Abstract
TMEM16A (ANO1) is a calcium-activated chloride channel (CaCC) expressed in secretory epithelia, smooth muscle, and other tissues. Cell-based functional screening of ∼110,000 compounds revealed compounds that activated TMEM16A CaCC conductance without increasing cytoplasmic Ca2+. By patch-clamp, N-aroylaminothiazole “activators” (Eact) strongly increased Cl− current at 0 Ca2+, whereas tetrazolylbenzamide “potentiators” (Fact) were not active at 0 Ca2+ but reduced the EC50 for Ca2+-dependent TMEM16A activation. Of 682 analogs tested, the most potent activator (Eact) and potentiator (Fact) produced large and more sustained CaCC Cl− currents than general agonists of Ca2+ signaling, with EC50 3–6 μM and Cl− conductance comparable to that induced transiently by Ca2+-elevating purinergic agonists. Analogs of activators were identified that fully inhibited TMEM16A Cl− conductance, providing further evidence for direct TMEM16A binding. The TMEM16A activators increased CaCC conductance in human salivary and airway submucosal gland epithelial cells, and IL-4 treated bronchial cells, and stimulated submucosal gland secretion in human bronchi and smooth muscle contraction in mouse intestine. Small-molecule, TMEM16A-targeted activators may be useful for drug therapy of cystic fibrosis, dry mouth, and gastrointestinal hypomotility disorders, and for pharmacological dissection of TMEM16A function.—Namkung, W., Yao, Z., Finkbeiner, W. E., Verkman, A. S. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction.
Keywords: CaCC, cystic fibrosis, dry mouth, intestinal motility, drug discovery
Calcium-activated Cl− channels (CaCCs) are widely expressed in epithelial and nonepithelial cell types, where they facilitate epithelial fluid secretion, smooth muscle contraction, neurosensory signaling, and other functions (1–3). TMEM16A (alternative name, anoctamin-1, ANO1) was identified as a CaCC, as its heterologous expression in oocytes and mammalian cells produced outwardly rectifying, Ca2+-sensitive Cl− currents (4–6). TMEM16A is expressed in epithelial cells in airways, salivary gland, intestine, and other tissues, as well as in arterial smooth muscle, intestinal pacemaker cells, sensory neurons, and various tumors (4, 7–9). Though TMEM16A-knockout mice die just after birth because of tracheomalacia (10), electrophysiological measurements in the neonatal knockout mice suggested TMEM16A involvement in chloride secretion in salivary gland (11) and airway (12) epithelia. Evidence has also been reported for TMEM16A involvement in intestinal and vascular smooth muscle contraction, nociception, and bile formation (9, 13–15).
We recently identified, by high-throughput screening, small-molecule inhibitors of TMEM16A chloride conductance. Some compounds, including tannic acid and related gallotannins (16) and the arylaminothiophene CaCCinh-A01 (17), function as nonselective CaCC inhibitors that inhibit TMEM16A and other, as yet unidentified, CaCCs in multiple cell types. We proposed that CaCC inhibition by gallotannins in red wines and green teas may account, in part, for their health benefits, including reduced risk of cardiovascular disease. TMEM16A-selective inhibitors were also identified, including the aminophenylthiazole, T16Ainh-A01 (18). T16Ainh-A01 inhibited CaCC Cl− current in TMEM16A-transfected cells and in cultures of human salivary gland and IL-4-treated bronchial epithelia, but not in intestine, providing pharmacological data on TMEM16A involvement in CaCC function in various tissues.
Here, we report the identification of small-molecule TMEM16A activators that target TMEM16A itself rather than upstream Ca2+ signaling. TMEM16A activators are useful research tools for pharmacological dissection of TMEM16A function, and potential drug candidates for treatment of salivary gland dysfunction, as in Sjogren's syndrome and following radiation injury, as well as for cystic fibrosis, dry eye syndrome, intestinal hypomotility, and other disorders associated with Cl− channel dysfunction (2, 19). In cystic fibrosis, the rationale for CaCC activator therapy is the activation of alternative, non-CFTR chloride channels in airway epithelium where CFTR is dysfunctional. Two CaCC activator therapies for cystic fibrosis have been in clinical trials, including a P2Y2 receptor antagonist (denufosol) (20), which acts through Ca2+ elevation, and a bacterial polycyclic peptide (duramycin) (21). A P2Y2 receptor agonist is also in clinical trials for dry eye disease (22). CaCC activators that target CaCCs directly without cytoplasmic Ca2+ elevation might offer more targeted therapy than general agonists of Ca2+ signaling and, unlike receptor agonist therapy, could produce more sustained CaCC activation and hence, offer greater efficacy. Here, we report the identification and activation mechanism of TMEM16A-targeted activators and evidence for their potential therapeutic utility in cystic fibrosis, dry mouth, and intestinal hypomotility.
MATERIALS AND METHODS
Chemicals and solutions
Amiloride, ATP, UTP, and other chemicals, unless otherwise indicated, were purchased from Sigma (St. Louis, MO, USA). 1-(2-Methoxyethyl)-2-thiourea was purchased from Oakwood Products (West Columbia, SC, USA). T16Ainh-A01 and CFTRinh-172 were synthesized as described previously (23). The compound collections used for screening included ∼100,000 synthetic small molecules from ChemDiv (San Diego, CA, USA) and Asinex (Winston Salem, NC, USA), and ∼7500 purified natural products from Analyticon (Potsdam, Germany), Timtek (Newark, NJ, USA), and Biomol (Plymouth Meeting, PA, USA). Compounds were maintained as DMSO stock solutions. Structure-activity analysis was done on analogs purchased from ChemDiv and Asinex. The HCO3−-buffered solution contained (in mM): 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 d-glucose, 5 HEPES, and 25 NaHCO3 (pH 7.4). In the half-Cl− solution, 65 mM NaCl in the HCO3−-buffered solution was replaced by Na gluconate.
Cell culture
Fisher rat thyroid (FRT) cells were stably transfected with human TMEM16A [TMEM16A(abc), cDNA provided by Dr. Luis Galietta, Gaslini Institute, Genoa, Italy] and the halide sensor YFP-H148Q/I152L/F46L. Cells were plated in 96-well black-walled microplates (Corning, Corning, NY, USA) at a density of 20,000 cells/well in Coon's modified F12 medium supplemented with 5% FCS, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The human submandibular cell line, A253 (HTB 41; American Type Culture Collection, Manassas, VA, USA) was cultured in complete McCoy's 5A medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin.
Tracheal and bronchial tissues were obtained from non-CF and CF patients following lung transplantation or from post- mortem examinations performed within 24 h after death. Non-CF tissues were from individuals without significant pulmonary airway disease. The Committee on Human Research at the University of California, San Francisco, approved the use of human tissues for these studies. Primary cultures of non-CF and CF human bronchial epithelial (HBE) cells were grown at an air-liquid interface as described previously (24). Cells were plated at a density of 5 × 105/cm2 onto 12-mm diameter, 0.4-μm pore polycarbonate cell culture inserts (Snapwell; Corning, Lowell, MA, USA) precoated with human placental collagen (15 μg/cm2; Sigma). Cultures were grown at an air-liquid interface in ALI medium at 37°C in 5% CO2/95% air (25). Medium was changed every 2–3 days. Cultures were used 21–30 days after plating, at which time transepithelial resistance (Rte) was 400–1000 Ω/cm2, and an airway surface liquid film was seen.
Primary cultures of non-CF human tracheal gland (HTG) serous cells were generated from the trachea and mainstem bronchi under conditions that induced serous cell differentiation (26). Briefly, after removal of surface epithelium, the gland-rich submucosal tissues were dissected from between the cartilaginous rings. Small segments of gland tubules and acinar structures were isolated by enzymatic digestion, as described previously. Gland fragments were plated in T-25 flasks in DMEM/F12 supplemented with 20% FBS, penicillin (105 mU/ml), streptomycin (100 μg/ml), gentamicin (100 μg/ml), and amphotericin B (2.5 μg/ml). The next day, cultures were rinsed with PBS, and plating medium was replaced with bronchial epithelial growth medium (BEGM; Lonza, Basel, Switzerland). Medium was changed every 24 h for 3 days, and every 2 days thereafter. When the outgrowths of cells from attached acini reached ∼80% confluence, they were removed by trypsinization (0.05% trypsin, 0.02% EDTA) and plated (3×105 cells) onto 12-mm cell culture inserts coated with human placental collagen (15 μg/cm2). Serous gland cells were grown at an air-liquid interface on 0.4-μm pore polyester cell culture inserts (Snapwell) in DMEM/F12 supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), retinoic acid (5×10−8 M), hydrocortisone (0.5 μg/ml), triidothyronine (20 ng/ml), BSA (2 mg/ml), 0.1% Ultroser G serum substitute (Pall Corp., Port Washington, NY, USA), and gentamicin (50 μg/ml). Cells were studied after 10–14 days, with Rte >100 Ω · cm2.
Screening procedures
High-throughput screening was done using an automated screening platform (Beckman, Brea, CA, USA) equipped with FluoStar fluorescence plate readers (BMG Lab Technologies, Durham, NC, USA) as described previously (23). Each well of a 96-well plate was washed 3 times with PBS (200 μl/wash), leaving 50 μl PBS. Test compounds (0.5 μl) were added to each well at 25 μM final concentration. After 10 min, 96-well plates were transferred to a plate reader for fluorescence assay. Each well was assayed individually for TMEM16A-mediated I− influx by recording fluorescence continuously (400 ms/point) for 2 s (baseline), then 50 μl of a 140 mM I− solution was added. The initial rate of I− influx was computed from fluorescence data by nonlinear regression.
Short-circuit current
Snapwell inserts containing TMEM16A-expressing FRT, HBE, CF HBE, or HTG cells were mounted in Ussing chambers (Physiological Instruments, San Diego, CA, USA). Amiloride, CFTRinh-172, UTP, ATP, T16Ainh-A01, and TMEM16A activators were added to the apical solution, and an equal volume of vehicle was added at the same time to the basolateral solution. Symmetrical HCO3−-buffered solutions were used for HBE, CF HBE, and HTG cells. For FRT cells, the hemichambers were filled with a half-Cl− solution (apical) and the HCO3−-buffered solution (basolateral), and the basolateral membrane was permeabilized with 250 μg/ml amphotericin B, as described previously (16). Cells were bathed for a 10-min stabilization period and aerated with 95% O2/5% CO2 at 37°C or room temperature. Apical membrane current (for FRT cells) and short-circuit current were measured using an EVC4000 multi-channel V/I clamp (World Precision Instruments, Sarasota, FL, USA) and recorded using PowerLab/8sp (AD Instruments, Castle Hill, NSW, Australia).
Patch clamp
Whole-cell recordings were made at room temperature on TMEM16A-expressing FRT cells and human submandibular A253 cells. The bath solution contained (in mM): 140 NMDG-Cl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4). The pipette solution contained (in mM): 130 CsCl, 0.5 EGTA, 1 MgCl2, 1 Tris-ATP, and 10 HEPES (pH 7.2). Different concentrations of free calcium in pipette solution were obtained by replacing 0.5 mM EGTA with 5 mM EGTA and using different amounts of CaCl2 in the pipette solution. Pipettes were pulled from borosilicate glass and had resistances of 3–5 MΩ after fire polishing. Seal resistances were between 3 and 10 GΩ. After establishing the whole-cell configuration, TMEM16A was activated by 100 μM ATP, TMEM16A activators, or different concentrations of free calcium in the pipette solution. Whole-cell currents were elicited by applying hyperpolarizing and depolarizing voltage pulses from a holding potential of 0 mV to potentials between −100 and +100 mV in steps of 20 mV. Recordings were made at room temperature using an Axopatch-200B (Axon Instruments). Currents were digitized with a Digidata 1440A converter (Axon Instruments), filtered at 5 kHz, and sampled at 1 kHz.
Cytoplasmic calcium measurements
FRT cells in 96-well black-walled microplates were loaded with Fluo-4 NW per the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Fluo-4 fluorescence was measured with a FLUOstar Optima fluorescence plate reader equipped with syringe pumps and custom Fluo-4 excitation/emission filters (485/538 nm).
Immunoblot
CF HBE and HTG cells were lysed with cell lysis buffer [50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and protease inhibitor mixture (Roche Applied Science, Indianapolis, IN, USA)]. Cell debris was removed by centrifugation, and proteins in the supernatant were resolved by SDS-PAGE and immunoblotted using standard procedures [transfer to polyvinylidene difluoride membrane, 1 h blocking in 5% nonfat dry milk, primary TMEM16A antibody (1:1000 dilution, ab16293, Abcam, Cambridge, MA, USA) and secondary antibody incubations, and enhanced chemiluminescence detection].
Immunohistochemistry
Paraffin tissue sections (5 μm) were dewaxed in two changes of Clear-Rite (Thermo Scientific, Waltham, MA, USA) then rehydrated through a series of graded alcohols. Slides were submerged in 3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity. Heat-induced antigen retrieval was performed by boiling slides in Borg antigen retrieval buffer (Biocare Medical, Concord, CA, USA) for 10 min at 125°C. Slides were blocked with protein block (Dako, Carpinteria, CA, USA) for 10 min and incubated for 60 min with TMEM16A primary antibody (NBP1–49559, Novus Biologicals, Littleton, CO, USA). Antibody detection was done using the SuperPicture Polymer Kit (Invitrogen). 3,3′-Diaminobenzidine) was used to develop the stains. Slides were counterstained with hematoxylin and photographed.
Gland fluid secretion
Human airways were obtained from human subjects following lung transplantation and the California Lung Transplantation Donation Network. For optical recording of mucus (fluid) secretion in airway glands, a fragment of human trachea or bronchus of ∼1 cm2 with underlying glands was dissected from the cartilage and mounted in a 37°C chamber allowing serosal solution exchange. The mucosal surface was rinsed and blotted dry with a cotton swab and further dried with an air stream, after which ∼100 μl of water-saturated mineral oil was placed on the surface. Agonists and inhibitors were added to the serosal side by complete bath replacement. Mucus bubbles in the oil layer were imaged using a Nikon SMZ stereozoom epifluorescence microscope (Nikon, Tokyo, Japan) equipped with P-HR Plan Apo ×1.6 objective lens (working distance 24 mm) and Hamamatsu ORCA-ER CCD camera (Hamamatsu Photonics, Hamamatsu, Japan). Mucus bubble volume was deduced from bubble size, as described previously (27).
Intestinal smooth muscle contraction
Wild-type CD1 mice (age 8–10 wk) were killed by avertin overdose (200 mg/kg). The ileum was removed and washed with ice-cold HCO3−-buffered solution. The ends of the ileal segments were tied with silk thread and connected to a force transducer. Ileal segments were equilibrated for 60 min with a resting force of ∼1 mN, with changes of the bathing solution every 15 min. Tension was monitored continuously with a fixed-range precision force transducer (TSD, 125 C; Biopac, Goleta, CA) connected to a differential amplifier (DA 100B; Biopac). Data were recorded using MP100, Biopac digital acquisition system, and analyzed using Acknowledge 3.5.7 software (Biopac).
Synthesis procedures
Flash chromatography was performed on a CombiFlash Companion chromatography system (Teledyne Isco, Lincoln, NE). 1H and 13C NMR images were obtained on a Bruker 300 MHz instrument (Bruker Corp., Billerica, MA, USA). High-resolution mass spectrometry was done at a core facility at the University of California (Riverside, CA, USA). Elemental analyses were done at the Micro-Mass Facility (University of California, Berkeley, CA, USA).
N-(2-methoxyethyl)-4-phenyl-2-thiazolamine (compound 1)
A solution of 2-bromoacetylphenone (1.59 g, 7.99 mmol) and 1-(2-methoxyethyl)-2-thiourea (1.02 g, 7.61 mmol) in ethanol (30 ml) was refluxed under argon for 4 h. After the reaction mixture was cooled to room temperature, saturated aqueous NaHCO3 was slowly added. Ethanol was removed under reduced pressure. The resulting suspension was extracted twice with CH2Cl2. The combined organic phase was washed with water, dried (Na2SO4), and concentrated. Chromatography [silica, hexanes:ethyl acetate (9:1 to 4:1)] yielded a white crystalline solid (1.62 g, 91%), melting point 57–60°C. 1H NMR (300 MHz, CDCl3) δ 3.39 (s, 3H), 3.53 (m, 2H), 3.64 (m, 2H), 5.46 (br, 1H), 6.70 (s, 1H), 7.27 (m, 1H), 7.37 (m, 2H), and 7.80 (m, 2H). 13C NMR (75 mHz, CDCl3) δ 45.2, 58.8, 70.7, 101.0, 126.0, 127.6, and 128.5. ESI-MS calculated for C12H15N2OS 235.0900; found 235.0902.
N-(2-methoxyethyl)-N-(4-phenyl-2-thiazolyl)-2,3,4-trimethoxybenzeneacetamide (Eact)
A solution of compound 1 (1.00 g, 4.27 mmol) and anhydrous pyridine (690 ml, 8.54 mmol) in anhydrous toluene (40 ml) was stirred for 5 min. To the reaction mixture was added a solution of 3,4,5-trimethoxybenzoyl chloride (1.47 g, 6.41 mmol) in anhydrous toluene (20 ml). The mixture was refluxed under argon for 4.5 h, cooled to room temperature, and poured into water and ethyl acetate. The organic phase was collected, dried (Na2SO4) and concentrated. Chromatography [silica, hexanes:ethyl acetate (4:1 to 3:1)] yielded a crystalline solid (1.33 g, 73%), melting point 97–100°C. 1H NMR (300 MHz, CDCl3) δ 3.28 (s, 3H), 3.86 (t, J = 5.4, 2H), 3.88 (s, 6H), 3.90 (s, 6H), 4.48 (t, J = 5.4, 2H), 6.92 (s, 2H), 7.26 (s, 1H), 7.33 (m, 1H), 7.43 (m, 2H), and 7.90 (m, 2H). 13C NMR (75 mHz, CDCl3) δ 49.6, 56.2, 58.9, 61.0, 69.7, 105.4, 109.4, 126.0, 127.9, and 128.7. ESI-MS is calculated for C22H25N2O5S 429.1479; found 429.1477.
Synthesis of Fact analogs, exemplified with N-(4-bromophenyl)-3-(1H-tetrazol-1-yl)benzamide (Fact)
Thionyl chloride (477 ml, 6.58 mmol) and 3-(1H-tetrazol-1-yl)benzoic acid (50 mg, 0.263 mmol) was heated to 80°C in a screw-cap vial. After a clear solution was observed, the residue solid on the wall was washed by gentle shaking. After 1.5 h, the reaction was cooled to room temperature. The reaction mixture was concentrated to dryness under reduced pressure. The resulting white solid was suspended in CH2Cl2 (1.5 ml) and treated with p-bromoaniline (90 mg, 0.526 mmol) and triethylamine (100 ml, 0.719 mmol). The reaction was stirred at room temperature for 14 h, mixed with silica gel, and concentrated to dryness. Chromatography [silica, CH2Cl2:methanol (98:2 to 1:3)] yielded an off-white solid (48 mg, 53%), decomposition point 203°C. 1H NMR (300 MHz, DMSO-d6) d 7.58 (d, J = 4.9, 2H), 7.79 (d, J = 4.9, 2H), 7.85 (δ, J = 8.0, 1H), 8.10–8.18 (m, 2H), 8.47–8.51 (m, 1H), 10.22 (s, 1H), and 10.62 (br, 1H, NH). 13C NMR (75 mHz, DMSO-d6) δ 120.18, 122.08, 123.92, 128.50, 130.11, 131.30, and 142.25. ESI-MS is calculated for C14H10BrN5O 344.0141; found 344.0152.
RESULTS
Discovery and characterization of TMEM16A activators
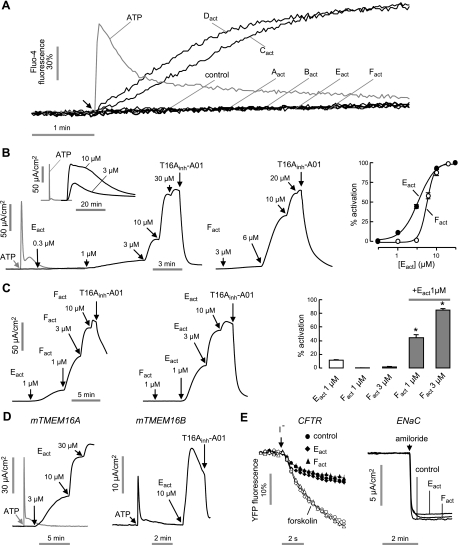
TMEM16A activators were identified from screening of ∼110,000 synthetic drug-like compounds, purified natural products, and approved/investigational drugs. Our cell-based screen utilized FRT cells coexpressing human TMEM16A and the I−-sensing yellow fluorescent protein YFP-H148Q/I152L/F46L. FRT cells were chosen because of their low basal I− and Cl− transport, rapid growth on uncoated plastic, strong stable expression of transfected proteins, and formation of tight junctions for measurements of transepithelial short-circuit current (23). As diagrammed in Fig. 1A, test compounds at 25 μM final concentration were added 10 min prior to I− addition. The 10-min incubation was chosen to allow for compound transport into cytoplasm and to minimize false positives from compounds that elevate cytoplasmic Ca2+ transiently. Fluorescence from individual wells of 96-well plates was measured just prior to and for 6 s after I− addition for computation of initial I− influx rate. Figure 1B shows representative fluorescence data from single wells showing positive (ionomycin) and negative (vehicle only) controls, and examples of inactive and active compounds.
Figure 1.
Identification of small-molecule TMEM16A activators by high-throughput screening. A) Screening protocol. FRT cells stably expressing TMEM16A and the halide-sensitive cytoplasmic fluorescent sensor YFP-H148Q/I152L/F46L were incubated for 10 min with test compound. Fluorescence was monitored in response to addition of iodide. B) Fluorescence measured in single wells of 96-well plates, showing vehicle and positive (ionomycin) controls and examples of inactive and active compounds. C) Structures of TMEM16A activators of six different chemical classes.
Primary screening yielded 40 compounds that increased I− influx by >2 mM/s at 25 μM (>50% of maximal I− influx produced by 100 μM ATP) and had EC50 of <10 μM. Figure 1C shows structures of active compounds from six chemical classes. Secondary screens were done to identify compounds that increased I− influx by targeting TMEM16A. Measurements on TMEM16A null cells (expressing YFP alone) showed that none of the active compounds increased I− influx. However, Cact and Dact increased TMEM16A-mediated I− efflux by producing sustained elevation of cytoplasmic Ca2+ (Fig. 2A). Though such Ca2+ agonists are of potential interest, they were not studied further. Of the remaining compounds that did not elevate Ca2+, we chose the N-aroylaminothiazole Eact and the tetrazolylbenzamide Fact for further characterization because they produced maximal TMEM16A activation (compared to ATP effect) in apical membrane current measurements, whereas Aact and Bact produced only ∼ 50% maximal activation.
Figure 2.
Characterization of TMEM16A activators. A) Cytoplasmic calcium measured by Fluo-4 fluorescence. Arrow indicates addition of 100 μM ATP (gray line) or 10 μM of indicated TMEM16A activators. B) Apical membrane current measured in TMEM16A-expressing FRT cells in the presence of a transepithelial chloride gradient and after basolateral membrane permeabilization. Left and center panels: representative current traces showing ATP (100 μM), Eact- or Fact-stimulated TMEM16A Cl− current. T16Ainh-A01 (10 μM) was added where indicated. Inset shows long-time Eact effect. Right panel: concentration-activation data summary (mean±se, n=4–6). C) Synergistic effect of Eact and Fact. Left and center panels: representative current traces showing synergy. Right panel: data summary of low doses of TMEM16A activation (mean±se, n=5). *P < 0.05. D) Apical membrane current measured in FRT cells transfected with mouse TMEM16A or TMEM16B. E) Effect of Eact and Fact on CFTR and ENaC. Left panel: FRT cells expressing wild-type CFTR and YFP indicator were pretreated for 5 min with 10 μM Eact and Fact. Forskolin (10 μM) was added as indicated. Right panel: HBE cells were pretreated for 5 min with 10 μM Eact and Fact, with amiloride (10 μM) added as indicated.
Figure 2B (left and center panels) shows measurements of apical membrane Cl− current in TMEM16A-expressing FRT cells in which the cell basolateral membrane was permeabilized with amphotericin B and a mucosal-to-serosal Cl− gradient was applied. The purinergic agonist ATP, which transiently elevates cytoplasmic Ca2+, produced a large but transient elevation in Cl− current. Eact and Fact produced large, concentration-dependent increases in Cl− current, which were inhibited by the TMEM16A-selective inhibitor T16Ainh-A01. The current increase was sustained for >10 min (Fig. 2B, inset). The concentration-activation data gave EC50 of ∼3 μM for Eact and ∼6 μM for Fact (Fig. 2B, right panel). Figure 2C shows synergy between Eact and Fact for TMEM16A activation, suggesting distinct mechanisms of action. Whereas 1–3 μM Fact produced little TMEM16A activation alone, it greatly increased TMEM16A current following 1 μM Eact.
Figure 2D (left panel) shows that Eact was effective in producing Cl− current in mouse TMEM16A, which supports its testing in mouse tissues. Figure 2D (right panel) shows activation by Eact of TMEM16B, the other TMEM16 isoform having CaCC activity. Neither Eact nor Fact affected CFTR Cl− conductance or ENaC Na+ conductance (Fig. 2E), which are often found in epithelial cell mucosal membranes where TMEM16A is expressed.
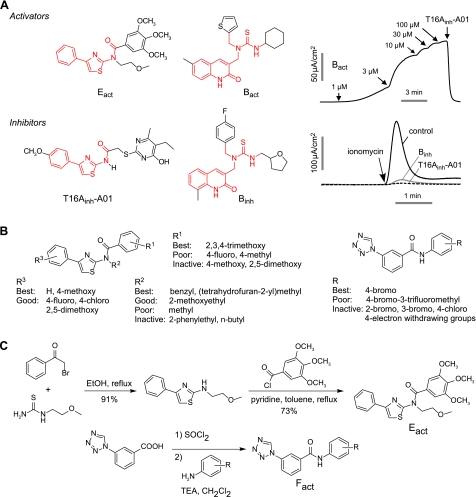
More than 1000 analogs of the B, E, and F classes were tested for TMEM16A inhibition activity, reasoning that small structural changes can convert an agonist into an antagonist. As shown in Fig. 3A, we found compounds of the B and E classes that fully inhibited TMEM16A Cl− conductance. In each case relatively minor chemical structural changes (common scaffolds shown in red in Fig. 3A) converted an activator to an inhibitor, supporting the conclusion that these compounds target TMEM16A directly.
Figure 3.
Structure-activity analysis and synthesis of TMEM16A activators. A) Structural similarities between TMEM16A inhibitors and activators (common scaffolds shown in red). Apical membrane current measurements show activation of TMEM16A by Bact (top panels), inhibition of ionomycin (1 μM)-induced TMEM16A currents by pretreatment B and E class analogs (each 10 μM; bottom panels). B) Summary of structural determinants for TMEM16A activation. Left panel: Eact class. Right panel: Fact class. C) Synthesis of Eact and Fact analogs (see Materials and Methods).
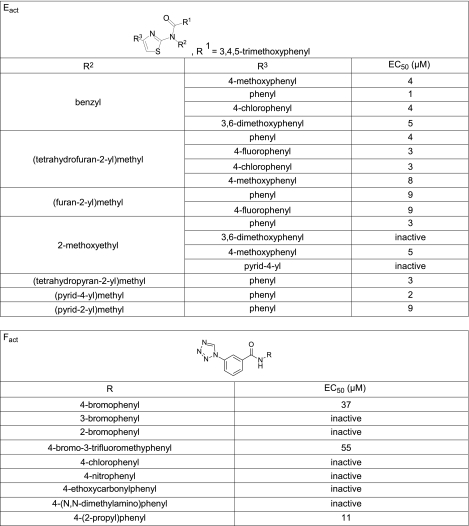
Eact and Fact analogs were assayed to establish structure-activity relationships and to select the best compound(s) for resynthesis in highly pure form for biological studies. Of 673 commercially available class E analogs screened, 18 compounds increased TMEM16A Cl− conductance. Nine of the 10 most potent compounds had a 2,3,4-trimethoxyphenyl (TMOP) group at the R1 position (Fig. 4). Similar compounds but with 4-methoxyphenyl or 2,5-dimethoxyphenyl at R1 were inactive, as summarized in Fig. 3B (left panel). Of the active compound with TMOP at R1, the most potent compounds had benzyl, (tetrahydrofuran-2-yl)methyl, or methoxyethyl groups at R2. Compounds containing an additional carbon on the benzyl group were inactive, as were compounds with methylene replacing the oxygen in methoxyethyl group. Methoxyethyl at R2 and phenyl group at R3 gave one of the most potent analogs. Limited SAR on the F class was done on 9 synthesized analogs, as analogs were not available from commercial sources. We found that while 4-bromo at R1 increased TMEM16A Cl− conductance, the 2-bromo or 3-bromo analogs did not, and that compounds containing 4-chloro, 4-nitro, 4-ethoxycarbonyl, or 4-dimethylamino were inactive.
Figure 4.
Structure-activity analysis of Eact and Fact analogs. EC50 values were determined from fluorescence plate reader assay.
The most potent compounds of the E and F classes were synthesized in highly pure form for further characterization and biological studies (Fig. 3C). Eact was synthesized in two steps. 2-Aminothiazole was obtained by reflux of 2-bromoacetyl-phenone and 1-(2-methoxyethyl)-2-thiourea in ethanol (Fig. 3C). Eact was obtained by reaction of 2-aminothiazole and 2,3,4-trimethoxybenzoyl chloride using anhydrous pyridine in anhydrous toluene. The yield (73%) was comparable to that reported for similar reactions between N-alkyl-2-aminothiazoles and benzoyl chloride (28). Fact analogs were synthesized from 3-(1H-tetrazol-1-yl)benzoic acid and the corresponding anilines. Because amide formation with 1,1′-carbonyldiimidazole as the coupling agent did not drive the reaction to completion, we adopted a two-step, one-pot procedure (29). The benzoic acid was first treated with neat SOCl2 at 80°C for 1.5 h. After removal of excess SOCl2 by rotary evaporation, the resulting acid chloride was suspended in CH2Cl2 and treated with anilines and TEA to yield Fact compounds in 48–65% yield.
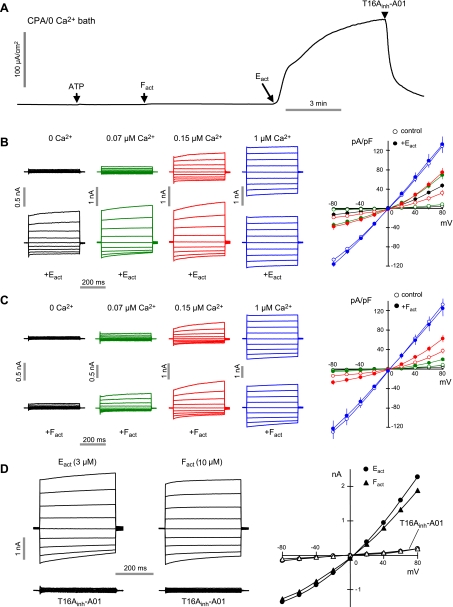
The Ca2+ dependence of TMEM16A activation by Eact and Fact was investigated. Apical membrane current measurements done with 0 Ca2+ apical and basolateral solutions in the presence of cycloplazonic acid (to deplete intracellular Ca2+ stores) showed TMEM16A Cl− currents induced by Eact, but not by ATP or Fact (Fig. 5A). Whole-cell currents were then recorded by patch clamp at different cytoplasmic (pipette) [Ca2+]. In the absence of activators, Fig. 5B (top panel) shows increasing TMEM16A Cl− current with outward rectification at relatively low [Ca2+] and near linear currents at high [Ca2+], in agreement with prior patch-clamp studies of TMEM16A (4). Eact strongly activated TMEM16A at 0 Ca2+, producing outwardly rectifying currents, with more linear currents at higher [Ca2+] (Fig. 5B, bottom and right panels). In contrast, Fact did not produce Cl− current at 0 Ca2+, but increased Cl− current (compared to no compound) at submaximal Ca2+ (Fig. 5C). Maximum TMEM16A Cl− current at high [Ca2+] was not further increased by Eact or Fact. Thus, Eact and Fact activate TMEM16A by different mechanisms: Eact as a largely Ca2+-independent activator, and Fact as a potentiator of Ca2+ activation. T16Ainh-A01 completely blocked Cl− currents produced by Eact or Fact (Fig. 5D), as expected.
Figure 5.
Patch-clamp analysis of Ca2+ requirements for TMEM16A activation by Eact and Fact. A) Apical membrane current measured in TMEM16A-expressing FRT cells. ER calcium stores were depleted by CPA (50 μM, 30 min) and 0 CaCl2 in bath. ATP (100 μM), Fact (10 μM), Eact (10 μM), and T16Ainh-A01 (10 μM) were added as indicated. B, C) Whole-cell TMEM16A currents were recorded at a holding potential at 0 mV, and pulsing to voltages between ± 80 mV (in steps of 20 mV) in the absence and presence of 3 μM Eact (B) or 10 μM Fact (C). Left panels: free calcium concentration of pipette solutions were clamped at 0 μM (black), 0.07 μM (green), 0.15 μM (red), and 1 μM (blue). Eact (3 μM) or Fact (10 μM) was added as indicated. Right panels: current-voltage (I/V) plots of mean currents at the middle of each voltage pulse. D) TMEM16A inhibited by 10 μM T16Ainh-A01 after stimulation by Eact or Fact.
Epithelial fluid secretion and intestinal smooth muscle contraction
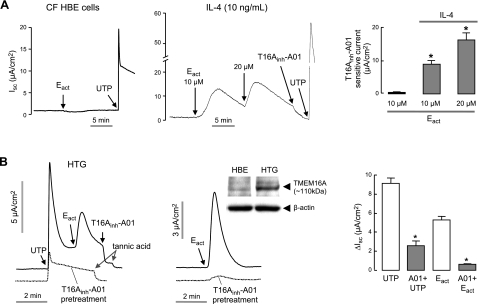
Prior studies using TMEM16A inhibitors and RNAi knockdown indicated that TMEM16A is a minor component of total CaCC conductance in human bronchial surface epithelial cell cultures under basal conditions, but that TMEM16A is strongly up-regulated after IL-4 treatment for 24 h (18). Supporting this conclusion, we found here that Eact did not induce Cl− current in untreated human CF bronchial epithelial cells, whereas UTP, which elevates cytoplasmic Ca2+ and hence non-TMEM16A CaCC(s), produced a large Cl− current (Fig. 6A, left panel). Remarkably, Eact induced Cl− current in IL-4-treated CF bronchial cells, which was blocked by the TMEM16A-selective inhibitor T16Ainh-A01 (Fig. 6A, center and right panels).
Figure 6.
Airway epithelial chloride secretion. A) Short-circuit current in CF HBE cells. Left and center panels: Eact and UTP (100 μM) were added in control (left) and IL-4 (10 ng/ml, 24 h, center) treated CF HBE cells. Right panels: summary of Eact-induced, T16Ainh-A01-sensitive peak current (mean±se, n=6–8). *P < 0.05. ENaC was inhibited by 10 μM amiloride. B) Eact (10 μM) and UTP (100 μM) induced CaCC Cl− current measured in primary cultures of non-CF human tracheal gland (HTG) serous cells. Left and center panels: TMEM16A, CFTR, and ENaC were inhibited by pretreatment with T16Ainh-A01, CFTRinh-172, and amiloride, respectively. Inset: TMEM16A immunoblot in whole cell homogenates of CF HBE and HTG cells. Right panel: summary of UTP and Eact-induced peak current in the presence and absence of T16Ainh-A01 (mean±se, n=3). *P < 0.05.
Prior reports suggested the involvement of TMEM16A in CaCC activity in airway submucosal glands (30, 31). To verify TMEM16A function, short-circuit current measurements were done in primary cultures of human tracheal submucosal gland epithelial cells that were grown under conditions that preserve serous-type phenotype (26). Figure 6B (left panel) shows increased Cl− current in response to UTP and Eact, which was largely abolished by T16Ainh-A01 pretreatment. Figure 6B (middle panel) shows increased Cl− conductance with Eact in the absence of UTP pretreatment. Immunoblot (Fig. 6B, inset) confirmed TMEM16A protein in the glandular epithelial cell cultures. Averaged peak Cl− currents are summarized in Fig. 6B (right panel).
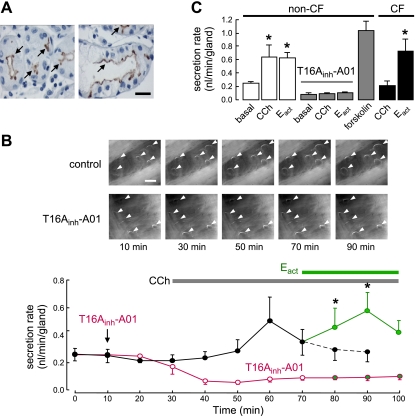
Immunostaining of human non-CF and CF bronchi showed TMEM16A expression at the luminal membrane of submucosal gland serous cells, but not gland mucous cells or surface epithelial cells (Fig. 7A). Similar staining was found in non-CF and CF human bronchi. Fluid secretion was measured in individual submucosal glands from the increasing size of mucus (fluid) bubbles in which airway fragments were mounted in a 37°C perfusion chamber and the mucosal solution was covered with oil. Figure 7B (top panel) shows mucus bubbles following addition of carbachol and Eact in human bronchi. Figure 7B (bottom panel) summarizes the secretion rate from many mucus droplets, showing nonzero basal secretion, and significantly increased secretion following serosal application of submaximal carbachol and Eact. T16Ainh-A01 pretreatment abolished basal and agonist-stimulated gland fluid secretion. The data summary in Fig. 7C shows Eact-induced gland fluid secretion in non-CF and CF bronchi of comparable magnitude to that induced by maximal cAMP stimulation by forskolin.
Figure 7.
Airway submucosal gland fluid secretion in human bronchi. A) TMEM16A immunohistochemistry in CF (left panel) and non-CF (right panel) human bronchi showing apical membrane expression in serous gland epithelial cells (arrows). Scale bar = 20 μm. B) Mucus (fluid) secretion in human bronchi. Top panels: images of mucus bubbles formed under oil in response to basolateral application of 300 nM carbachol (CCh) and 20 μM Eact. TMEM16A was inhibited by 30 μM T16Ainh-A01. Individual fluid bubbles marked with arrowheads. Scale bar = 0.5 mm. Bottom panels: CCh and Eact-induced secretion rates. Where indicated, tissues were pretreated with T16Ainh-A01 (30 μM). Each point is the average of measurements made from 20 glands (mean±se). C) Summary of human gland fluid secretion rates measured at 20 min after addition of 20 μM Eact, and 30 min after application of 300 nM carbachol (CCh) and 10 μM forskolin (20–66 glands from 3 tracheas and 4 bronchi). In CF-bronchi, 6 glands from one donor were stimulated by Eact. *P < 0.05.
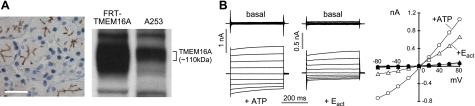
Prior studies implicated TMEM16A as the principle CaCC in salivary gland epithelium (4, 11, 18). TMEM16A immunostaining showed that TMEM16A is expressed on the apical surface of acinar epithelial cells in human parotid gland (Fig. 8A, left panel). A253 cells, a human salivary gland epithelial cell line, express TMEM16A (Fig. 8A, right panel). By whole-cell patch-clamp Eact strongly increased Cl− current in A253 cells (Fig. 8B).
Figure 8.
Salivary gland epithelial cell Cl− secretion. A) Expression of TMEM16A in human salivary gland. Left panel: TMEM16A immunostaining in human parotid gland. Scale bar = 20 μm. Right panel: immunoblot of TMEM16A in FRT-TMEM16A and A253 cells. B) Whole-cell patch-clamp recordings in A253 cells. Left panel: CaCC and TMEM16A chloride current induced by 100 μM ATP and 10 μM Eact, respectively. Right panel: current/voltage (I/V) plot of mean currents at the middle of each voltage pulse (voltages between −80 and +80 mV in steps of 20 mV).
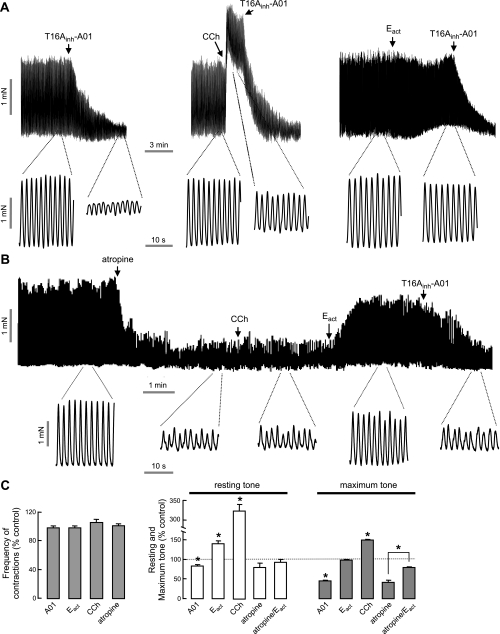
TMEM16A in expressed in the interstitial cells of Cajal, the pacemaker cells that control smooth muscle contraction dynamics in stomach and intestine (8, 32). We investigated the effect of Eact on mouse ileal smooth muscle contraction in an ex vivo intestinal preparation. Figure 9A shows considerable constitutive activity of mouse ileal muscle segments at baseline, with large, spontaneous intestinal contractions that were inhibited by T16Ainh-A01 (left panel). Resting and maximum tone were increased by carbachol, without change in contraction frequency (Fig. 9, middle panel). Eact produced a very small increase in resting but not maximum tone (Fig. 9, right panel). We reasoned that the constitutive TMEM16A activity in this ex vivo preparation could obscure Eact activity. To reveal Eact effects, atropine was first added to inhibit basal contractions. Figure 9B shows that atropine prevented the carbachol effect, which is Ca2+ dependent, and revealed a large increase in contraction amplitude following Eact, whose action is Ca2+ independent. Figure 9C summarizes data on contraction frequency, resting tone, and maximal tone.
Figure 9.
Intestinal smooth muscle contraction. A) Representative traces from mouse ileal segments showing effects of T16Ainh-A01 (10 μM), carbachol (CCh, 1 μM) and Eact (10 μM). B) Effect of CCh (1 μM) and Eact (10 μM) following atropine (1 μM). C) Summary of contraction frequency (left panel) and resting and maximum tone (right panel; mean±se, n=4–7). *P < 0.05.
DISCUSSION
A functional, cell-based screen of small-molecule collections revealed several chemical classes of TMEM16A activators that produced strong and more sustained Cl− currents than Ca2+-elevating purinergic agonists in multiple cell types without elevating cytoplasmic Ca2+. Two classes of compounds with distinct activating mechanisms, activators and potentiators, were identified. Though various agonists of cytoplasmic Ca2+ have been available and studied in clinical trials, direct-acting CaCC modulators have not been reported previously. The more sustained CaCC activation produced by the compounds identified here could translate to improved efficacy compared to Ca2+ agonists, such as P2Y2 agonists, which generally produce only transient elevation in cytoplasmic Ca2+ and, consequently, in Cl− secretion. The recently reported phase 3 trial of the P2Y2 agonist denufosol (20), which failed to show clinical efficacy, may be related to its limited duration of action. In addition to producing more sustained activation of Cl− conductance, direct-acting CaCC activators also have the theoretical advantage over Ca2+ agonists of greater target specificity.
Action of Eact and Fact probably involves direct interaction with the TMEM16A protein, as they did not elevate cytoplasmic Ca2+ and were effective in patch-clamp studies in which [Ca2+] was clamped by the pipette solution. Further, the generation of TMEM16A inhibitors by minor structural modification of activators strongly support a direct binding mechanism. The molecular site of activator interaction on the TMEM16A protein is not known and will require mutagenesis or purification/binding studies. On the basis of the assumed topography and domain structure of TMEM16A (4), we speculate that Eact interacts at or very near the Ca2+ binding site, perhaps at the cluster of four contiguous glutamic acid residues localized in the first intracellular loop (7). Fact, which increased the Ca2+ sensitivity of TMEM16A activation, might act by an allosteric mechanism at a site distinct from the Ca2+ binding site.
Lung disease pathogenesis in CF is thought to involve distinct defects in airway submucosal gland fluid secretion, causing secretion of hyperviscous mucus (33), and in airway surface transport, causing reduced airway surface liquid volume (34). Both related defects are likely attributed to defective CFTR-mediated Cl− secretion, and perhaps ENaC hyperactivity, though the evidence remains controversial (35). Small-molecule therapies under development to correct the underlying CFTR defect include correctors, potentiators, and read-though enhancers to restore Cl− conductance in cells expressing CF-causing mutant CFTRs (2, 36), as well as gene replacement (37). Therapies targeting the activation of alternative Cl− channels, the CaCCs, have received considerable attention as well, as CaCCs are robustly expressed in non-CF and CF airways where CFTR is normally expressed. Our results here provide evidence that TMEM16A activators increase secretion by submucosal glands, as well as by airway surface cells exposed to a proinflammatory milieu. The long-acting TMEM16A activators identified here may thus be useful for CF therapy.
Dry mouth due to salivary gland dysfunction is seen following radiation therapy for head and neck cancers, in Sjogren's syndrome, and most commonly, with unknown (idiopathic) etiology. Dry eye (keratoconjunctivis sicca) is a related disorder that is very common in the elderly, which results from lacrimal or meibomian gland dysfunction. TMEM16A is the major ion channel regulating saliva secretion by salivary gland acinar epithelial cells (11). As such, TMEM16A activators are predicted to potentiate, or amplify, endogenous cholinergic signals to maximize saliva secretion from functional salivary gland acini. Because Cl− and saliva fluid secretion require activity of basolateral membrane K+ channels, TMEM16A-targeted activators are potentially advantageous over nonselective Ca2+-elevating agonists, as they would primarily amplify the effect of physiological stimuli rather than cause fluid secretion on their own. Follow-on studies are indicated of TMEM16A activator therapy in rodent models of dry mouth involving radiation- or immune-mediated salivary gland injury.
Interstitial cells of Cajal (ICC) generate slow-wave pacemaker activity in smooth muscle in the gastrointestinal (GI) tract. TMEM16A is expressed in ICC but not in the GI smooth muscle cells (8, 9, 38). A recent report showed that pharmacological inhibition or genetic deletion of TMEM16A abolished slow waves in murine small intestine (9). We found that the TMEM16A-selective inhibitor T16Ainh-01 greatly reduced smooth muscle contraction in mouse intestine ex vivo, and that the TMEM16A activator Eact increased contraction, restoring contraction following atropine inhibition. These data suggest that GI smooth muscle motility can be modulated by pharmacological activation or inhibition of TMEM16A. TMEM16A activators may be of therapeutic utility for GI motility disorders such as slow transit constipation, and inhibitors for disorders associated with hypermotility. Of interest, TMEM16A modulators changed the strength but not the frequency of intestinal smooth muscle contraction. Pharmacological inhibition of CaCCs by nonspecific inhibitors of CaCCs (niflumic acid, DIDS) reduce the frequency and blocked slow waves in murine intestine (9). Thus, TMEM16A may be involved in the regulation of intestinal smooth muscle contractility and other CaCCs in the regulation of contraction frequency.
There is prior literature on the chemistry and biological properties of analogs of the class E and F compounds identified here as TMEM16A activators. 4-Aryl-1,3-thiazol-2-yl-N-methylbenzamide analogs have been reported as inhibitors of mGluR1 (28) malonyl-CoA decarboxylase (39), and HCV RNA polymerase (40). Analysis of 2-aminothiazole analogs having a secondary amide structure (rather than the tertiary amide in Eact), have revealed analogs as thrombopoietin agonists (41), VEGFR-2 kinase inhibitors (42), adenosine receptor antagonists (43), RNA polymerase inhibitors (40), and mGluR1 antagonists (28). These compounds also have antimicrobial (44), hypolipidemic (45), and antiatherogenic (46) activities. The published data suggest favorable pharmacological properties and low toxicity of compounds related to class E and F activators, though pharmacological testing of the specific TMEM16A activators identified here will be needed for their further preclinical development.
In summary, the TMEM16A activators identified here by high-throughput screening likely act by binding to the TMEM16A protein. They produced large and more sustained Cl− currents than Ca2+-elevating purinergic agonists in salivary gland and airway submucosal gland epithelial cells and IL-4-treated bronchial surface epithelial cells, and stimulated submucosal gland secretion in human bronchi and smooth muscle contraction in mouse intestine. Small-molecule, TMEM16A-targeted activators may have broad utility for treatment of cystic fibrosis, dry mouth, and dry eye syndromes, and motility disorders of the gastrointestinal tract.
Acknowledgments
The authors thank Dr. Dennis W. Nielson (Department of Pediatrics, University of California, San Francisco) and Dr. Jae-Woo Lee (Department of Anesthesia and Perioperative Care, University of California, San Francisco) for human airway tissues.
This work was supported by NIH grants DK72517, HL73856, DK35124, DK86125, EB00415, and EY13574 and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.
REFERENCES
- 1. Hartzell C., Putzier I., Arreola J. (2005) Calcium-activated chloride channels. Annu. Rev. Physiol. 67, 719–758 [DOI] [PubMed] [Google Scholar]
- 2. Verkman A. S., Galietta L. J. (2009) Chloride channels as drug targets. Nat. Rev. Drug. Discov. 8, 153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eggermont J. (2004) Calcium-activated chloride channels: (un) known, (un)loved? Proc. Am. Thorac. Soc. 1, 22–27 [DOI] [PubMed] [Google Scholar]
- 4. Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 [DOI] [PubMed] [Google Scholar]
- 5. Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 [DOI] [PubMed] [Google Scholar]
- 6. Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrera L., Caputo A., Galietta L. J. (2010) TMEM16A protein: a new identity for Ca2+-dependent Cl channels. Physiology 25, 357–363 [DOI] [PubMed] [Google Scholar]
- 8. Huang F., Rock J. R., Harfe B. D., Cheng T., Huang X., Jan Y. N., Jan L. Y. (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. U. S. A. 106, 21413–21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hwang S. J., Blair P. J., Britton F. C., O'Driscoll K. E., Hennig G., Bayguinov Y. R., Rock J. R., Harfe B. D., Sanders K. M., Ward S. M. (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 587, 4887–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rock J. R., Futtner C. R., Harfe B. D. (2008) The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev. Biol. 321, 141–149 [DOI] [PubMed] [Google Scholar]
- 11. Romanenko V. G., Catalan M. A., Brown D. A., Putzier I., Hartzell H. C., Marmorstein A. D., Gonzalez-Begne M., Rock J. R., Harfe B. D., Melvin J. E. (2010) Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J. Biol. Chem. 285, 12990–13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rock J. R., O'Neal W. K., Gabriel S. E., Randell S. H., Harfe B. D., Boucher R. C., Grubb B. R. (2009) Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J. Biol. Chem. 284, 14875–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta A. K., Khimji A. K., Kresge C., Bugde A., Dougherty M., Esser V., Ueno Y., Glaser S. S., Alpini G., Rockey D. C., Feranchak A. P. (2011) Identification and functional characterization of TMEM16A, a Ca2+-activated Cl− channel activated by extracellular nucleotides, in biliary epithelium. J. Biol. Chem. 286, 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manoury B., Tamuleviciute A., Tammaro P. (2010) TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 588, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu B., Linley J. E., Du X., Zhang X., Ooi L., Zhang H., Gamper N. (2010) The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J. Clin. Invest. 120, 1240–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Namkung W., Thiagarajah J. R., Phuan P. W., Verkman A. S. (2010) Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 24, 4178–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De La Fuente R., Namkung W., Mills A., Verkman A. S. (2008) Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol. Pharmacol. 73, 758–768 [DOI] [PubMed] [Google Scholar]
- 18. Namkung W., Phuan P. W., Verkman A. S. (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 286, 2365–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tabbara K. F., Vera-Cristo C. L. (2000) Sjogren syndrome. Curr. Opin. Ophthalmol. 11, 449–454 [DOI] [PubMed] [Google Scholar]
- 20. Kellerman D., Rossi Mospan A., Engels J., Schaberg A., Gorden J., Smiley L. (2008) Denufosol: a review of studies with inhaled P2Y2 agonists that led to Phase 3. Pulm. Pharmacol. Ther. 21, 600–607 [DOI] [PubMed] [Google Scholar]
- 21. Steiner I., Errhalt P., Kubesch K., Hubner M., Holy M., Bauer M., Muller M., Hinterberger S., Widmann R., Mascher D., Freissmuth M., Kneussl M. (2008) Pulmonary pharmacokinetics and safety of nebulized duramycin in healthy male volunteers. Naunyn Schmiedebergs Arch. Pharmacol. 378, 323–333 [DOI] [PubMed] [Google Scholar]
- 22. Nichols K. K., Yerxa B., Kellerman D. J. (2004) Diquafosol tetrasodium: a novel dry eye therapy. Expert Opin. Investig. Drugs 13, 47–54 [DOI] [PubMed] [Google Scholar]
- 23. Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levin M. H., Sullivan S., Nielson D., Yang B., Finkbeiner W. E., Verkman A. S. (2006) Hypertonic saline therapy in cystic fibrosis: Evidence against the proposed mechanism involving aquaporins. J. Biol. Chem. 281, 25803–25812 [DOI] [PubMed] [Google Scholar]
- 25. Fulcher M. L., Gabriel S., Burns K. A., Yankaskas J. R., Randell S. H. (2005) Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 107, 183–206 [DOI] [PubMed] [Google Scholar]
- 26. Finkbeiner W. E., Zlock L. T., Mehdi I., Widdicombe J. H. (2010) Cultures of human tracheal gland cells of mucous or serous phenotype. In Vitro Cell. Dev. Biol. Anim. 46, 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiagarajah J. R., Song Y., Haggie P. M., Verkman A. S. (2004) A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 18, 875–877 [DOI] [PubMed] [Google Scholar]
- 28. Satoh A., Nagatomi Y., Hirata Y., Ito S., Suzuki G., Kimura T., Maehara S., Hikichi H., Satow A., Hata M., Ohta H., Kawamoto H. (2009) Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 19, 5464–5468 [DOI] [PubMed] [Google Scholar]
- 29. DiMauro E. F., Newcomb J., Nunes J. J., Bemis J. E., Boucher C., Buchanan J. L., Buckner W. H., Cee V. J., Chai L., Deak H. L., Epstein L. F., Faust T., Gallant P., Geuns-Meyer S. D., Gore A., Gu Y., Henkle B., Hodous B. L., Hsieh F., Huang X., Kim J. L., Lee J. H., Martin M. W., Masse C. E., McGowan D. C., Metz D., Mohn D., Morgenstern K. A., Oliveira-dos-Santos A., Patel V. F., Powers D., Rose P. E., Schneider S., Tomlinson S. A., Tudor Y. Y., Turci S. M., Welcher A. A., White R. D., Zhao H., Zhu L., Zhu X. (2006) Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: synthesis, SAR, and in vivo anti-inflammatory activity. J. Med. Chem. 49, 5671–5686 [DOI] [PubMed] [Google Scholar]
- 30. Fischer H., Illek B., Sachs L., Finkbeiner W. E., Widdicombe J. H. (2010) CFTR and calcium-activated chloride channels in primary cultures of human airway gland cells of serous or mucous phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L585–L594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee R. J., Foskett J. K. (2010) Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L210–L231 [DOI] [PubMed] [Google Scholar]
- 32. Gomez-Pinilla P. J., Gibbons S. J., Bardsley M. R., Lorincz A., Pozo M. J., Pasricha P. J., Van de Rijn M., West R. B., Sarr M. G., Kendrick M. L., Cima R. R., Dozois E. J., Larson D. W., Ordog T., Farrugia G. (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boucher R. C. (2007) Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 [DOI] [PubMed] [Google Scholar]
- 34. Verkman A. S., Song Y., Thiagarajah J. R. (2003) Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell. Physiol. 284, C2–C15 [DOI] [PubMed] [Google Scholar]
- 35. Donaldson S. H., Boucher R. C. (2007) Sodium channels and cystic fibrosis. Chest 132, 1631–1636 [DOI] [PubMed] [Google Scholar]
- 36. Sloane P. A., Rowe S. M. (2010) Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr. Opin. Pulm. Med. 16, 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White A. F., Ponnazhagan S. (2006) Airway epithelium directed gene therapy for cystic fibrosis. Med. Chem. 2, 499–503 [DOI] [PubMed] [Google Scholar]
- 38. Kashyap P., Gomez-Pinilla P. J., Pozo M. J., Cima R. R., Dozois E. J., Larson D. W., Ordog T., Gibbons S. J., Farrugia G. (2011) Immunoreactivity for Ano1 detects depletion of Kit-positive interstitial cells of Cajal in patients with slow transit constipation. Neurogastroenterol. Motil. 23, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng J. F., Chen M., Wallace D., Tith S., Haramura M., Liu B., Mak C. C., Arrhenius T., Reily S., Brown S., Thorn V., Harmon C., Barr R., Dyck J. R., Lopaschuk G. D., Nadzan A. M. (2006) Synthesis and structure-activity relationship of small-molecule malonyl coenzyme A decarboxylase inhibitors. J. Med. Chem. 49, 1517–1525 [DOI] [PubMed] [Google Scholar]
- 40. Shipps G. W., Jr., Deng Y., Wang T., Popovici-Muller J., Curran P. J., Rosner K. E., Cooper A. B., Girijavallabhan V., Butkiewicz N., Cable M. (2005) Aminothiazole inhibitors of HCV RNA polymerase. Bioorg. Med. Chem. Lett. 15, 115–119 [DOI] [PubMed] [Google Scholar]
- 41. Munchhof M. J., Antipas A. S., Blumberg L. C., Brissette W. H., Brown M. F., Casavant J. M., Doty J. L., Driscoll J., Harris T. M., Wolf-Gouveia L. A., Jones C. S., Li Q., Linde R. G., Lira P. D., Marfat A., McElroy E., Mitton-Fry M., McCurdy S. P., Reiter L. A., Ripp S. L., Shavnya A., Thomasco L. M., Trevena K. A. (2009) The identification of orally bioavailable thrombopoietin agonists. Bioorg. Med. Chem. Lett. 19, 1428–1430 [DOI] [PubMed] [Google Scholar]
- 42. Lee K., Jeong K. W., Lee Y., Song J. Y., Kim M. S., Lee G. S., Kim Y. (2010) Pharmacophore modeling and virtual screening studies for new VEGFR-2 kinase inhibitors. Eur. J. Med. Chem. 45, 5420–5427 [DOI] [PubMed] [Google Scholar]
- 43. Scheiff A. B., Yerande S. G., El-Tayeb A., Li W., Inamdar G. S., Vasu K. K., Sudarsanam V., Muller C. E. (2010) 2-Amino-5-benzoyl-4-phenylthiazoles: Development of potent and selective adenosine A1 receptor antagonists. Bioorg. Med. Chem. 18, 2195–2203 [DOI] [PubMed] [Google Scholar]
- 44. Stadelmann B., Scholl S., Muller J., Hemphill A. (2010) Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 65, 512–519 [DOI] [PubMed] [Google Scholar]
- 45. Mokale S. N., Sanap P. T., Shinde D. B. (2010) Synthesis and hypolipidemic activity of novel 2-(4-(2-substituted aminothiazole-4-yl) phenoxy) acetic acid derivatives. Eur. J. Med. Chem. 45, 3096–3100 [DOI] [PubMed] [Google Scholar]
- 46. Kawamatsu Y., Sohda T., Imai Y. (1981) 2-Amino-4-phenylthianzole derivatives as anti-atherogenic agents. Eur. J. Med. Chem. 16, 355–362 [Google Scholar]