Term Care; 2008. [accessed 2011 June 26]. http://www.phs.utoronto.ca/ohemu/doc/PHERO2006_report_final.pdf. [Google Scholar]
- 62.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS. 2005;19(Suppl 2):S67–S72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 63.Williams Peter, Narciso Lea, Browne Gina, Roberts Jacqueline, Weir Robin, Gafni Amiram. Characteristics of people living with HIV who use community-based services in Ontario, Canada: implications for service providers. J Assoc Nurses AIDS Care. 2005;16(4):50–63. doi: 10.1016/j.jana.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Lo Re Vincent, Frank Ian, Gross Robert, Synnestvedt Marie, Localio A Russell, Kostman Jay R, Strom Brian L. Self-reported hepatitis B and C virus infections had low sensitivity among HIV-infected patients. J Clin Epidemiol. 2006 Nov 13;60(3):294–299. doi: 10.1016/j.jclinepi.2006.06.020. [DOI] [PubMed] [Google Scholar]