Abstract
The physiological and pathophysiological implications of the expression of vimentin, a type III intermediate filament protein, in alveolar epithelial cells (AECs) are unknown. We provide data demonstrating that vimentin is regulated by TGFβ1, a major cytokine released in response to acute lung injury and that vimentin is required for wound repair and remodeling of the alveolar epithelium. Quantitative real-time PCR shows a 16-fold induction of vimentin mRNA in TGFβ1-treated transformed AECs. Luciferase assays identify a Smad-binding element in the 5′ promoter of vimentin responsible for TGFβ1-induced transcription. Notably, TGFβ1 induces vimentin protein expression in AECs, which is associated with a 2.5-fold increase in cell motility, resulting in increased rates of migration and wound closure. These effects are independent of cell proliferation. TGFβ1-mediated vimentin protein expression, cell migration, and wound closure are prevented by a pharmacological inhibitor of the Smad pathway and by expression of Ad-shRNA against vimentin. Conversely, overexpression of mEmerald-vimentin is sufficient for increased cell-migration and wound-closure rates. These results demonstrate that vimentin is required and sufficient for increased wound repair in an in vitro model of lung injury.—Rogel, M. R., Soni, P. N., Troken, J. R., Sitikov, A., Trejo, H. E., Ridge, K. M. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells.
Keywords: TGF-β1, migration, scratch wound assay, intermediate filaments, lung injury
Alveolar epithelial barrier integrity is a principal determinant of normal lung function. Injury to the lung compromises epithelial barrier integrity, leading to pulmonary edema, diffuse epithelial damage, and inflammation. Widespread necrosis of the alveolar epithelial cells (AECs) leaves behind a denuded basement membrane, allowing edema fluid to accumulate in the alveolar space. Increased production and release of cytokines within the lung and other inflammatory molecules found in the edema fluid also accompany the acute phase of lung injury.
Surviving alveolar epithelial type II cells migrate into the denuded basement membrane and proliferate and differentiate into the alveolar epithelial type I cells (which comprise 90% of the alveolar epithelial surface area due to their flat morphology) in order to restore epithelial barrier integrity (1). The alveolar type II cells at the margin of the denuded area become hyperplastic and exhibit increased mitotic activity and/or enhanced cell motility. The process likely is mediated in the initial stages by cell spreading and migration, as suggested by in vitro wound-repair models, followed by cell proliferation after 1 or 2 d (2–5). For migrating cells, the requirements appear to be fulfilled by transitioning to a more plastic, dedifferentiated, activated state (6–8).
Following lung injury, TGF-β1 is the predominant cytokine produced by inflammatory cells, fibroblasts, and epithelial cells alike. TGF-β1 is also implicated in a number of other physiological functions, including an epithelial-mesenchymal transition (EMT; refs. 9, 10) and homeostatic cellular processes, such as proliferation, differentiation, and migration (11–13). Once activated by this cytokine, fibroblasts and myofibroblasts begin secreting TGF-β1, so that levels increase in both a paracrine and autocrine fashion. Elevated levels of TGF-β1 gene expression and activated protein have been found in AECs and macrophages in animal lungs and patients with idiopathic pulmonary fibrosis and fibroproliferative acute respiratory distress syndrome (14–17). Transient adenoviral-mediated overexpression of TGF-β1 in rat lungs induces prolonged and severe pulmonary fibrosis (18). On the other hand, mice lacking either the αvβ6 integrin, or the TGF-β1-dependent Smad3 signaling pathway, are protected from bleomycin-induced fibrosis (19, 20).
Among the many unresolved questions regarding acute lung injury and its counterpart, fibrosis, is the question of how AECs actually meet requirements for efficient wound repair and remodeling. One important effector of epithelial cell integrity, function, and plasticity is the cellular cytoskeleton. In particular, the intermediate filament (IF) network within epithelial cells integrates and organizes the cytoplasm to effectively provide the mechanical integrity that is crucial to functionality at the tissue level. In fact, AECs owe much of their resilience during environmental stress to the unique properties of IFs (21, 22). IFs have long half-lives and are biochemically stable, yet the networks are very dynamic and routinely rearrange by disassembly and reassembly during wound healing and cell migration, as well as during various environmental stresses (21, 23–27). IFs are classified into 5 major families expressed in cell-, tissue-, differentiation-, and development stage-specific patterns. In general, epithelium-derived cells (e.g., ATII) express type I and II keratin IFs, while mesenchymal cells (e.g., fibroblasts) express type III vimentin IFs. Vimentin has been observed in epithelium-derived cells during physiological and pathological processes requiring migration. TGF-β1 has been linked to vimentin up-regulation, specifically during periods of increased cellular plasticity, such as those mentioned (6–8). Therefore, in this study, we focused on the role of vimentin during wound repair and remodeling in an in vitro lung injury model. Our results show that expression of vimentin IF protein in AECs via the TGF-β1 pathway increases the rate of cell migration, promoting more efficient wound closure, which demonstrates that vimentin is required and sufficient for increased wound repair in an in vitro model of lung injury.
MATERIALS AND METHODS
Reagents
Recombinant TGF-β1 (human, Chinese hamster ovary cell line) was purchased from Calbiochem (Darmstadt, Germany), reconstituted according to manufacturer's recommendations, and used at a concentration of 2 ng/ml. SB431542 (10 mM), mitomycin C (10 μM) and bromo-2′-deoxyuridine (BrdU, 10 μM), monoclonal vimentin (clone V9), polyclonal E-cadherin, monoclonal actin, and monoclonal BrdU antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal GAPDH antibody, used for Western blot analysis, was purchased from Cell Signaling (Danvers, MA, USA). Goat anti-mouse fluorescein-tagged immunoglobulins (GAM 488) secondary antibody and Hoechst 33342 were obtained from Molecular Probes (Eugene, OR, USA). Secondary antibodies for immunoblotting were peroxidase-labeled goat anti-mouse or goat anti-rabbit immunoglobulins (Bio-Rad Laboratories, Hercules, CA, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA) at a concentration of 1 mg/ml. Insulin-transferrin sodium selenite (ITS) medium supplement, purchased from Sigma-Aldrich, was added to the experimental cell culture medium (1% v/v).
Cell culture
Human bronchoalveolar carcinoma cell line (H358) and human epithelial cancer cell line (A549) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI medium and DMEM, respectively, supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% (v/v) sodium pyruvate (in the case of RPMI medium only). Cells were incubated in a humidified atmosphere of 5% CO2–95% air at 37°C. During experiments, H358 cells were cultured in RPMI containing 5% charcoal-stripped (CS) FBS and ITS supplement.
Alveolar epithelial type II (ATII) cells were isolated from pathogen-free male Sprague-Dawley rats (200–225 g). Briefly, the lungs were perfused via the pulmonary artery, lavaged, and digested with elastase (30 U/ml). AECs were purified by differential adherence to IgG-pretreated dishes, and cell viability was assessed by trypan blue exclusion (>95%). Cells were suspended in DMEM containing 10% FBS with 2 mM l-glutamine, 40 μg/ml gentamicin, 100 U/ml penicillin, and 100 μg/ml streptomycin, and they were placed in culture for 24 h before the start of all experimental conditions. Cells were incubated in a humidified atmosphere of 5% CO2–95% air at 37°C.
Scratch wound assay
The scratch wound assay is used in wound closure rate experiments, live cell imaging, indirect immunofluorescence, and Western blot analyses. For Western blot and wound closure rate experiments, AECs were plated in 10 cm2 dishes or 6-well plates in complete medium, respectively. Cells were seeded on 22-mm glass coverslips for immunofluorescence. For experiments with H358 cells, complete medium was replaced with medium containing CS FBS at 48 h before wounding. Crisscross (Western blotting) or single scratches (wound closure rate experiments, immunofluorescence, live cell imaging) were made in confluent cell monolayers using either 250- or 20-μl pipette tips, respectively; cells were then rinsed 3 times with PBS, and the medium was replaced. TGF-β1 was added to the medium prior to its addition to cells at a concentration of 2 μg/ml. For experiments involving SB431542, cells were pretreated for 30 min, and fresh medium containing both SB431542 and TGF-β1 was added to cells following wounding. In the case of mitomycin C experiments, cells were synchronized by serum starvation for 48 h prior to treatment, and confluent monolayers were incubated with mitomycin C for 3 h on ice at 5% CO2. After removing the medium and allowing cells to recover, monolayers were scratched and treated as described above. To confirm the inhibition of proliferation in these experiments, BrdU was added to the medium at the time of wounding; after 48 h, cells were processed for immunofluorescence, as described below. Every 24 h postwounding, the 6-well plates were placed on the stage of a Nikon inverted microscope (Nikon Instruments, Tokyo, Japan) connected to a camera in order to capture images of the wounds. The perimeter of the wound in each image was traced using MetaMorph software (Molecular Devices, Downingtown, PA, USA), which output a corresponding area. The area of every wound at each time point was then normalized to its respective area at time 0.
Reverse transcriptase and quantitative real-time PCR
AECs were incubated with TGF-β1 (2 ng/μl) for 24 h before lysing and extracting RNA using the TriZOL method. Reverse transcriptase was performed using the RETROscript kit (Ambion, Austin, TX, USA), following the manufacturer's instructions. Total RNA (1 μg) was converted into cDNA, after denaturing at 80°C for 3 min with oligo dT primers, then incubating with a buffer containing dNTPs, RNase inhibitor, and RT enzyme as follows: 55°C for 5 min 55 s; 44°C for 22 min 22 s (2 cycles); and 92.2°C for 10 min. The resulting cDNA was amplified by PCR using vimentin and RPL19 primers. Quantitative real-time PCR reactions were performed using the SYBR-Green PCR Mastermix (Bio-Rad Laboratories) with the primers listed below, and analyzed using a Bio-Rad iCycler iQ Multicolor real-time PCR detection system. The sequences for the human vimentin and RPL19 primers are vimentin (VIM): forward, TGTCCAAATCGATGTGGATGTTTC, and reverse, TTGTACCATTCTTCTGCCTCCTG; and RPL19: forward, AGTATGCTCAGGCTTCAGAAGA, and reverse, CATTGGTCTCATTGGGGTCTAAC.
Luciferase assay
Vimentin promoter luciferase constructs, wild type (WT; full length), Δ1881 (truncated), Δ Smad-binding element (SBE) 1, and ΔSBE2 were made with pGL4.12 vector (Promega, Madison, WI, USA) and Human Bac Clone RPCI-11 (Invitrogen). Point mutations to the SBE of the vimentin promoter were made using Quikchange XLII site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The constructs were then transiently transfected into AECs (A549) in a 24-well plate, serum starved for 18 h prior to treatment with TGF-β1 for 24 h, and then lysed with passive lysis buffer. Firefly luciferase activity and Renilla luciferase activity were measured using the Dual Luciferase Reporter Assay System (Promega), according to the manufacturer's instructions.
Protein isolation and Western blot analysis
Cells were scraped into PBS and centrifuged (16,000 g, 10 min). The pellet was resuspended in lysis buffer containing 1% Triton X-100, 0.6 M NaCl, 10 mM MgCl2, a protease inhibitor mix (6 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 10 μg/ml N-tosyl-l-phenylal-anine chloromethyl ketone), and 6 μg/ml DNase I (Sigma-Aldrich) prior to homogenization or sonication. Protein concentrations were determined using the standard Bradford assay (Bio-Rad Laboratories). β-Mercaptoethanol (1%) was added to the samples, and this mixture was boiled for 3 min. Equal amounts of proteins were loaded on 10% SDS-PAGE gels, transferred to nitrocellulose membranes, and blotted with the primary antibodies as follows: anti-vimentin monoclonal antibody (1:200), anti-actin polyclonal antibody (1:200), anti-E-cadherin monoclonal antibody (1:200), or anti-GAPDH monoclonal antibody (1:1000). Membranes were washed 3 times with TBS containing 0.1% Tween 20 for 30 min, incubated with secondary antibodies coupled to horseradish peroxidase (1:25,000), and visualized using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA).
Indirect immunofluorescent confocal microscopy
Cells on coverslips were rinsed 3 times in PBS, fixed in 4% formaldehyde at room temperature for 9 min, and then permeabilized with 1% Triton X-100 in PBS for 30 min. Cells were then washed 3 times with PBS and processed for indirect immunofluorescence using an anti-vimentin antibody (1:100), followed by GAM 488 (1:200) secondary antibody. After staining, the glass slides were washed in PBS and mounted on glass slides using Aqua Poly/Mount (Polysciences, Warrington, PA, USA). Images of fixed, stained preparations were taken with a Zeiss UV LSM510 META laser-scanning confocal microscope (Carl Zeiss, Jena, Germany). For BrdU staining, cells were fixed and permeabilized as described, washed 3 times with PBS, and incubated with 1.5 M HCl for 30 min to denature the DNA. Borate buffer (0.2 M) was used to neutralize the acid. Cells were then imaged using a Nikon inverted microscope (Nikon Instruments) connected to a camera.
Live-cell imaging
H358 cells were cultured to confluence on 40-mm Bioptechs coverslips (Bioptechs, Butler, PA, USA). At 24 h prior to the experiment, monolayers were wounded with a single scratch, and medium with or without TGF-β1 (2 ng/ml) was added to the cells. Coverslips were sealed into a Bioptechs FCS2 chamber with serum-free RPMI medium, and both were maintained at 37°C. To ensure cell viability for the duration of the experiment, samples of medium were taken from the Bioptechs chamber during the experiment to measure pH, pCO2, and pO2 using a NovaMed blood gas analyzer (NovaMed Corp., Trumbull, CT, USA). No significant changes in pH, pCO2, or pO2 were observed in the cell imaging medium from the start to the end of experiment. Time-lapse phase microscopy was performed using a Nikon TE2000 (Nikon Instruments) equipped with a 100-W halogen lamp and a Planapo ×10 1.4-NA objective (Nikon Instruments). Images were acquired every 60 s for 6 h with an exposure time of 32 ms using a Cascade EMCCD camera with on-chip multiplication gain (Photometrics, Tucson, AZ, USA) driven by MetaMorph software. The movement of each cell was tracked and quantified using MetaMorph software. The trajectory for each uninterrupted movement of each cell was calculated; trajectories that could not be followed for ≥10 frames were discarded. At least 6 cells were randomly selected from along the wound edge and analyzed. The displacement in micrometers per unit of time was obtained as follows: for each trajectory, the individual step displacements were added to obtain the total contour length as a function of time.
Transient transfections
For transient transfection of H358 cells, Lipofectamine was used according to the manufacturer's protocol. Cells were seeded on 6-well plates and transfected either concurrently or 24 h later by incubating overnight with a mixture of 3–4 μg mEmerald-tagged vimentin DNA (gift from Dr. Robert Goldman, Northwestern University, Chicago, IL, USA) and 36 μl Lipofectamine (per 6-well plate) in serum-free medium. Medium was changed on the subsequent day. For knockdown experiments of primary rat AECs, recombinant adenovirus vectors expressing shRNA against vimentin (Ad-Vimentin shRNA) or an empty vector (Ad-Null shRNA) were designed. Infection of primary rat AECs was carried out 24 h after isolation. Primary rat AECs were infected with adenovirus vectors expressing shRNA against vimentin (10 MOI) or an empty vector (10 MOI) for 24 h, with agitation throughout the first hour.
Statistical analysis
Data are represented as means ± se or means ± sd, as noted. A 2-way ANOVA with a Bonferroni posttest was used to analyze the mEmerald-vimentin data. An unpaired t test was used to analyze all other data. Values of P < 0.05 were considered significant.
RESULTS
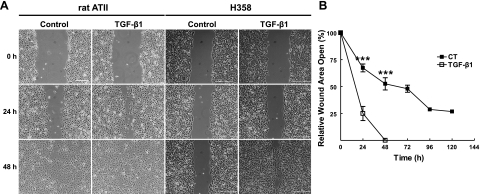
To determine whether the migration rates of AECs are regulated by TGF-β1, a standard in vitro scratch wound assay was used (28). In this assay, a single wound was made in the center of a confluent monolayer of either primary rat AECs or H358 cells (a lung epithelial cell line) in the presence or absence of TGF-β1. TGF-β1 accelerated the rate of wound closure in both primary rat AECs and H358 cells by 3- and 2.7-fold at 24 h, respectively, as compared to untreated control H358 cells (Fig. 1), which required upwards of 72 and 96 h to achieve 100% wound closure (Fig. 1). The TGF-β1-mediated increase in wound closure was associated with changes in AEC morphology. Cells at the wound edge exhibited changes from their typical epithelial phenotype of round, cuboidal cells to an elongated, mesenchymal cell-like phenotype.
Figure 1.
A) Primary rat AECs and H358 cells were wounded with a single scratch, cultured under control conditions (DMEM or RPMI plus 5% charcoal-stripped FBS) or in the presence of TGF-β1 (2 ng/ml) for up to 48 h. Representative photomicrographs are shown at 0, 24, and 48 h. Scale bars = 400 μm. B) Rate of wound closure was determined by using customized MetaMorph software; results were normalized to the initial wound area. Line graph represents means ± se of 7 determinations performed independently in duplicate. ***P < 0.001.
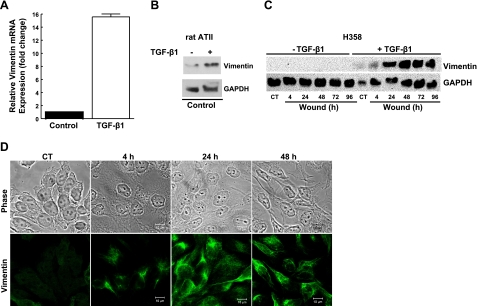
We then determined whether these changes in AEC phenotype and the acquisition of migratory properties were associated with the induction of vimentin protein. Under basal conditions, neither primary rat AECs nor human H358 cells express vimentin mRNA or protein, as shown by qRT-PCR and Western blot analysis (Fig. 2). Following exposure to TGF-β1, H358 cells demonstrate a 15.6-fold increase in mRNA levels, as compared to untreated control cells (Fig. 2A). The TGF-β1-mediated increase in vimentin mRNA levels was associated with a time-dependent increase in vimentin IF protein (Fig. 2B). Immunofluorescent laser-scanning confocal microscopy confirmed the Western blot results. Cells treated with TGF-β1 began to express vimentin protein within 4 h of treatment; the cells expressed mainly nonfilamentous vimentin particles (Fig. 2C, D). By 24 h, the majority of the cells expressed an intact filamentous vimentin IF network, while only a few cells expressed short vimentin filaments and particles. All cells expressed intact vimentin IF networks at 48 h after wounding and treatment with TGF-β1. In contrast, untreated control H358 cells did not have intact vimentin IF networks. Taken together, these data show that vimentin is transcriptionally regulated by TGF-β1 and that vimentin IF network assembly occurs in a time-dependent manner.
Figure 2.
A) H358 cells were cultured under control conditions (RPMI plus 5% charcoal-stripped FBS) or in the presence of TGF-β1 (2 ng/ml) for 24 h. Total RNA was isolated, and steady-state mRNA levels were determined by real-time PCR. B, C) Primary rat AECs (B) and H358 cells (C) were cultured under control conditions (DMEM or RPMI plus 5% charcoal-stripped FBS) or in the presence of TGF-β1 (2 ng/ml) for 24 h. Cells were then wounded by crisscross scratch assays, washed 3 times with PBS, and then cultured under control conditions (DMEM or RPMI plus 5% charcoal-stripped FBS) or in the presence of TGF-β1 (2 ng/ml) for up to 96 h. Total cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-vimentin (V9) antibody or anti-GAPDH antibody. Representative autoradiogram is shown. CT, control. D) Representative photomicrograph of indirect immunofluorescence microscopy of H358 cells following exposure to TGF-β1. Cells were wounded and treated with TGF-β1 for 4, 24, and 48 h prior to fixing with formaldehyde and immunostaining with anti-vimentin antibody. Scale bars = 10 μm.
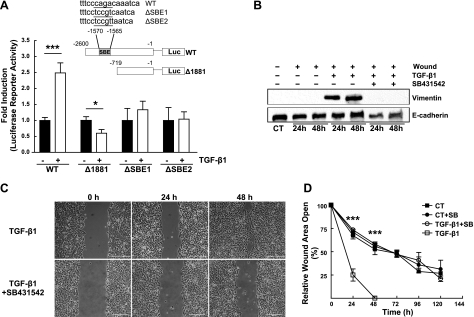
There are several conflicting reports regarding whether vimentin is regulated by TGF-β1 via the canonical ALK5/Smad pathway (29). To determine whether the vimentin gene has a functional SBE in the 5′ promoter region and whether this SBE is required for the TGF-β1-mediated induction of vimentin in AECs, we designed 4 luciferase reporter vectors. WT is 2600 bp in length and contains a putative SBE between −1570 and −1565 in the 5′ flanking region of the vimentin gene upstream from the ATG translation initiation codon. ΔSBE1 and ΔSBE2 contain the putative SBE each with a unique 6-point mutation, whereas Δ1881 is 719 bp in length and lacks the putative SBE. These constructs were transiently transfected into A549 cells and subsequently exposed to TGF-β1 for 24 h. The luciferase activity of cells transfected with WT (full length+SBE) increased 2.5-fold with TGF-β1, but the luciferase activity of cells transfected with either ΔSBE1 or ΔSBE2 (6-point mutation) or Δ1881 (−SBE) was not increased (Fig. 3A), indicating that the SBE is required for TGF-β1-mediated increase in vimentin mRNA.
Figure 3.
A) Inset: structures of the pGL4.12 luciferase reporter vectors containing various lengths of 5′-flanking regions of the human vimentin gene; WT (−2600 → −1) possesses the putative SBE, while ΔSBE1 and ΔSBE2 possess the putative SBE with distinct 6-point mutations. Δ1881 (−719 → −1) does not contain the putative SBE. Graph: reporter constructs and pRL-SV40 were transiently transfected into the A549 cells. At 24 h post-transfection, cells were exposed to control conditions (serum-free DMEM) or to TGF-β1 (2 ng/ml) for 24 h. Induction ratio of the luciferase activity by TGF-β1 for each construct was calculated. Bars represent means ± sd of >3 determinations performed independently in duplicate. B) H358 cells were wounded by crisscross scratch assays, washed 3 times with PBS, and then exposed to control conditions (RPMI plus 5% charcoal-stripped FBS) or to TGF-β1 (2 ng/ml) for 24 and 48 h in the presence or absence of the ALK5/Smad inhibitor, SB431542 (10 μM). Equal amounts of protein from cell lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-vimentin and anti-E-cadherin antibodies. Representative autoradiogram is shown; n = 4. C) Cells were wounded with a single scratch, washed with PBS, and then exposed to TGF-β1 (2 ng/ml) for up to 120 h in the presence or absence of the ALK5/Smad inhibitor, SB431542 (10 μM). Representative photomicrographs are shown at 24 and 48 h. Scale bars = 400 μm. D) Rate of wound closure was determined by using customized MetaMorph software; results were normalized to initial wound area. Line graph represents means ± se of 8 determinations performed independently in duplicate. CT, control; SB, SB431542. *P < 0.05, ***P < 0.001.
To determine whether the ALK5/Smad pathway was responsible for the increased wound closure rates and vimentin protein expression, we treated confluent monolayers of H358 cells with SB431542, a pharmacological inhibitor of the ALK5 pathway. SB431542 prevented the TGF-β1-mediated up-regulation of vimentin IF protein, as demonstrated by Western blot analysis (Fig. 3B). Additionally, pretreatment with the ALK5 inhibitor prevented the TGF-β1-mediated increase in wound closure; ∼75% of the wound area in the SB431542-treated cells remained open at 48 h, and wounds did not close by 96 h, similar to control, untreated wounds (Fig. 3C, D). Thus, we show that blocking the TGF-β1 pathway leads to inhibition of vimentin IF expression and impaired wound closure in AECs.
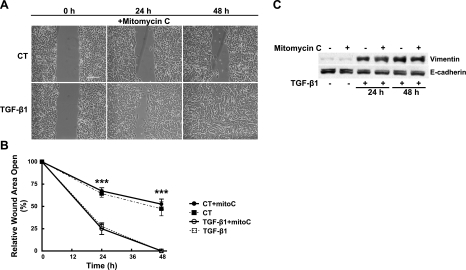
AEC wound repair can involve both migration and proliferation (1–5). To determine whether cellular proliferation contributes to wound repair, we treated cells with mitomycin C, a drug known to inhibit cell proliferation, prior to wounding and exposure to TGF-β1. Cells treated with mitomycin C were unable to proliferate, as assessed by BrdU incorporation (data not shown), and they became more spread following treatment. Inhibition of cell proliferation had no effect on the rate of wound closure in either the presence or absence of TGF-β1 as compared to cells not treated with mitomycin C (Fig. 4A, B). Mitomycin C treatment also had no effect on the ability of the cells to maintain a confluent monolayer as the wounds closed. As expected, cells treated with mitomycin C continued to express vimentin protein (Fig. 4C). These results indicate that wound closure occurs independently of cellular proliferation.
Figure 4.
A, B) Cells were pretreated with mitomycin C (10 μM, 3 h) and then washed with PBS before wounding with a single scratch, washing with PBS, and exposure to control conditions (RPMI plus 5% charcoal-stripped FBS) or to TGF-β1 (2 ng/ml) for up to 48 h. A) Representative photomicrographs at 0, 24, and 48 h. Scale bars = 400 μm. B) Rate of wound closure was determined by using customized MetaMorph software; results were normalized to initial wound area. Line graph represents means ± se of 6 determinations performed independently in duplicate. CT, control; mitoC, mitomycin C. ***P < 0.001. C) Cells were pretreated with or without mitomycin C (10 μM, 3 h) and then washed with PBS before wounding by crisscross scratch assays, washing 3 times with PBS, and exposure to control conditions (RPMI plus 5% charcoal-stripped FBS) or to TGF-β1 (2 ng/ml) for up to 48 h. Equal amounts of protein from cell lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-vimentin (V9) and anti-E-cadherin antibodies. Representative autoradiogram is shown.
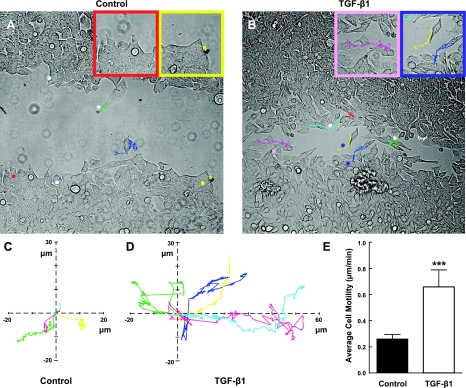
The data thus far show that cell migration is the major contributing factor to wound healing in our in vitro model. We wanted to better understand the dynamics of individual cells during collective migration, so we performed live-cell imaging to track individual cells at the edge of wounds over a period of 24 h. Untreated control cells moved at an average speed of ∼0.26 μm/min (Fig. 5A, C, E), whereas cells exposed to TGF-β1 moved at an average speed of ∼0.66 μm/min (Fig. 5B, D, E and Supplemental Movies S1 and S2). The lack of wound closure by 96 h in untreated cells is, in part, a function of slow, undirected cell movement. Most of the control cells moved in a circle with little to no net forward motion, in contrast to cells treated with TGF-β1, which progressively moved forward into the denuded space (Supplemental Movie S2). For the most part, cells maintained cell-to-cell contact and migrated as a collective sheet, even in the TGF-β1 condition. Motion of individual cells was generally parallel (rather than perpendicular) to the wound edge, with a few cells dissolving cell-cell contacts and migrating into the denuded area. Overall, there was variability among individual TGF-β1-treated cells. These results indicate that TGF-β1-treated cells have a 150% increase in average speed (Fig. 5E).
Figure 5.
A, B) H358 cells were cultured on 40-mm Bioptechs coverslips before wounding with a single scratch and culturing under control conditions (RPMI plus 5% charcoal-stripped FBS; A) or in the presence of TGF-β1 (2 ng/ml; B) for up to 48 h. Time-lapse phase microscopy was performed using a Nikon TE2000; culture medium was saturated with a gas mixture containing 5% CO2 and 21% O2, perfused through an environmental control system chamber. Images were collected with a Cascade EMCCD camera with on-chip multiplication gain driven by MetaMorph software. Images were acquired every 60 s, with an exposure time of 32 ms. Representative photomicrographs are shown at ∼30 h postwounding after tracking the positions of cells for 6 h. Insets: higher-magnification views of cells. Asterisks indicate cells that were tracked and shown in the graphs in C, D. C, D) Rose plots show individual cell velocities in the x and y directions. Colored lines correspond to individual cells marked by asterisks in A, B. E) Graph represents means ± sd of 6 cells tracked in 2 independent determinations. **P < 0.005.
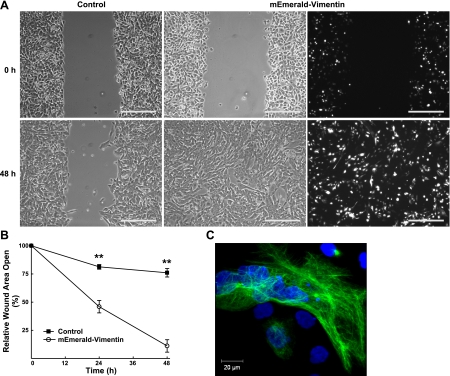
To determine whether vimentin is sufficient to increase cell motility and migration in AECs, cells were transiently transfected with mEmerald-tagged vimentin. At 24 h post-transfection, >50% of the cells expressed mEmerald-vimentin, which resulted in a filamentous vimentin IF network (Fig. 6A, C). Cells expressing mEmerald-vimentin had an accelerated rate of wound closure as compared to control, nontransfected cells (Fig. 6A, B). At 48 h, the wound in mEmerald-vimentin-expressing cells was completely closed; in contrast, the wound in the nontransfected control cells was <20% closed. Interestingly, mEmerald-vimentin expressing cells closed at the same rate as cells treated with TGF-β1 (Figs. 1 and 6), which suggests that vimentin is sufficient to increase AEC migration.
Figure 6.
A) At subconfluence, H358 cells were transiently transfected with mEmerald-tagged vimentin cDNA (3 μg). After reaching confluence, cells were wounded with a single scratch, washed with PBS, and cultured under control conditions (RPMI plus 5% charcoal-stripped FBS). Representative bright-field photomicrographs of the scratch wound are shown at 0 and 48 h. Scale bars = 200 μm. Representative direct immunofluorescent photomicrograph demonstrates that >50% of the cells expressed mEmerald-tagged vimentin. B) Rate of wound closure was determined by using customized MetaMorph software; results were normalized to initial wound area. Line graph represents means ± se of 3 determinations performed independently in triplicate. **P < 0.001. C) Representative direct immunofluorescent photomicrograph of mEmerald-tagged vimentin cells demonstrates expression of an intact vimentin IF network.
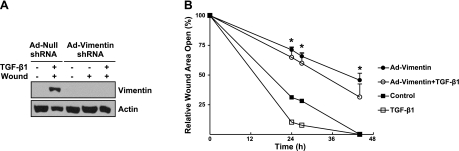
To determine whether vimentin would be required for increased rates of migration, leading to faster wound closure, vimentin expression was suppressed in primary AECs. The shRNA against vimentin (Ad-vimentin shRNA) successfully knocked down vimentin expression, as shown by Western blot analysis of untreated monolayers, wounded monolayers, and wounded monolayers treated with TGF-β1 for 24 h (Fig. 7A). In contrast, primary AECs transfected with the empty vector (Ad-null shRNA) prior to wounding and treating with TGF-β1 retained the ability to induce vimentin expression. Nonwounded, nontreated primary AECs did not express vimentin protein. The inability to induce vimentin protein expression in primary AECs significantly impaired cell migration and prevented wound closure; even following treatment with TGF-β1 for 24 h (Fig. 7B), wounds remained ∼75% open. Cells transfected with the null adenovirus exhibited significantly greater migration rates, leading to faster wound closure at 24 h following scratch wounding.
Figure 7.
Primary rat AECs were infected with adenovirus vectors expressing shRNA against vimentin (Ad-Vimentin shRNA, 10 MOI) or an empty vector (Ad-Null shRNA, 10 MOI). A) At 24 h postinfection, cells were wounded by crisscross scratch assays, washed 3 times with PBS, and then exposed to control conditions (DMEM plus 5% charcoal-stripped FBS) or to TGF-β1 (2 ng/ml) for 24 h. Equal amounts of protein from cell lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-vimentin and anti-E-actin antibodies. Representative autoradiogram is shown; n = 2. B) At 24 h postinfection, cells were wounded with a single scratch, washed with PBS, and exposed to control conditions (DMEM plus 5% charcoal-stripped FBS) or to TGF-β1 (2 ng/ml). Rate of wound closure was determined by using customized MetaMorph software; results were normalized to initial wound area. Line graph represents means ± sd in triplicate. *P < 0.05.
DISCUSSION
In the present study, we investigated the effects of TGF-β1-mediated vimentin expression on AEC migration. We demonstrated that vimentin is sufficient and required for increased cell motility, leading to faster wound repair in an in vitro model of wound healing. TGF-β1 is one of the dominant cytokines released during lung injury and also has been linked to vimentin IF expression during periods of increased cellular migration. In the presence of TGF-β1, both primary rat AECs and H358 cells behave similarly by inducing vimentin expression and increasing cell motility. The result is a similar rate of wound closure between primary AECs and H358 cells in the presence of TGF-β1.
Early studies correlated vimentin expression with wound healing or other processes requiring migration (6, 8). More recent studies demonstrate a direct functional role for vimentin in cell migration (30–33). Expression of an intact vimentin network causes an increase in cellular migration in both primary rat AECs and human H358 cells, leading to wound closure. In contrast to other studies, in our experimental system, wounding by itself is insufficient to increase vimentin expression in H358 cells, and TGF-β1-induced vimentin expression in cells is similar regardless of distance from the wound edge. We determined that vimentin IFs are sufficient to increase the rate of wound closure compared to control wounded cells. Indeed, the wound closure rate of mEmerald-vimentin-expressing cells is similar to the TGF-β1-treated cells. There is a small lag in vimentin assembly in cells transfected with mEmerald-vimentin, supporting the existing literature showing that vimentin must be assembled in order to increase cell motility.
Despite the studies demonstrating a requirement for vimentin IFs in cell migration, the mechanism by which vimentin works to facilitate and/or maintain migration in AECs or other cells is not completely understood. However, there is some insight from the literature. In endothelial cells, vimentin associates with focal adhesions through the mechanism of the β3 integrin (34, 35). In MCF-7 cells transiently transfected with RFP-vimentin, focal adhesions not only associate with vimentin, but exhibit an increased rate of turnover in the presence of vimentin IFs (33). Conversely, focal adhesions appear to be disrupted in primary vimentin-null fibroblasts, where wound healing is impaired (30), and vimentin may also have a signaling function in transitioning stationary cells to motile cells. In leukocytes, for example, phosphorylation and reorganization of vimentin by the PI3Kγ pathway are required for transmigration (36). Vimentin is also a downstream phosphorylation target of molecules in other pathways, such as p21-activated kinase, a downstream target of Rac (37, 38). We did not pursue whether wound closure was Rac- or Rho-dependent, nor did we delve into the specific differences in cell motility between cells at the wound edge compared to cells far from the wound. Finally, vimentin IFs may affect cellular mechanics, potentially increasing traction forces exerted by cells through focal adhesions at precise areas. Little is known about the role of vimentin IFs in creating and/or altering cellular traction forces during migration, and future studies beyond the scope of this paper are imperative to elucidate this role. Collectively, our results demonstrate a functional role for TGF-β1-mediated vimentin expression in cell migration without establishing the precise mechanism by which this effect occurs.
There are conflicting reports as to whether vimentin gene regulation via the canonical Smad pathway requires an SBE. Wu et al. (29) reported that the vimentin gene does not contain any known TGF-β1 response elements; rather that tandem AP-1 elements located upstream of the transcription start site are required. Other studies suggest that TGF-β1-stimulated migration can occur through either a Smad2/3-dependent or independent mechanism (39, 40). We demonstrate that TGF-β1 directly mediates vimentin expression via the Smad pathway and that an SBE located between −1570 and −1565 in the 5′ flanking region in the vimentin promoter is required for transcription. This was not the result of decreased activity of the promoter itself. Inhibition of TGF-β1 signaling by SB431542, an ALK5-selective and specific inhibitor of the canonical Smad pathway, completely abrogated the positive effects of TGF-β1, as would be expected. Another study has shown that ALK5 kinase is required for TGF-β1-stimulated cell migration (41), confirming our finding that SB431542 prevents TGF-β1-mediated vimentin up-regulation and increased migration.
Some in vitro studies have suggested that the start of AEC proliferation may actually begin subsequent to increased cell spreading and migration, as much as 2 d following the initial onset of injury (5, 42, 43). Our results are consistent with this literature and other studies showing that mitomycin C treatment has no significant effect on the wound-closure rate of other epithelial cell lines, in the presence or absence of growth factors (44, 45). Although we did not investigate the individual contributions of cell spreading, migration, and proliferation to the wound closure rate, this has been mathematically modeled and verified by experiment, indicating that AEC spreading and migration dominates wound closure (46). Yet, a few other studies have shown that cell proliferation plays a dominant role in the wound closure rate of cells in vitro (47). The contributions of cell proliferation, spreading, and migration to wound repair appear to be distinct and unequal, and we have effectively modeled one aspect of alveolar epithelial injury in the scratch wound assay. Since the severity of lung injury is influenced by the extent of damage to the alveolar epithelial barrier, interventional therapies to accelerate the repair process in the earliest stages of repair may reduce the amount of permanent damage to the lung tissue (48).
The acquisition of a hyperplastic phenotype following acute lung injury is characterized, in part, by up-regulation of the mesenchymal cell-derived protein, vimentin, and concomitant with down-regulation of epithelial markers such as E-cadherin and keratin IFs, and changes in cell morphology and motility. Because of its complexity, we did not intend to study EMT as an end point, nor did we study all aspects of the EMT phenotype; rather, we focused on the specific contribution of vimentin, a key IF protein in AEC wound repair and remodeling. While this IF protein has been long appreciated as a classic marker of cell migration and EMT, our studies determine a causal role of vimentin in the cellular activities associated with normal or aberrant wound repair and remodeling. We observed changes in cellular morphology following treatment with TGF-β1, but we did not see decreased levels of E-cadherin protein, according to our Western blot analyses. Singh et al. (49) showed that 10 d of chronic TGF-β1 treatment was required to down-regulate E-cadherin expression in H358 cells (the cell line used in this study), consistent with our data. Our results suggest that although the phenotype changes and migration of the AECs increases, we are not inducing EMT in these cells. Rather, exposure to TGF-β1 is sufficient to exploit the ability of these cells to become hyperplastic according to their microenvironment. Thus, these cells should still be considered AECs, not mesenchymal cells. Studies on other differentiation markers, such as SP-C, would be required to determine whether the primary AECs still function as AECs.
In summary, our results demonstrate the requirement for and sufficiency of vimentin to increase wound repair in an in vitro model of wound repair. We have contributed evidence to strengthen the connection between vimentin IF expression and TGF-β1 in the context of alveolar epithelial wound repair. Furthermore, our results indicate that vimentin is transcriptionally regulated via the Smad pathway, and an SBE is required for increased cell migration rates leading to faster wound closure. Further study into the effect of the Smad pathway on wound healing will add to our understanding of wound repair in the lung. In addition, it will be important to study the regulation of vimentin IFs after repair. Do AECs down-regulate vimentin following complete repair, or does vimentin expression continue? The answer to this question will have important implications for treatment of acute lung injury, but also for prevention and/or treatment of chronic lung injury, such as fibrosis. Therapeutically, it may be desirable to up-regulate vimentin in AECs for a period of time and then down-regulate or disrupt the vimentin network in these cells following complete wound repair. Withaferin A is one agent recently proposed to disrupt the vimentin network, but the literature is still sparse on this compound, and more work needs to be done to characterize this and other compounds (50–52).
Supplementary Material
Acknowledgments
The authors thank Dr. Robert D. Goldman (Northwestern University, Evanston, IL, USA) for the mEmerald-vimentin vector construct, Dr. Robert B. Hamanaka for technical help and suggestions, and Mr. Luke Skertich for assistance with wound closure data analysis.
This work was supported by grants from the U.S. National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (PO1 HL71643 NIH-NHLBI), the Department of Veterans Affairs (MERIT Award), the Training Program in Lung Science (T32-076139-02 NIH-NHLBI; to M.R.R.), and the American Heart Association (AHA 10PRE4210064; to M.R.R.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Uhal B. D. (1997) Cell cycle kinetics in the alveolar epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 272, L1031–L1045 [DOI] [PubMed] [Google Scholar]
- 2. Zahm J. M., Chevillard M., Puchelle E. (1991) Wound repair of human surface respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 5, 242–248 [DOI] [PubMed] [Google Scholar]
- 3. Adamson I. Y., Hedgecock C., Bowden D. H. (1990) Epithelial cell-fibroblast interactions in lung injury and repair. Am. J. Pathol. 137, 385–392 [PMC free article] [PubMed] [Google Scholar]
- 4. Lesur O., Arsalane K., Lane D. (1996) Lung alveolar epithelial cell migration in vitro: modulators and regulation processes. Am. J. Physiol. Lung Cell. Mol. Physiol. 270, L311–L319 [DOI] [PubMed] [Google Scholar]
- 5. Kheradmand F., Folkesson H. G., Shum L., Derynk R., Pytela R., Matthay M. A. (1994) Transforming growth factor-alpha enhances alveolar epithelial cell repair in a new in vitro model. Am. J. Physiol. Lung Cell. Mol. Physiol. 267, L728–L738 [DOI] [PubMed] [Google Scholar]
- 6. Buisson A. C., Gilles C., Polette M., Zahm J. M., Birembaut P., Tournier J. M. (1996) Wound repair-induced expression of a stromelysins is associated with the acquisition of a mesenchymal phenotype in human respiratory epithelial cells. Lab. Invest. 74, 658–669 [PubMed] [Google Scholar]
- 7. Gilles C., Polette M., Zahm J. M., Tournier J. M., Volders L., Foidart J. M., Birembaut P. (1999) Vimentin contributes to human mammary epithelial cell migration. J. Cell Sci. 112, 4615–4625 [DOI] [PubMed] [Google Scholar]
- 8. SundarRaj N., Rizzo J. D., Anderson S. C., Gesiotto J. P. (1992) Expression of vimentin by rabbit corneal epithelial cells during wound repair. Cell Tissue Res. 267, 347–356 [DOI] [PubMed] [Google Scholar]
- 9. Willis B. C., Liebler J. M., Luby-Phelps K., Nicholson A. G., Crandall E. D., du Bois R. M., Borok Z. (2005) Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 166, 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyer B., Valles A. M., Edme N. (2000) Induction and regulation of epithelial-mesenchymal transitions. Biochem. Pharmacol. 60, 1091–1099 [DOI] [PubMed] [Google Scholar]
- 11. Dennler S., Goumans M. J., ten Dijke P. (2002) Transforming growth factor beta signal transduction. J. Leukoc. Biol. 71, 731–740 [PubMed] [Google Scholar]
- 12. Bierie B., Moses H. L. (2006) TGF-beta and cancer. Cytokine Growth Factor Rev. 17, 29–40 [DOI] [PubMed] [Google Scholar]
- 13. Siegel P. M., Massague J. (2003) Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 3, 807–821 [DOI] [PubMed] [Google Scholar]
- 14. Bergeron A., Soler P., Kambouchner M., Loiseau P., Milleron B., Valeyre D., Hance A. J., Tazi A. (2003) Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-β and IL-10. Eur. Respir. J. 22, 69–76 [DOI] [PubMed] [Google Scholar]
- 15. Dhainaut J. F., Charpentier J., Chiche J. D. (2003) Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit. Care Med. 31, S258–S264 [DOI] [PubMed] [Google Scholar]
- 16. Khalil N., Corne S., Whitman C., Yacyshyn H. (1996) Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am. J. Respir. Cell Mol. Biol. 15, 252–259 [DOI] [PubMed] [Google Scholar]
- 17. Khalil N., O'Connor R. N., Flanders K. C., Unruh H. (1996) TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 14, 131–138 [DOI] [PubMed] [Google Scholar]
- 18. Sime P. J., Xing Z., Graham F. L., Csaky K. G., Gauldie J. (1997) Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 100, 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pittet J. F., Griffiths M. J., Geiser T., Kaminski N., Dalton S. L., Huang X., Brown L. A., Gotwals P. J., Koteliansky V. E., Matthay M. A., Sheppard D. (2001) TGF-beta is a critical mediator of acute lung injury. J. Clin. Invest. 107, 1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonniaud P., Kolb M., Galt T., Robertson J., Robbins C., Stampfli M., Lavery C., Margetts P. J., Roberts A. B., Gauldie J. (2004) Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J. Immunol. 173, 2099–2108 [DOI] [PubMed] [Google Scholar]
- 21. Sivaramakrishnan S., DeGiulio J. V., Lorand L., Goldman R. D., Ridge K. M. (2008) Micromechanical properties of keratin intermediate filament networks. Proc. Natl. Acad. Sci. U. S. A. 105, 889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sivaramakrishnan S., Schneider J. L., Sitikov A., Goldman R. D., Ridge K. M. (2009) Shear stress induced reorganization of the keratin intermediate filament network requires phosphorylation by protein kinase C zeta. Mol. Biol. Cell 20, 2755–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ridge K. M., Linz L., Flitney F. W., Kuczmarski E. R., Chou Y. H., Omary M. B., Sznajder J. I., Goldman R. D. (2005) Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J. Biol. Chem. 280, 30400–30405 [DOI] [PubMed] [Google Scholar]
- 24. Yoon K. H., Yoon M., Moir R. D., Khuon S., Flitney F. W., Goldman R. D. (2001) Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J. Cell Biol. 153, 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prahlad V., Yoon M., Moir R. D., Vale R. D., Goldman R. D. (1998) Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon M., Moir R. D., Prahlad V., Goldman R. D. (1998) Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eriksson J. E., He T., Trejo-Skalli A. V., Harmala-Brasken A. S., Hellman J., Chou Y. H., Goldman R. D. (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 117, 919–932 [DOI] [PubMed] [Google Scholar]
- 28. Liang C. C., Park A. Y., Guan J. L. (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333 [DOI] [PubMed] [Google Scholar]
- 29. Wu Y., Zhang X., Salmon M., Lin X., Zehner Z. E. (2007) TGFbeta1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim. Biophys. Acta 1773, 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckes B., Colucci-Guyon E., Smola H., Nodder S., Babinet C., Krieg T., Martin P. (2000) Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113, 2455–2462 [DOI] [PubMed] [Google Scholar]
- 31. Eckes B., Dogic D., Colucci-Guyon E., Wang N., Maniotis A., Ingber D., Merckling A., Langa F., Aumailley M., Delouvee A., Koteliansky V., Babinet C., Krieg T. (1998) Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907 [DOI] [PubMed] [Google Scholar]
- 32. Brown M. J., Hallam J. A., Colucci-Guyon E., Shaw S. (2001) Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J. Immunol. 166, 6640–6646 [DOI] [PubMed] [Google Scholar]
- 33. Mendez M. G., Kojima S., Goldman R. D. (2009) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Godsel L. M., Hsieh S. N., Amargo E. V., Bass A. E., Pascoe-McGillicuddy L. T., Huen A. C., Thorne M. E., Gaudry C. A., Park J. K., Myung K., Goldman R. D., Chew T. L., Green K. J. (2005) Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J. Cell Biol. 171, 1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattacharya R., Gonzalez A. M., Debiase P. J., Trejo H. E., Goldman R. D., Flitney F. W., Jones J. C. (2009) Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J. Cell Sci. 122, 1390–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barberis L., Pasquali C., Bertschy-Meier D., Cuccurullo A., Costa C., Ambrogio C., Vilbois F., Chiarle R., Wymann M., Altruda F., Rommel C., Hirsch E. (2009) Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur. J. Immunol. 39, 1136–1146 [DOI] [PubMed] [Google Scholar]
- 37. Goto H., Tanabe K., Manser E., Lim L., Yasui Y., Inagaki M. (2002) Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK). Genes Cells 7, 91–97 [DOI] [PubMed] [Google Scholar]
- 38. Li Q. F., Spinelli A. M., Wang R., Anfinogenova Y., Singer H. A., Tang D. D. (2006) Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 281, 34716–34724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dumont N., Bakin A. V., Arteaga C. L. (2003) Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells. J. Biol. Chem. 278, 3275–3285 [DOI] [PubMed] [Google Scholar]
- 40. Tian F., Byfield S. D., Parks W. T., Stuelten C. H., Nemani D., Zhang Y. E., Roberts A. B. (2004) Smad-binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 64, 4523–4530 [DOI] [PubMed] [Google Scholar]
- 41. Liu I. M., Schilling S. H., Knouse K. A., Choy L., Derynck R., Wang X. F. (2009) TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 28, 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berthiaume Y., Lesur O., Dagenais A. (1999) Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 54, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geiser T., Jarreau P. H., Atabai K., Matthay M. A. (2000) Interleukin-1beta augments in vitro alveolar epithelial repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1184–L1190 [DOI] [PubMed] [Google Scholar]
- 44. Howat W. J., Holgate S. T., Lackie P. M. (2002) TGF-beta isoform release and activation during in vitro bronchial epithelial wound repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L115–L123 [DOI] [PubMed] [Google Scholar]
- 45. Farooqui R., Fenteany G. (2005) Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell Sci. 118, 51–63 [DOI] [PubMed] [Google Scholar]
- 46. Savla U., Olson L. E., Waters C. M. (2004) Mathematical modeling of airway epithelial wound closure during cyclic mechanical strain. J. Appl. Physiol. 96, 566–574 [DOI] [PubMed] [Google Scholar]
- 47. Draper B. K., Komurasaki T., Davidson M. K., Nanney L. B. (2003) Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J. Cell. Biochem. 89, 1126–1137 [DOI] [PubMed] [Google Scholar]
- 48. Ware L. B., Matthay M. A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 49. Singh A., Greninger P., Rhodes D., Koopman L., Violette S., Bardeesy N., Settleman J. (2009) A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell 15, 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thaiparambil J. T., Bender L., Ganesh T., Kline E., Patel P., Liu Y., Tighiouart M., Vertino P. M., Harvey R. D., Garcia A., Marcus A. I. (2011) Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. [E-pub ahead of print] Int. J. Cancer doi: 10.1002/ijc.25938 [DOI] [PubMed] [Google Scholar]
- 51. Bargagna-Mohan P., Paranthan R. R., Hamza A., Dimova N., Trucchi B., Srinivasan C., Elliott G. I., Zhan C. G., Lau D. L., Zhu H., Kasahara K., Inagaki M., Cambi F., Mohan R. Withaferin A targets intermediate filaments glial fibrillary acidic protein and vimentin in a model of retinal gliosis. J. Biol. Chem. 285, 7657–7669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bargagna-Mohan P., Hamza A., Kim Y. E., Khuan Abby Ho Y., Mor-Vaknin N., Wendschlag N., Liu J., Evans R. M., Markovitz D. M., Zhan C. G., Kim K. B., Mohan R. (2007) The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 14, 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.