Abstract
Abnormal tau accumulation can lead to the development of neurodegenerative diseases. P301S mice overexpress the human tau mutated gene, resulting in tau hyperphosphorylation and tangle formation. Mice also develop synaptic deficits and microglial activation prior to any neurodegeneration and tangles. Oxidative stress can also affect tauopathy. We studied the role of oxidative stress in relationship to behavioral abnormalities and disease progression in P301S mice at 2, 7, and 10 mo of age. At 7 mo of age, P301S mice had behavioral abnormalities, such as hyperactivity and disinhibition. At the same age, we observed increased carbonyls in P301S mitochondria (∼215 and 55% increase, males/females), and deregulation in the activity and content of mitochondrial enzymes involved in reactive oxygen species formation and energy metabolism, such as citrate synthase (∼19 and ∼5% decrease, males/females), MnSOD (∼16% decrease, males only), cytochrome C (∼19% decrease, females only), and cytochrome C oxidase (∼20% increase, females only). These changes in mitochondria proteome appeared before tau hyperphosphorylation and tangle formation, which were observed at 10 mo and were associated with GSK3β activation. At that age, mitochondria proteome deregulation became more apparent in male P301S mitochondria. The data strongly suggest that oxidative stress and mitochondrial abnormalities appear prior to tau pathology.—Dumont, M., Stack, C., Elipenahli, C., Jainuddin, S., Gerges, M., Starkova, M. N., Yang, L., Starkov, A. A., Beal, F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice.
Keywords: tauopathy, exploration, tangles
Tauopathies constitute a subset of neurodegenerative diseases, which result from the aggregation and accumulation of tau protein within neurons (1, 2). Several mutations of the tau gene responsible for frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) have been discovered (3–5). These human mutations were then used to create new mouse models of tau aggregation and neurodegeneration (6, 7).
A number of lines of transgenic mice have been developed that express human mutations of the tau protein and mimic the human illnesses of frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Like other models, the P301S transgenic mice have been generated to study the effects of tau aggregation in the brain and the spinal cord (8). The tau positive neurofibrillary tangles are globose or flame-shaped and stain with many different phospho-tau antibodies. The P301S mice derived from the PS19 line overexpress the human tau gene with the P301S mutation in exon 10. In these mice, human tau expression is about 5-fold greater than endogenous mouse tau. Interestingly, they develop synaptic deficits and microglial activation prior to any sign of neurodegeneration and tangle formation (8).
Oxidative stress, which is involved in neurodegenerative diseases (9), can also affect tauopathy. Therefore, we studied the role of oxidative stress in relationship to behavioral abnormalities and disease progression in P301S mice. In P301L tau transgenic mice, proteomic analysis revealed that metabolism-related and synaptic proteins were altered, and evidence indicated mitochondrial dysfunction (10). In addition, increased oxidative stress induces tau phosphorylation (11). This finding suggests that mitochondrial dysfunction might play an important role in the disease pathogenesis and could contribute to the disease phenotype. In this study, we examined the effect of the P301S mutation on behavior, tau pathology, oxidative stress, and mitochondrial enzymes at 2, 7, and 10 mo of age, corresponding to three important stages of the disease: preonset, onset, and late stage.
MATERIALS AND METHODS
Animals
Male P301S transgenic and female wild-type mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and bred to generate 3 independent cohorts. These 3 cohorts, composed of P301S mice and their wild-type littermates, were evaluated at 2, 7, or 10 mo, respectively. All experiments were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee.
Behavior
One week prior to behavioral testing, the experimenter handled the mice daily in order to habituate them. Body weights were recorded at the end of the week. Locomotor activity and exploration were assessed in the open-field test and the elevated plus maze, as described previously (12). Briefly, mice were placed for a single trial of 5 min in the open-field test or the elevated plus maze. For the open-field test, total distance traveled and rearing frequency were recorded using a video tracking system (Ethovision 3.1; Noldus Technology, Attleborough, MA, USA). For the elevated plus maze, frequency of entries into closed and open arms and percentage of time spent in open arms were calculated.
Spatial learning and memory were assessed using the Morris water maze test, as described previously (12). For the acquisition period, mice were given 4 trials/d over 5 d to determine the location of a hidden platform (placed in the northwest quadrant of the pool). The latency before reaching the platform was recorded (Ethovision 3.1). A probe trial was conducted 24 h after the acquisition period, in which the percentage of time spent in each quadrant was measured for 60 s. Animals were also tested for 4 trials/d over 2 d in a visible-platform version of the Morris water maze. General grip strength was measured by the grid test. Animals were placed on a metal grid (10×5 cm; height, 30 cm), which was inverted after a few seconds. Latency to fall was recorded during a single trial of 3 min.
Motor coordination was assessed using the balance beam and the accelerated rotorod, for which latencies before falling were recorded. For the balance beam (diameter, 2.5 cm; length, 110 cm; height, 33 cm; division into 11 segments of 10 cm each), mice were placed in the center of the beam for 4 trials of 60 s each. In the accelerated rotorod, mice were placed on the rotating beam for 3 trials/d over 4 d at high speed (4 to 40 rpm in 2 min; 0.3 rpm/s) and at low speed (4 to 40 rpm in 5 min; 0.1 rpm/s). Two tests at constant speeds, consisting of 3 trials at 5 rpm and 3 trials at 10 rpm, were also conducted on a single day.
Western blotting
After behavioral testing, half of the mice in each cohort were sacrificed by decapitation. Tissues were collected, dissected, snap-frozen in liquid nitrogen, and stored at −80°C for biochemical studies. Cortex, hippocampus, and spinal cord were homogenized in RIPA buffer with protease and phosphatase inhibitors to extract soluble proteins (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Equal amounts of protein from the homogenates were electrophoresed through 4–12% Tri-Bis NuPage (Invitrogen, Carlsbad, CA, USA). After transfer to polyvinylidene fluoride (PVDF), membranes were blocked in 5% nonfat dry milk in phosphate buffered saline with 0.05% Tween 20 (PBST) and exposed overnight to the primary antibody at 4°C. Horseradish peroxidase-conjugated (HRP) secondary antibody binding was visualized with enhanced chemiluminescence.
Primary antibodies and concentrations used for Western blotting were DA9 mouse monoclonal anti-total Tau aa 102–140 (1:1000; gift from Dr. Peter Davies; Albert Einstein College of Medicine, Manhasset, NY, USA); CP13 mouse monoclonal anti-human tau pSer202 (1:1000; gift from Dr. Peter Davies); PHF1 mouse monoclonal anti-human tau pSer396/Ser-404 (1:1000; gift from Dr. Peter Davies); AT8 mouse monoclonal anti-human tau pSer202/Thr205 (1:250; Thermo Fisher Scientific, Rockford, IL, USA); mouse monoclonal anti-GSK3β (1:500; Abcam, Cambridge, MA, USA); and mouse monoclonal anti-β-actin (1:10,000; Sigma, St. Louis, MO).
Immunohistochemistry
After behavioral testing, the other half of the mice from each cohort were deeply anesthetized using sodium pentobarbital and transcardially perfused with ice-cold 0.9% sodium chloride and 4% paraformaldehyde. Tissues were removed and postfixed in 4% paraformaldehyde followed by gradient sucrose (15 and 30%) and storage in cryoprotectant for immunohistochemical studies.
Sections were cut at 40 μm thickness and stained with MC1 mouse monoclonal anti-human tau (N-terminal conformational change, exon 10; 1:500; gift from Dr. Peter Davies). The secondary antibody was goat anti-mouse IgG1, Biotin labeled (1:1000; Pierce, Rockford, IL, USA). Immunolabeling was detected by the streptavidin-HRP method and visualized after diaminobenzidine (DAB) incubation for 30 s (Vector, Burlingame, CA, USA). Sections were viewed with the ×10 and ×20 objective on a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan). Digital images were captured using Stereo Investigator 4.35 (Microbrightfield, Burlington, VT, USA).
Mitochondria characterization
Sample preparation
Dissected nonperfused frontal cortex samples (∼30–55 mg) were stored frozen at −80°C until assaying. Before assays, tissue samples were thawed on ice and homogenized with a Dounce-type (glass vessel/glass pestle) 2-ml homogenizer. The homogenate was centrifuged at 1000 g for 5 min to get rid of nuclear fraction and cell debris; the resulting supernatant was centrifuged at 14,000 g for 5 min. The pellet was collected and centrifuged again at 14,000 g for 5 min; the pellet obtained in this step was resuspened in 20 mM HEPES (pH 7.8) and used for all assays.
Immunoblot analysis: mitochondria-enriched fraction
The protein lysates containing equal amounts of protein were separated by SDS-PAGE, electroblotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA), and immunoreacted with an appropriate primary antibody (see below), followed by HRP-conjugated secondary antibodies (Kierkegaard Perry Labs Inc., Gaithersburg, MD, USA). The immunoreactive proteins were visualized by incubating the blots in the chemiluminescence substrate (Pierce). Quantitative analysis of the immunoreactive proteins was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Statistical analysis was performed using the ratios of the densitometric value of each band normalized to β-actin as loading control.
Assays
All samples were assayed for the following: complex I (CI) activity (NADH:CoQ reductase, rotenone-sensitive; ref. 13), CI subunit expression (immunoblotting with anti-CI subunit NDUFB8, 1:1000; Invitrogen), complex III (CIII) subunit expression (immunoblotting with anti-CIII core 2 subunit UQCR2/QCR2, 1:1000; Invitrogen), complex IV (COXIV) subunit expression (immunoblotting with anti-COXIV MT-CO1 subunit, 1:1000; Invitrogen), ATPase subunit expression (immunoblotting with anti-ATPase ATP5A1 subunit, 1:1000; Invitrogen), cytochrome c content by quantitative ELISA (Rat/Mouse Cytochrome c Quantikine ELISA Kit; R&D Systems, Minneapolis, MN, USA), succinate dehydrogenase (SDH) activity (succinate:CoQ:DCIP reductase, TTFA sensitive; ref. 14), citrate synthase (CS) activity (15), aconitase (ACO) activity (Aconitase Assay Kit; Cayman Chemical, Ann Arbor, MI, USA), protein carbonyls by DNP derivatization followed by immunoblotting (Oxyblot kit; Cayman), glutathione reductase (GR) activity (Glutathione Reductase Assay Kit; Cayman), superoxide dismutase (SOD) activity (Superoxide Dismutase Assay Kit; Cayman), manganese SOD (MnSOD) content (immunoblotting with anti-MnSOD, 1:1000; Novus Biologicals, Littleton, CO, USA). All the activities and content values were normalized by protein content in the sample (measured with BCA protein assay; Thermo Scientific, Waltham, MA, USA) and (when appropriate) by CS activity of the sample. The emission of reactive oxygen species (ROS) by isolated brain mitochondria was measured as described earlier (16). For these experiments, mitochondria were isolated from mouse brain cortex by Sims' isopycnic centrifugation method in Percoll gradient (17) with modifications described in Thomas et al. (18).
High-performance liquid chromatography (HPLC)
The HPLC determination of malondialdehyde (MDA) was performed by a method modified from a previous report by Agarwal and Chase (19). Fresh brain tissues were homogenized in 40% ethanol solution. To 50 μl of sample homogenate or MDA standard prepared in 40% ethanol, 50 μl of 0.05% butylated hydroxytoluene (BHT), 400 μl of 0.44 M H3PO4, and 100 μl of 0.42 mM 2-thiobarbituric acid (TBA) were added, vortexed, heated for 1.5 h at 100°C, and immediately cooled with iced water to stop the derivative reaction. The MDA-TBA derivative was extracted by adding 250 μl n-butanol, followed by vortexing and centrifugation. n-Butanol extract (50 μl)was used for the HPLC assay. The HPLC mobile phase used acetonitrile buffer (20:80, v/v, buffer 50 mM KH2PO4, pH 6.8). The column was an ESA 150- × 3-mm C18 column with particle size of 3 μm (ESA Inc., Bedford, MA, USA). The fluorescence detector was set at an excitation wavelength of 515 nm and emission wavelength of 553 nm. MDA was eluted out in 2 min. Protein in tissue homogenates was assessed using a Bio-Rad kit.
Statistical analysis
Two-tailed unpaired t tests were used to compare P301S mice and their wild-type littermates. ANOVA was used to compare P301S mice at 2, 7, and 10 mo. After the ANOVA, post hoc Fisher's PLSD test was used for further comparison between groups. (Statview 5.0.1; SAS Institute Inc., Cary, NC, USA).
RESULTS
P301S transgenic mice showed hyperactivity and disinhibition without major motor and cognitive defects
For all behavioral measurements, independent groups of P301S mice and their wild-type littermates were tested at 2, 7, or 10 mo. Comparisons were done within groups. Prior to behavioral testing, body weight was recorded. At 2 mo, male P301S mice had lower body weight than age-matched nontransgenic mice (data not shown). Similar results were found at 10 mo, where both male and female P301S mice had lower body weight than wild-type littermates (data not shown). No significant difference was observed at 7 mo (data not shown).
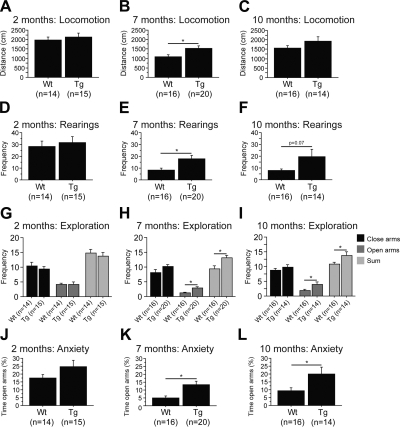
To assess locomotor activity, we used the open-field test. There was no difference in the performance of 2-mo-old P301S mice compared to nontransgenic mice [Fig. 1A, D; wild type (Wt), n=14; P301S (Tg), n=15]. Starting at 7 mo, P301S mice were hyperactive as compared to wild-type littermates, as shown by the increased distance traveled and rearing frequency at 7 mo (Fig. 1B, E; Wt, n=16; Tg, n=20) and 10 mo (P=0.07; Fig. 1F; Wt, n=16; Tg, n=14). At 10 mo, P301S mice traveled longer distances in the open-field test as compared to their wild-type littermates. However, data did not quite reach significance (Fig. 1C; Wt, n=16; Tg, n=14). Further analysis revealed that this hyperactivity was reflective of a gender effect by which only female P301S mice were significantly more active than female wild-type mice (Supplemental Table S1).
Figure 1.
P301S transgenic mice showed hyperactivity and disinhibition. A–F) Total distance traveled (A–C) and rearing frequency (D–F) in the open-field test at 2 mo (A, D), 7 mo (B, E), and 10 mo (C, F). P301S (Tg) mice were hyperactive compared to wild-type (Wt) littermates at 7 and 10 mo. G–L) Frequency of entries in closed and open arms (G–I), as well as percentage of time spent in open arms (J–L), in the elevated plus maze at at 2 mo (G, J), 7 mo (H, K), and 10 mo (I, L). P301S mice were hyperactive and disinhibited compared to wild-type littermates at 7 and 10 mo. Data are expressed as means ± se.
Hyperactivity was also observed in the elevated plus maze, in which 7- and 10-mo-old P301S mice visited more arms than their wild-type littermates [Fig. 1H (Wt, n=16; Tg, n=20), I (Wt, n=16; Tg, n=14)]. In addition, these same P301S mice were disinhibited compared to nontransgenic mice, as evidenced by the percentage of time spent in the open arms [Fig. 1K (Wt, n=16; Tg, n=20), L (Wt, n=16; Tg, n=14)]. This difference was more prominent in male P301S mice (Supplemental Table S1). Similar to the open-field test, no difference was found in the elevated plus maze at 2 mo (Fig. 1G, J; Wt, n=14; Tg, n=15).
Several tests were conducted to evaluate motor skills of P301S mice, such as the grid (muscular strength), the balance beam, and accelerated rotorod (motor coordination and balance). We did not observe motor deficits at 2 mo (Supplemental Fig. S1A, D, G; Wt, n=14; Tg, n=15), 7 mo (Supplemental Fig. S1E, H, J; Wt, n=16; Tg, n=19), or 10 mo [Supplemental Fig. S1C, F (Wt, n=16; Tg, n=14); I, K (Wt, n=16; Tg, n=12)] in P301S mice relative to wild-type mice. It should be noted that 7-mo-old P301S mice had a shorter latency to fall from the grid relative to nontransgenic mice (Supplemental Fig. S1B; Wt, n=16; Tg, n=19). The rotorod was also used at various settings. For constant speeds at 5 and 10 rpm, P301S mice did not show any motor impairment [Supplemental Fig. S1L (Wt, n=16; Tg, n=19), M (Wt, n=16; Tg, n=12)].
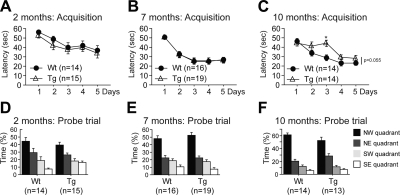
Finally, we assessed spatial learning and memory using the Morris water maze. We found no significant deficit in P301S mice at 2 mo (Fig. 2A, D; Wt, n=14; Tg, n=15), 7 mo (Fig. 2B, E; Wt, n=16; Tg, n=19), or 10 mo [Fig. 2C (Wt, n=14; Tg, n=14), F (Wt, n=14; Tg, n=13)]. However, we found a trend toward decreased latency to reach the platform during the acquisition period in 10-mo-old P301S mice compared to their age-matched wild-type littermates (Fig. 2C; Wt, n=14; Tg, n=14). This finding was explained by the poor performance of P301S mice on day 3. Interestingly, at 10 mo, gender analysis revealed that male P301S mice exhibited learning impairment when compared to male nontransgenic mice (Supplemental Table S1). To ensure that P301S mice did not show any swimming or visual inability, the swim speed and the latency to reach a visible platform were measured respectively. No abnormalities were found for these two parameters (data not presented).
Figure 2.
P301S transgenic mice did not present major cognitive deficits. Acquisition period (A–C) and probe trial (D–F) of the Morris water maze in which latency to reach the hidden platform and percentage of time spent in each quadrant were recorded at 2 mo (A, D), 7 mo (B, E), and 10 mo (C, F). No difference was found between P301S (Tg) mice and wild-type (Wt) littermates in the Morris water maze. However, trend indicated increased latency to reach the platform in P301S mice at 10 mo. Data are expressed as means ± se.
Tau phosphorylation and accumulation were augmented in aged P301S transgenic mice, together with GSK3β levels
To determine whether the tau pathology increased with age, P301S mice at 2, 7, and 10 mo were compared. Total tau and hyperphosphorylated tau were assessed using the following antibodies: DA9 anti-total Tau aa 102–140, CP13 anti-human tau pSer202, PHF1 anti-human tau pSer396/Ser-404, AT8 anti-human tau pSer202/Thr205. These antibodies were tested previously in both P301S mice and wild-type littermates (Supplemental Fig. S2A).
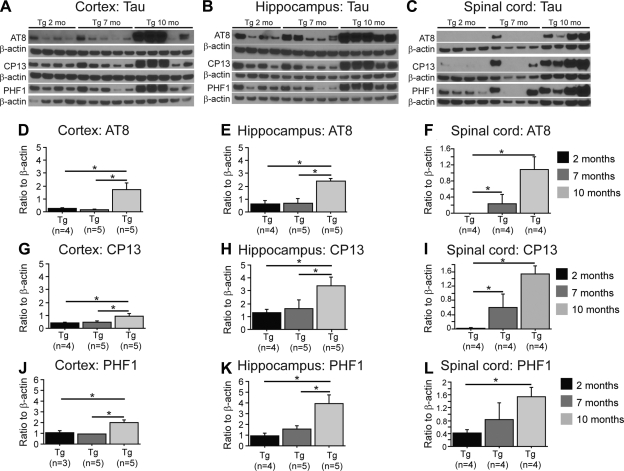
Our data showed that levels of total tau remained unchanged between 2, 7, and 10 mo in the brain or the spinal cord of P301S mice (Supplemental Fig. S2B–G; Tg, n=2–5). Levels of AT8, CP13, and PHF1 were increased between 2 and 10 mo in P301S mice in the cerebral cortex (Fig. 3A, D, G, J; Tg, n=3–5), hippocampus (Fig. 3B, E, H, K; Tg, n=4–5), and spinal cord (Fig. 3C, F, I, L; Tg, n=4). In addition, we found an increase in AT8, CP13, and PHF1 levels between 7- and 10-mo-old P301S mice in the cortex (Fig. 3D, G, J; Tg, n=3–5) and hippocampus (Fig. 3E, H, K; Tg, n=4–5). Finally, no significant differences in hyperphosphorylated tau levels were found between 2- and 7-mo-old P301S mice, with exception to AT8 and CP13 in the spinal cord (Fig. 3F, I; Tg, n=4). After gender analysis, we found that increased levels of phosphorylated tau were only present in male P301S mice (Supplemental Table S1).
Figure 3.
Tau phosphorylation was augmented in aged P301S transgenic mice. A–C) Western blots of AT8, CP13, and PHF1 in the cortex (A), hippocampus (B), and spinal cord (C) of P301S (Tg) mice at 2, 7, and 10 mo of age. D–L) Quantification of AT8 (D–F), CP13 (G–I), and PHF1 (J–L) levels in the cortex (D, G, J), hippocampus (E, H, K), and spinal cord (F, I, L), using ratio of each antibody to β-actin. P301S mice (10 mo old) had increased AT8, CP13, and PHF1 levels compared to 2- and 7-mo-old P301S mice. Data are expressed as means ± se.
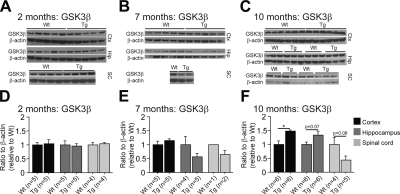
Together with the high level of phosphorylated tau at 10 mo, we also observed an increase of glycogen synthase kinase-3-beta (GSK3β) levels in both the cortex and hippocampus of P301S mice as compared to wild-type littermates (Fig. 4C, F; Wt, n=4–6; Tg, n=5–6). GSK3β is a serine/threonine protein kinase mediating the addition of phosphate molecules on specific serine and threonine amino acids in cellular substrates like for the protein tau. Surprisingly, levels of GSK3β were reduced in the spinal cord of 10-mo-old P301S mice as compared to wild-type mice (Fig. 4C, F; Wt, n=4–6; Tg, n=5–6). No differences in GKS3β levels were found at 2 or 7 mo between P301S mice and age-matched nontransgenic mice [Fig. 4A, B, D (Wt, n=4–5; Tg, n=4–5), E (WT n=1–5; Tg, n=2–5)].
Figure 4.
GSK3β level was augmented in aged P301S transgenic mice. A–C) Western blots of GSK3β at 2 mo (A), 7 mo (B), and 10 mo (C) in P301S (Tg) mice and their wild-type (Wt) littermates. D–F) Quantification of GSK3β level at 2 mo (D), 7 mo (E), and 10 mo (F) using ratio of the protein to β-actin. P301S mice (10 mo) had increased GSK3β levels compared to nontransgenic mice. Data are expressed as means ± se.
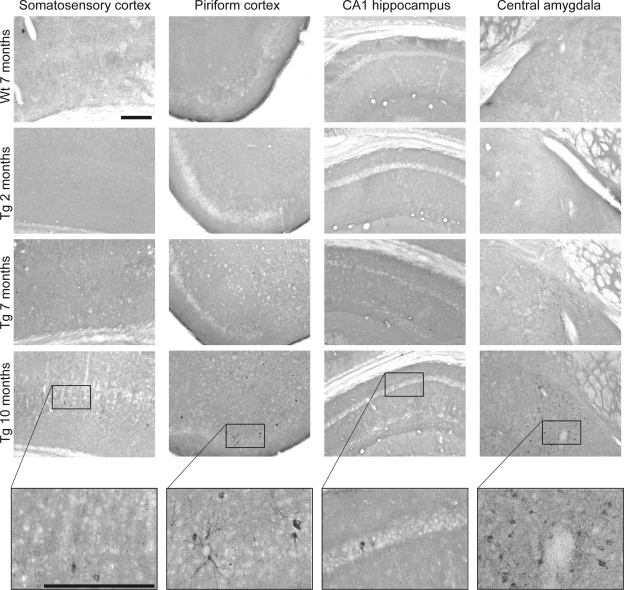
In addition to tau hyperphosphorylation, the accumulation of tau and formation of tangles increased from 2 to 10 mo in P301S mice, as evidenced by MC1 immunoreactivity in the somatosensory and piriform cortices, the CA1 region of the hippocampus, and the central amygdala (Fig. 5). These brain regions have been identified previously as bearing more tangles compared to other regions.
Figure 5.
Tau accumulation and tangles were increased with age in P301S transgenic mice. MC1 immunoreactivity in the somatosensory and piriform cortices, the CA1 region of the hippocampus, and the central amygdala of 7-mo-old wild-type (Wt) mice, as well as 2-, 7-, and 10-mo-old P301S (Tg) mice. Scale bars = 250 μm. Higher-magnification photographs were taken in 10-mo-old P301S mice to visualize tangles. MC1 immunoreactivity increased from 2 to 10 mo in P301S mice.
Oxidative stress was increased in the brain of P301S transgenic mice
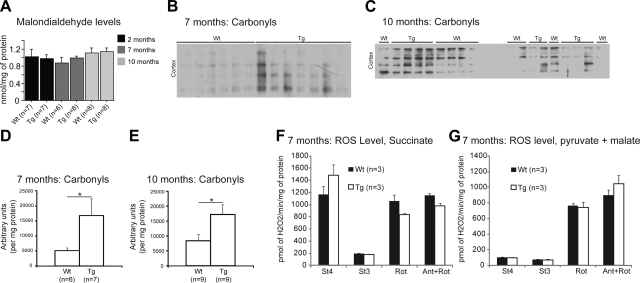
For this assessment, analysis was done comparing each specific group at 2, 7, or 10 mo. Increased oxidative damage was investigated by measuring cortical levels of MDA (lipid peroxidation) and carbonyls (protein oxidation). At all ages, MDA levels were unchanged in P301S mice relative to wild-type littermates (Fig. 6A; Wt, n=6–8; Tg, n=7–8). However, protein carbonyls were significantly elevated in the mitochondria fraction from 7- and 10-mo-old P301S mice (Fig. 6B–E; Wt, n=6–9; Tg, n=7–9). We also measured the emission of ROS in isolated brain cortex mitochondria of P301S mice at 7 mo (Fig. 7F, G) and 10 mo (data not presented). No differences were found between P301S mice and their wild-type littermates at both ages.
Figure 6.
Oxidative stress was enhanced in the brain of P301S transgenic mice. A) Levels of MDA at 2, 7, and 10 mo. No significant difference was found between P301S (Tg) mice and their wild-type (Wt) littermates. B–D) Western blots of carbonyls (B, C) and quantification of carbonyl level (D, E) at 7 mo (B, D) and 10 mo (C, E). Levels of carbonyls in 7- and 10-mo-old P301S mice were increased in the cortex and compared to nontransgenic mice. F, G) ROS generation with FADH-linked (F) and NADH-linked substrates (G) at 7 mo. No differences were found in ROS generation. St4, state 4; St3, state 3; Rot, rotenone; Ant, antimycin. Data are expressed as means ± se.
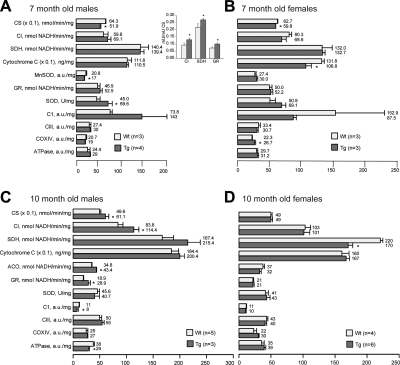
Figure 7.
Deficits in the respiratory chain and the tricarboxyl acid cycle were present in P301S transgenic mice. Mitochondrial enzyme activity of CS, CI, SDH, GR, and ACO, and protein expression of cytochrome c, MnSOD, total SOD (SOD), CI, CIII, CIV (COXIV) and ATPase at 7 mo (A, B) and 10 mo (C, D) in male (A, C) and female (B, D) P301S (Tg) mice and their wild-type (Wt) littermates. Data are normalized per milligram of protein and expressed as means ± se. Inset: mitochondrial enzyme activity of CI, SDH, and GR after normalization by CS at 7 mo of age between male P301S mice and their wild-type male littermates (A). Starting at 7 mo, P301S mice had mitochondrial abnormalities compared to their wild-type littermates.
In addition, enzymatic activities of SOD and GR, two important indicators of oxidative stress within mitochondria, were measured. At 7 mo, P301S mice had somewhat elevated GR and SOD activities (Fig. 7A; Wt, n=3; Tg, n=4) and B; Wt, n=3; Tg, n=3) as compared to nontransgenic mice. The difference in SOD activity reached the statistical significance in 7 mo P301S male mitochondria (Fig. 7A; Wt, n=3; Tg, n=4). No differences were found at 2 mo (data not presented) or 10 mo (Fig. 7C; Wt, n=5; Tg, n=3) and D; Wt, n=4; Tg, n=6).
P301S transgenic mice exhibited multiple changes in the respiratory chain and the tricarboxylic acid cycle enzyme activities and contents
We measured the activities of respiratory chain CI, SDH, CS, and ACO to study mitochondrial enzymes in P301S mice with age. We also assessed protein expression levels of CI, CIII, COXIV, mitochondrial MnSOD, and ATPase by immunoblotting, and cytochrome c content by ELISA, as described in Materials and Methods.
No significant differences were found between P301S and wild-type mitochondria at 2 mo (data not presented)
At 7 mo, we found a reduction in CS activity in both male and female mitochondria of P301S mice (Fig. 7A; Wt, n=3; Tg, n=4) and B; Wt, n=3; Tg, n=3); cytochrome c level was reduced only in female P301S mitochondria, which also exhibited a small but significant increase in the content of cytochrome oxidase subunit I (Fig. 7B; Wt, n=3; Tg, n=3). In addition to changes in CS and total SOD activities, male P301S mitochondria exhibited a decrease in MnSOD content as assessed by immunobloting (Fig. 7A; Wt, n=3; Tg, n=4). However, after normalizing the enzyme activity data by CS activity, male P301S mitochondria showed a statistically significant increase in CI, SDH, and GR activity (Fig. 7A, inset), which indicates a change in the composition of mitochondrial proteome in male P301S mitochondria.
At 10 mo, only the activity of SDH was significantly decreased in female P301S mitochondria (Fig. 7D; Wt, n=4; Tg, n=6). However, brain mitochondria from male P301S mice exhibited significant increases in CS, CI, and ACO activities, whereas CI content and ATPase content were decreased significantly (Fig. 7C; Wt, n=5; Tg, n=3), as compared to wild-type mitochondria.
DISCUSSION
The importance of oxidative stress early in disease progression has been demonstrated in several neurodegenerative diseases (9). In diseases manifesting tauopathy, the disease is characterized by tau hyperphosphorylation and tangle formation within neurons, which leads to neurodegeneration (1, 2).
Transgenic mouse models of tauopathy have been developed and used to further investigate the role of tau accumulation and its effects on the central nervous system (6). P301S and P301L transgenic mice, which carry the human tau gene with the P301S or P301L mutations, develop tau pathology in both the brain and the spinal cord. Remarkably, P301S mice derived from the PS19 line show synaptic impairment, synapse loss, and microglial activation before the presence of neurodegeneration, cell death, or even tangle formation (8). These findings indicated that other key factors may contribute to the disease pathogenesis.
In the present work, we used P301S mice and evaluated the role of oxidative stress in relationship to behavioral abnormalities and disease progression. These mice have been engineered specifically to overexpress the protein tau only, thus we considered that the observed effects were due to this protein. However, it would be important to verify that no other protein is being overproduced in mouse models of tauopathy.
In P301S mice, we found elevated tau hyperphosphorylation in the cortex, the hippocampus, and the spinal cord at 10 mo compared to 2 and 7 mo of age. Furthermore, 10-mo-old P301S mice had increased MC1 immunoreactivity mainly in the cortex, the CA1 region of the hippocampus, and the amygdala, which indicated the presence of neurofibrillary tangles in these regions. These tau abnormalities were associated with elevated GSK3β levels in the cerebral cortex and the hippocampus. Several reports have identified GSK3β as a key player in Alzheimer's disease (AD) (20) because of its role in promoting tau phosphorylation and tangle formation (21, 22).
As early as 7 mo, P301S mice had behavioral deficits, including hyperactivity and disinhibition. This hyperactivity has been documented previously in P301S homozygous mice as an early disease marker (23). We did not observe any major motor or cognitive decline in these mice. Mild muscular weakness was found at 7 mo. Later on, at 10 mo of age, both wild-type and P301S mice had reduced ability to hang onto the grid. This may have been due to increased body weight for the wild-type mice and to increased muscular fatigue for the P301S mice. In addition, at 10 mo of age, the P301S mice had mild learning impairment during the acquisition period of the Morris water maze, as evidenced by their poor performance on the third day of training. However, they did learn the task and did not show memory deficits during the probe trial. A lack of cognitive decline during the Morris water maze was also reported in P301S homozygous mice by Scattoni et al. (23). Similar to these researchers, we demonstrated that behavioral abnormalities were present prior to tau pathology.
In addition to behavioral deficits, P301S mice exhibited signs of elevated oxidative stress. At both 7 and 10 mo, increased protein carbonyl levels in the cerebral cortex of P301S mice were found. We found no change in MDA levels. Similar findings in P301L mice were reported by David et al. (10). Liu et al. (24) showed that carbonyl modification of 4-hydroxy-nonenal (4HNE) can induce normal tau to be phosphorylated and can maintain its phosphorylation state. It can also facilitate phosphorylated tau isomers to form PHF-like filaments in vitro (25) and in neuroblastoma cells or primary cortical neurons (26).
As a potential mechanism to explain elevated oxidative stress, mitochondrial enzymes were examined in P301S mice at 2, 7, and 10 mo. Indeed, mitochondria play a key role in ROS formation but also in the antioxidant response (27). As early as 7 mo, P301S mice had dysregulated mitochondrial enzymes, as evidenced by the alteration key mitochondrial enzymes (Fig. 7). In addition, levels of CI and COXIV were affected. Our data are consistent with the findings in P301L tau transgenic mice, where proteomic analysis showed alterations of metabolism-related and synaptic proteins (10). In addition, functional analysis confirmed the presence of mitochondrial dysfunction, as shown by the reduction of complex V and ATP levels, as well as NADH-ubiquinone oxidoreductase activities (10).
We also measured ROS generation in isolated mitochondria but did not find differences at 7 or 10 mo in P301S mice. Our data are in line with the report by David et al. (10), which demonstrated that in P301L brain cells, ROS levels measured by DCF were markedly elevated at 12 and 24 mo (∼168%). However, ROS were elevated by only ∼17% when measured by DHE, which primarily responds to mitochondrial ROS. The same researchers have demonstrated elevated SOD and GR activity at 24 mo, which is what we observed in our mice but at earlier age. As suggested by David et al. (10), an increase in the levels of protein carbonyls may likely be due to the reduction of CI content. Considering that isolated mitochondria do not show elevated ROS production, our data together with those of David et al. (10) indicate that the source of oxidative stress is likely not of the mitochondrial origin.
The P301L tau mitochondria showed increased vulnerability to Aβ42 administration (28). Moreover, exposure to truncated tau protein induced oxidative stress in rats (29). In Drosophila, tau-induced neurodegeneration was proven to be mediated by oxidative stress (30). Furthermore increased oxidative stress induces tau phosphorylation in transgenic AD mice (11). Therefore, oxidative stress and mitochondrial dysfunction are present in P301S mice, even before abnormal tau accumulation. Similarly, in the triple transgenic mice carrying human mutations of the amyloid precursor protein, presenilin 1, and tau, mitochondrial dysfunction was observed before the formation of amyloid plaques and neurofibrillary tangles (31).
It should be noted that observed deficits, including mitochondrial abnormalities, were predominantly found in male P301S mice. Gender differences have formerly been reported in human and animal studies of neurodegenerative diseases. In AD transgenic mice, females had increased amyloid pathology but not tau pathology (32). In addition, dysregulation of the mitochondrial proteome was found in male triple transgenic mice (31). In neurons, cell death induced by 6-hydroxydopamine was caused by increased oxidative stress, which was more pronounced in males (33). Several reports have shown that female hormones, such as estrogens, are neuroprotective (34, 35). By regulating mitochondrial function and biogenesis, estrogens can reduce oxidative stress (36).
To summarize, we demonstrated that behavioral deficits, elevated oxidative stress, and mitochondrial abnormalities preceded tau pathology in P301S mice. This finding suggests that oxidative stress plays a crucial role in disease pathogenesis. Interestingly, Du et al. (37) have shown that in AD transgenic mice, synaptic mitochondria had increased amyloid-β accumulation and mitochondrial alterations relative to nonsynaptic mitochondria. Considering that tau can also accumulate abnormally at synapses (38), it is possible that oxidative stress and mitochondrial abnormalities present at synapses, enhances synaptic deficits, leading to behavioral abnormalities and tangle formation and neurodegeneration.
Supplementary Material
Acknowledgments
This work was supported by the Tau Consortium. The authors thank Dr. Peter Davies (Albert Einstein College of Medicine, Manhasset, NY, USA) for providing the tau antibodies (CP13, PHF1, MC1, and DA9). A.A.S was supported by the National Institute of Aging (PO AG014930).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Lee V. M., Goedert M., Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 2. Yoshiyama Y., Lee V. M., Trojanowski J. Q. (2001) Frontotemporal dementia and tauopathy. Curr. Neurol. Neurosci. Rep. 1, 413–421 [DOI] [PubMed] [Google Scholar]
- 3. Gasparini L., Terni B., Spillantini M. G. (2007) Frontotemporal dementia with tau pathology. Neurodegener. Dis. 4, 236–253 [DOI] [PubMed] [Google Scholar]
- 4. Goedert M., Jakes R. (2005) Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta 1739, 240–250 [DOI] [PubMed] [Google Scholar]
- 5. Ingram E. M., Spillantini M. G. (2002) Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol. Med. 8, 555–562 [DOI] [PubMed] [Google Scholar]
- 6. Lee V. M., Kenyon T. K., Trojanowski J. Q. (2005) Transgenic animal models of tauopathies. Biochim. Biophys. Acta 1739, 251–259 [DOI] [PubMed] [Google Scholar]
- 7. Noble W., Hanger D. P., Gallo J. M. (2010) Transgenic mouse models of tauopathy in drug discovery. CNS Neurol. Disord. Drug Targets 9, 403–428 [DOI] [PubMed] [Google Scholar]
- 8. Yoshiyama Y., Higuchi M., Zhang B., Huang S. M., Iwata N., Saido T. C., Maeda J., Suhara T., Trojanowski J. Q., Lee V. M. (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 [DOI] [PubMed] [Google Scholar]
- 9. Lin M. T., Beal M. F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 10. David D. C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Drose S., Brandt U., Muller W. E., Eckert A., Gotz J. (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 280, 23802–23814 [DOI] [PubMed] [Google Scholar]
- 11. Melov S., Adlard P. A., Morten K., Johnson F., Golden T. R., Hinerfeld D., Schilling B., Mavros C., Masters C. L., Volitakis I., Li Q. X., Laughton K., Hubbard A., Cherny R. A., Gibson B., Bush A. I. (2007) Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE 2, e536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dumont M., Wille E., Calingasan N. Y., Nathan C., Flint Beal M., Lin M. T. (2010) N-iminoethyl-L-lysine improves memory and reduces amyloid pathology in a transgenic mouse model of amyloid deposition. Neurochem. Int. 56, 345–351 [DOI] [PubMed] [Google Scholar]
- 13. Degli Esposti M., Ghelli A., Crimi M., Estornell E., Fato R., Lenaz G. (1993) Complex I and complex III of mitochondria have common inhibitors acting as ubiquinone antagonists. Biochem. Biophys. Res. Commun. 190, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 14. Arrigoni O., Singer T. P. (1962) Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature 193, 1256–1258 [DOI] [PubMed] [Google Scholar]
- 15. Srere P. A. (1969) Citrate synthase. Methods Enzymol. 13, 3–11 [Google Scholar]
- 16. Starkov A. A. (2010) Measurement of mitochondrial ROS production. Methods Mol. Biol. 648, 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sims N. R. (1990) Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J. Neurochem. 55, 698–707 [DOI] [PubMed] [Google Scholar]
- 18. Thomas B., Banerjee R., Starkova N. N., Zhang S. F., Calingasan N. Y., Yang L., Wille E., Lorenzo B. J., Ho D. J., Beal M. F., Starkov A. (2011) Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson's disease. [E-pub ahead of print] Antioxid. Redox Signal. doi:10.1089/ars.2010.3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agarwal R., Chase S. D. (2002) Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 775, 121–126 [DOI] [PubMed] [Google Scholar]
- 20. Hooper C., Killick R., Lovestone S. (2008) The GSK3 hypothesis of Alzheimer's disease. J. Neurochem. 104, 1433–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernandez F., Gomez de Barreda E., Fuster-Matanzo A., Lucas J. J., Avila J. (2010) GSK3: a possible link between beta amyloid peptide and tau protein. Exp. Neurol. 223, 322–325 [DOI] [PubMed] [Google Scholar]
- 22. Sperber B. R., Leight S., Goedert M., Lee V. M. (1995) Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci. Lett. 197, 149–153 [DOI] [PubMed] [Google Scholar]
- 23. Scattoni M. L., Gasparini L., Alleva E., Goedert M., Calamandrei G., Spillantini M. G. (2010) Early behavioural markers of disease in P301S tau transgenic mice. Behav. Brain Res. 208, 250–257 [DOI] [PubMed] [Google Scholar]
- 24. Liu Q., Smith M. A., Avila J., DeBernardis J., Kansal M., Takeda A., Zhu X., Nunomura A., Honda K., Moreira P. I., Oliveira C. R., Santos M. S., Shimohama S., Aliev G., de la Torre J., Ghanbari H. A., Siedlak S. L., Harris P. L., Sayre L. M., Perry G. (2005) Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 38, 746–754 [DOI] [PubMed] [Google Scholar]
- 25. Perez M., Cuadros R., Smith M. A., Perry G., Avila J. (2000) Phosphorylated, but not native, tau protein assembles following reaction with the lipid peroxidation product, 4-hydroxy-2-nonenal. FEBS Lett. 486, 270–274 [DOI] [PubMed] [Google Scholar]
- 26. Gomez-Ramos A., Diaz-Nido J., Smith M. A., Perry G., Avila J. (2003) Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. J. Neurosci. Res. 71, 863–870 [DOI] [PubMed] [Google Scholar]
- 27. Starkov A. A. (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 1147, 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eckert A., Hauptmann S., Scherping I., Meinhardt J., Rhein V., Drose S., Brandt U., Fandrich M., Muller W. E., Gotz J. (2008) Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. J. Mol. Med. 86, 1255–1267 [DOI] [PubMed] [Google Scholar]
- 29. Cente M., Filipcik P., Pevalova M., Novak M. (2006) Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur. J. Neurosci. 24, 1085–1090 [DOI] [PubMed] [Google Scholar]
- 30. Dias-Santagata D., Fulga T. A., Duttaroy A., Feany M. B. (2007) Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 117, 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou J. L., Shenoy D. V., Thomas N., Choudhary P. K., Laferla F. M., Goodman S. R., Breen G. A. (2011) Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer's disease. J. Proteomics 74, 466–479 [DOI] [PubMed] [Google Scholar]
- 32. Hirata-Fukae C., Li H. F., Hoe H. S., Gray A. J., Minami S. S., Hamada K., Niikura T., Hua F., Tsukagoshi-Nagai H., Horikoshi-Sakuraba Y., Mughal M., Rebeck G. W., LaFerla F. M., Mattson M. P., Iwata N., Saido T. C., Klein W. L., Duff K. E., Aisen P. S., Matsuoka Y. (2008) Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 1216, 92–103 [DOI] [PubMed] [Google Scholar]
- 33. Misiak M., Beyer C., Arnold S. (2010) Gender-specific role of mitochondria in the vulnerability of 6-hydroxydopamine-treated mesencephalic neurons. Biochim. Biophys. Acta 1797, 1178–1188 [DOI] [PubMed] [Google Scholar]
- 34. Correia S. C., Santos R. X., Cardoso S., Carvalho C., Santos M. S., Oliveira C. R., Moreira P. I. (2010) Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer's disease? Curr. Aging Sci. 3, 113–126 [DOI] [PubMed] [Google Scholar]
- 35. Simpkins J. W., Yi K. D., Yang S. H., Dykens J. A. (2010) Mitochondrial mechanisms of estrogen neuroprotection. Biochim. Biophys. Acta 1800, 1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J. Q., Cammarata P. R., Baines C. P., Yager J. D. (2009) Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta 1793, 1540–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Du H., Guo L., Yan S., Sosunov A. A., McKhann G. M., Yan S. S. (2010) Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc. Natl. Acad. Sci. U. S. A. 107, 18670–18675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoover B. R., Reed M. N., Su J., Penrod R. D., Kotilinek L. A., Grant M. K., Pitstick R., Carlson G. A., Lanier L. M., Yuan L. L., Ashe K. H., Liao D. (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.