Abstract
Activation of matrix metalloproteinase-9 (MMP-9) is involved in HIV-1-induced disruption of the blood-brain barrier (BBB). In the present study, we hypothesize that peroxisome proliferator-activated receptor (PPAR)-α or PPARγ can protect against HIV-1-induced MMP-9 overexpression in brain endothelial cells (hCMEC cell line) by attenuating cellular oxidative stress and down-regulation of caveolae-associated redox signaling. Exposure to HIV-1-infected monocytes induced phosphorylation of ERK1/2 and Akt in hCMEC by 2.5- and 3.6-fold, respectively; however, these effects were attenuated by overexpression of PPARα or PPARγ and by silencing of caveolin-1 (cav-1). Coculture of hCMEC with HIV-1-infected monocytes significantly induced MMP-9 promoter and enzyme activity by 3- to 3.5-fold. Promoter mutation studies indicated that SP-1 (g1940t_g1941t) is an essential transcription factor involved in induction of MMP-9 promoter by HIV-1. In addition, HIV-1-stimulated activity of MMP-9 promoter was inhibited by mutation of AP-1 site 2 (c1918t_a1919g) and both (but not individual) NF-κB binding sites (g1389c and g1664c). PPAR overexpression, ERK1/2 or Akt inhibition, and silencing of cav-1 all effectively protected against HIV-1-induced MMP-9 promoter activity, indicating a close relationship among HIV-1-induced cerebrovascular toxicity, redox-regulated mechanisms, and functional caveolae. Such a link was further confirmed in MMP-9-deficient mice exposed to PPARα or PPARγ agonist and injected with the HIV-1-specific protein Tat into cerebral vasculature. Overall, our results indicate that ERK1/2, Akt, and cav-1 are involved in the regulatory mechanisms of PPAR-mediated protection against HIV-1-induced MMP-9 expression in brain endothelial cells.—Huang, W., András, I. E., Rha, G. B., Hennig, B., Toborek, M. PPARα and PPARγ protect against HIV-1-induced MMP-9 overexpression via caveolae-associated ERK and Akt signaling.
Keywords: peroxisome proliferator-activated receptor, matrix metalloproteinase, human immunodeficiency virus-1, blood-brain barrier
The blood-brain barrier (BBB) separates and protects the brain from the blood-borne factors (1). Such a position at the interface between the blood and the central nervous system (CNS) is chiefly responsible for the strategic functions of the BBB in the entry of HIV-1 from the bloodstream into the brain, followed by the generation of the virus reservoirs in the CNS, and the development of neurological pathology associated with HIV-1 infection (2). There is strong evidence indicating the BBB alterations in HIV-1 infection (3, 4). Notably, alterations of the BBB integrity are associated with increased monocyte infiltration into the brain and the degree of HIV-1-associated dementia (5–7).
Pathological changes of the BBB, such as thinning of the basal lamina and decreased content of collagen IV, observed in AIDS patients (8) suggest an increase in proteolytic activity (9). Indeed, HIV-1 infection is associated with increased expression and activity of matrix metalloproteinases (MMPs) in the CNS. Elevated levels of MMP-9 are reported in the cerebrospinal fluid of HIV-1-infected children (10) and adult patients (11). In addition, increased CNS levels of MMP-2 and MMP-9 are more frequent in patients with HIV-1-associated dementia than in HIV-1 patients without dementia (12–13). Overexpression of MMP-2 and MMP-9 was also reported in the brains of a severe combined immunodeficiency mouse model of HIV-1 encephalitis (14). These alterations appear to be brain specific, because production of MMP-9 by monocyte-derived macrophages is decreased in HIV-1 infection (15–16). Experimental research additionally indicated that HIV-1-induced disruption of the BBB is associated with up-regulation of MMPs, especially MMP-9 (17–18). Research from our laboratory demonstrated that HIV-1-inducted alterations of tight junction protein expression in brain endothelial cells can be protected by inhibition of MMP and proteasome activities (19).
HIV-1-infected patients are in a state of persistent oxidative stress (20–22). For example, lipid peroxidation pathways are increased both in asymptomatic and symptomatic patients with HIV-1 as determined by elevated serum levels of malondialdehyde. These changes are associated with reduced serum superoxide dismutase activity, levels of vitamins C and E, and decreased total antioxidant capacity (20, 22). Increased oxidative stress in vascular tissues was also demonstrated in HIV-1 transgenic animals (23).
Elevated tissue oxidation in HIV-1 infection provides a strong justification for the use of a supplemental antioxidative therapy to alleviate pathological changes associated with oxidative damage. Research in our laboratory (19, 24) and others (25, 26) indicated a protective role of peroxisome proliferator-activated receptors (PPARs) in HIV-1-related BBB alterations. For example, we indicated that PPARα and PPARγ can protect against HIV-1-induced dysfunction of brain endothelial cells partly by inhibition of proinflammatory responses (24) and downregulating of MMP-2 and MMP-9 activities (19). However, the detailed mechanisms of PPAR-mediated protection against MMP overexpression are not fully understood. In addition, limited information is available on the role of caveolae and caveolae-associated signaling pathways in regulation of MMP activity at the BBB level. Therefore, the aim of the present study was to evaluate the signaling and transcriptional mechanisms by which PPARs can protect against HIV-1-induced MMP-9 overexpression. Our data indicate that PPARα and PPARγ can influence the modulatory effects of caveolin-1 (cav-1) on HIV-1-induced activation of redox-regulated ERK1/2 and Akt signaling pathways and thus influence transcriptional induction of MMP-9 expression.
MATERIALS AND METHODS
Cell cultures and treatment
Human cerebral microvascular endothelial cells (hCMEC/D3 cell line, called here hCMECs; ref. 27) were cultured in EBM-2 medium (Cambrex BioScience, Walkersville, MD, USA) as described previously (24). Human monocytes (U937 cells) were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Invitrogen). Generation of the stable lines of hCMECs overexpressing PPARα or PPARγ (hCMEC/PPARα or hCMEC/PPARγ, respectively) was described previously (24). In selected experiments, cells were exposed to inhibitors of ERK1/2 or Akt (10 μM U0126 or 120 nM KP3721, respectively). The concentrations of individual inhibitors used in this study did not affect cell viability as determined by the MTT conversion assay (data not shown).
Generation of HIV-1 stock and exposure to HIV-1-infected monocytes
HIV-1 stock was generated by transfection of human embryonic kidney (HEK) 293T cells (American Type Culture Collection, Manassas, VA, USA) with the HIV-1 NL4–3 plasmid carrying full-length proviral DNA. p24 antigen was determined by ELISA (ZeptoMetrix, Buffalo, NY, USA) as described previously (24) as a marker of HIV-1 infection. HIV-1 stock was then used to infect U937 monocytes. Briefly, 5 × 106 U937 cells in T-25 flasks (Corning, Corning, sNY, USA) were infected with viral isolate containing 120 ng of p24 in a final volume of 5 ml. After an overnight incubation, cells were resuspended in fresh medium and maintained for an additional 3–5 d for viral replication. For the majority of the experiments, confluent hCMECs cultured in the wells of the Transwell plates (Costar; Corning) were exposed to HIV-1-infected or uninfected U937 cells added to the upper chambers. The ratio of added U937 cells to hCMECs was ∼1:1, and the two cell types were separated by filters with a pore size of 0.4 μm. Typically, 1.5 × 106 HIV-1-infected or uninfected U937 cells were used per each well of the 6-well plate or 0.5 × 106 U937 cells per each well of the 12-well plate. At the time U937 cells were added to the Transwell systems, cell culture media from infected cultures were collected, and p24 levels were determined by ELISA. p24 levels were at the level of 75 pg/ml, which corresponds to that present in serum of patients with AIDS (28, 29).
Cav-1 silencing
Cav-1 silencing was performed using LipofectAMINE-plus (Invitrogen) as a transfection agent, as described previously (30). Small interfering RNA (siRNA) oligomers specifically targeting cav-1 were obtained from Dharmacon (Chicago, IL, USA). Cells were transfected overnight with cav-1 siRNA-1 (40 nM), followed by transfection with cav-1 siRNA-2 (40 nM) for an additional 5 h.
Western blotting
Western blotting was performed as described previously (24). Protein was extracted using the RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and centrifuged at 15,000 g for 15 min. The supernatants were collected, and protein concentrations were determined using a BCATM protein assay kit (Pierce, Rockford, IL, USA). Samples were separated on 4–15% Tris-HCl Ready SDS-polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA). Cav-1 antibody was obtained from Affinity Bioreagents (Rockford, IL, USA). MEK1/2, p44/42 MAPK (ERK1/2), phospho-ERK1/2, and phospho-Akt (Ser473; D9E) were purchased from Cell Signaling (Danvers, MA). Anti-Akt1/2/3 (H-136) and all secondary antibodies were from Santa Cruz Biotechnology. Anti-actin antibody was purchased from Sigma (St. Louis, MO, USA).
MMP-9-promoter activity and enzyme activity assays
To generate firefly luciferase reporter constructs of MMP-9 (pGL3 MMP-9), the 5′-flanking region of human MMP-9 (−1729 to +3) was amplified by PCR from human genomic DNA. The fragment was then cloned to the pGL3-Basic vector (Promega, Madison, WI, USA) by inserting between MluI and NcoI sites. In addition, binding sites of NF-κB, AP1, and SP1 in the MMP-9 promoter were mutated with the Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA; Table 1). These mutations were verified by DNA sequencing after subcloning the mutated MMP-9 promoters into firefly luciferase basic vector pGL3. hCMEC, hCMEC/PPARα, and hCMEC/PPARγ cells were transfected with 0.5 μg wild-type (pGL3 MMP-9) or individual mutated MMP-9 promoter constructs using Lipofectin (Invitrogen) as a transfection reagent. To normalize for transfection efficiency, cells were cotransfected with 0.05 μg pRL-TK construct (Promega) encoding for Renilla luciferase. Firefly and Renilla luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega) as described previously (24).
Table 1.
Mutants and primers of the binding sites in human MMP-9 promoter used in site-directed mutagenesis
| Binding site | Mutant | Primer sequence |
|---|---|---|
| NF-κB site 1 | g1389c | 5′-ggttgccccagtgcaattccccagcct-3′ (sense) |
| NF-κB site 2 | g1664c | 5′-ccaagggatggggcatccctccagctt-3′ (sense) |
| AP-1 site 1 | c1460t_a1461g | 5′-gggagaggaagctgagttgaagaaggctgtcaggga-3′ (sense) |
| AP-1 site 2 | c1918t_a1919g | 5′-acacaccctgacccctgagttggcacttgcctg-3′ (sense) |
| SP-1 site | g1940t_g1941t | 5′-tgcctgtcaaggaggtttggggtcacaggagc-3′ (sense) |
MMP-9 activity was detected by gelatin zymography (19) on premade 10% polyacrylamide gels containing 0.1% gelatin (Invitrogen) using 10 μl aliquots of serum-free medium from treated cultures.
EMSA
Nuclear extracts were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) and analyzed by EMSA, as described previously (31). The double-stranded oligonucleotides (Integrated DNA Technologies, Coralville, IA, USA) containing the consensus or mutated sequences of the binding sites for NF-κB, AP-1, or SP-1 (Table 2) were end labeled with 5′-Biotin. Binding reactions were performed in a 20-μl volume using LightShift EMSA Optimization and Control Kit (Pierce) according to the manufacturer's instructions. Protein-DNA complexes were analyzed on a nondenaturing 5% polyacrylamide gel using 0.5× Tris, boric acid, and ethylenediaminetetraacetic acid (TBE) buffer (USB Corp., Cleveland, OH, USA). The binding reactions were transferred to nylon membrane (Pierce). Cross-linking transferred DNA to membrane with UV Stratalinker (Stratagene). The biotin-labeled DNA was detected by Chemiluminescent Nucleic Acid Detection Module (Pierce).
Table 2.
Oligonucleotide sequences of the probes used in DNA-binding activity
| Probe | Oligonucleotide sequence |
|---|---|
| NF-κB site 1 | Consensus 5′-ccccagtggaattccccagc-3′ |
| Mutant 5′-ccccagtgcaattccccagc-3′ | |
| NF-κB site 2 | Consensus 5′-gatgggggatccctccagc-3′ |
| Mutant 5′-gatggggcgatccctccagc-3′ | |
| AP-1 site 1 | Consensus 5′-gaagctgagtcaaagaaggctgt-3′ |
| Mutant 5′-gaagctgagttgaagaaggctgt-3′ | |
| AP-1 site 2 | Consensus 5′-acccctgagtcagcacttgcc-3′ |
| Mutant 5′-acccctgagttggcacttgcc-3′ | |
| SP-1 site | Consensus 5′-tcaaggaggggtggggtcac-3′ |
| Mutant 5′-tcaaggaggtttggggtcac-3′ |
MMP-9-knockout mice and isolation of brain microvessels
MMP-9-knockout mice, generated on the FVB/NJ genetic background, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of the University of Kentucky. Four-month-old male FVB/NJ wild-type or MMP-9-knockout mice were treated 1×/d for 7 d with PPARα agonist fenofibrate (100 mg/kg) or PPARγ agonist rosiglitazone (10 mg/kg), administered intraperitoneally. Both fenofibrate and rosiglitazone were obtained from Sigma and dissolved in DMSO. At the end of fenofibrate or rosiglitazone treatment procedure, saline or HIV-1-specific Tat protein (20 μg/mouse) was administered in a single dose into the internal carotid artery using our previously described technique (32) and allowed to circulate for 24 h. Then, mice were anesthetized and perfused with 15 ml saline, and brain microvessels were isolated for Western blotting or immunofluorescent staining as described previously (32). Immunofluorescence microscopy was performed using anti-cav-1 antibody (1:2000 dilution).
Statistical analysis
The intensity of the bands corresponding to specific protein or DNA binding was determined by ImageJ software (U.S. National Institutes of Health, Baltimore, MD, USA). Routine statistical analysis was completed using Sigma-Stat 2.03 (SPSS, Chicago, IL, USA). One- or 2-way ANOVA was used to compare mean responses among the treatments. Statistical probability of P < 0.05 was considered significant.
RESULTS
PPARα and PPARγ protect against HIV-1-induced phosphorylation of ERK1/2 and Akt
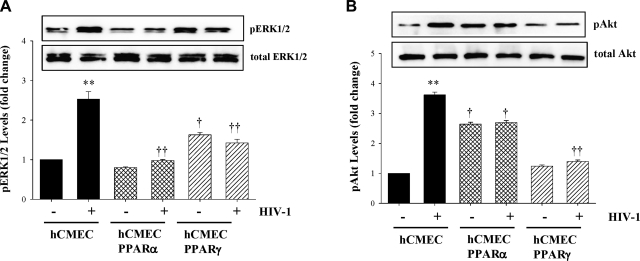
Alterations of the cellular redox status are involved in HIV-1-induced cerebrovascular pathology. In addition, MMP-9 expression can be regulated by the redox-regulated Ras-ERK1/2 and PI3K-Akt signaling pathways (33, 34). Therefore, we determined the effects of a 1 h exposure to HIV-1-infected monocytes on phosphorylation of ERK1/2 and Akt in normal hCMECs and hCMEC clones overexpressing PPARα or PPARγ (hCMEC, hCMEC/PPARα, and hCMEC/PPARγ, respectively). As shown in Fig. 1, levels of phosphorylated ERK1/2 (pERK1/2) and Akt (pAkt) were markedly (∼3-fold) upregulated in response to HIV-1 in control hCMEC cultures. Notably, these effects were attenuated in hCMECs overexpressing PPARα or PPARγ.
Figure 1.
Overexpression of PPARα and PPARγ protects against HIV-1-induced activation of the ERK1/2 and Akt signaling. Confluent hCMECs and clones overexpressing PPARα or PPARγ (hCMEC/PPARα or hCMEC/PPARγ cells, respectively) cultured on 6-well-plate Transwell systems were exposed to control or HIV-1-infected monocytes (U937 cells); 1.5 × 106/well for 1 h. Levels of phosphorylated ERK1/2 (pERK1/2; A) and Akt (pAkt; B) were determined by Western blotting. Levels of pERK1/2 or pAkt are normalized to the total level of these kinases. Blots are representative images from 3 independent experiments; quantified results are depicted as bar graphs. Results are means ± se of 3 separate experiments. **P < 0.01 vs. uninfected control; †P < 0.05, ††P < 0.01 vs. corresponding control group.
HIV-1-induced phosphorylation of ERK1/2 and Akt is influenced by functional caveolae
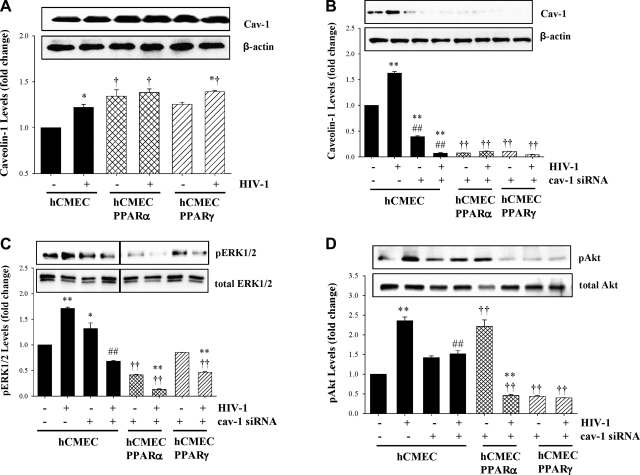
Because the ERK1/2 and Akt pathways are localized, at least partially, in caveolae (35), our experiments then centered on the role of cav-1 in HIV-1-induced activation of these signaling pathways. In the first series of experiments, we determined cav-1 levels in cells exposed to HIV-1-infected monocytes for 1 h. Such experimental conditions resulted in upregulated cav-1 levels in hCMECs. Overexpression of PPARα in hCMECs increased the baseline levels of cav-1; however, it also protected against HIV-1-induced up-regulation of this protein. In contrast, overexpression of PPARγ did not affect HIV-1-induced cav-1 levels (Fig. 2A).
Figure 2.
HIV-1-induced phosphorylation of ERK1/2 and Akt is regulated by PPARs and functional caveolae. A) Confluent hCMEC, hCMEC/PPARα, and hCMEC/PPARγ cultures were exposed to HIV-1-infected monocytes as in Fig. 1, and levels of cav-1 protein were determined by Western blotting and normalized to actin levels. B–D). In addition, functional caveolae were disrupted by silencing of cav-1, resulting in ∼90% reduction of cav-1 protein (B), followed by determination of pERK1/2 (C) and pAkt (D) by Western blotting, as in Fig. 1. Results are means ± se of 3 separate experiments. *P < 0.05, **P < 0.01 vs. uninfected control; †P < 0.05, ††P < 0.01 vs. corresponding control group; ##P < 0.01 vs. corresponding clone groups.
Expression of cav-1 was then silenced by cav-1 siRNA, followed by exposure to HIV-1-infected or uninfected monocytes. Successful cav-1 silencing procedure is reflected in Fig. 2B, which illustrates that treatment with cav-1 siRNA resulted in a 90–95% reduction of cav-1 protein levels in all experimental groups. Notably, cav-1 silencing decreased HIV-1-induced phosphorylation of ERK1/2 (Fig. 2C), illustrating the dependence of ERK1/2 activation on functional caveolae. While phosphorylation of ERK1/2 was increased in hCMECs exposed to cav-1 siRNA as compared with control, this effect may result from a stress response following cav-1 silencing. Overexpression of PPARα and PPARγ reduced the baseline phosphorylation of ERK1/2 and further enhanced the protective effects of cav-1 silencing.
Similar to the effects on pERK1/2, silencing of cav-1 markedly protected against HIV-1-stimulated phosphorylation of Akt in hCMECs. These effects were further potentiated in hCMEC clones overexpressing PPARα. In addition, overexpression of PPARγ reduced the baseline pAkt levels below control levels and protected against HIV-1-induced Akt phosphorylation (Fig. 2D).
PPARs modulate ERK1/2- and Akt-mediated regulation of MMP-9 enzyme and promoter activities
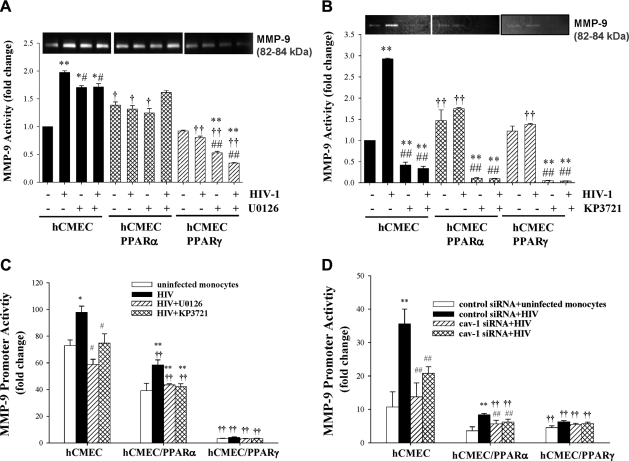
To address the hypothesis that the ERK1/2 and Akt pathways are important for HIV-1-induced up-regulation of MMP-9 activity, hCMEC, hCMEC/PPARα, and hCMEC/PPARγ cells were cocultured with HIV-1-infected monocytes for 24 h in the presence of MEK/ERK inhibitor U0126 or Akt inhibitor KP3721 (Fig. 3A, B, respectively). Gelatinase assays revealed that exposure to HIV-1-infected monocytes significantly increased MMP-9 activity in hCMECs. The observed band corresponded to 82–84 kDa, reflecting an active form of MMP-9, and distinct from a 92-kDa zymogen. Notably, HIV-1-induced activation of MMP-9 was attenuated by U0126 and dramatically reduced by KP3721. Overexpression of PPARα in hCMECs reduced the baseline activity of MMP-9, attenuated HIV-1-mediated stimulation of MMP-9 activity, and slightly potentiated the protective effects of U0126. Moreover, the protective influence of U0126 was dramatically enhanced in hCMEC/PPARγ cells (Fig. 3A). KP3721 was highly effective in decreasing HIV-1-induced MMP-9 enzymatic activity, and this effect was further potentiated in hCMEC clones overexpressing PPARα or PPARγ (Fig. 3B).
Figure 3.
Transcriptional regulation of MMP-9 expression requires functional caveolae and is modulated by PPARα or PPARγ. Confluent hCMEC, hCMEC/PPARα, and hCMEC/PPARγ cells were exposed to HIV-1-infected monocytes as in Fig. 1, and cav-1 silencing was performed as in Fig. 2. In addition, cultures were exposed to U0126 (10 μM, inhibitor of ERK1/2) and KP3721 (120 nM, inhibitor of Akt), added at the same time as HIV-1-infected monocytes. A, B) MMP-9 activity was determined by zymography in serum-free medium from treated cultures exposed to U0126 (A) or KP3721 (B). C, D) MMP-9 promoter activity was assessed in cells transfected with the firefly luciferase reporter constructs containing the promoter sequences of human MMP-9, followed by coexposure to HIV-1-infected monocytes, U0126, or KP3721 for 24 h (C) or silencing of cav-1 (D). Results are means ± se of 3 separate experiments. *P < 0.05, **P < 0.01 vs. uninfected control; †P < 0.05, ††P < 0.01 vs. corresponding control group; #P < 0.05, ##P < 0.01 vs. corresponding clone groups.
To evaluate whether PPARs, ERK1/2, and/or Akt signaling pathways are involved in transcriptional regulation of HIV-1-induced MMP-9 activation, control hCMECs and the clones overexpressing PPARα or PPARγ were transfected with the firefly luciferase reporter construct containing the promoter sequence of human MMP-9. The transfected cells were then exposed for 24 h to HIV-1-infected monocytes in the presence or absence of U0126 or KP3721. Exposure to HIV-1 significantly elevated promoter activity of MMP-9; however, treatments with U0126 or KP3721 markedly protected against these effects. Overexpression of PPARα slightly, and overexpression of PPARγ dramatically, lowered activity of MMP-9 promoter in all treatment groups (Fig. 3C). The protective effects of U0126 and KP3721 on MMP-9 promoter activity were largely mirrored by cav-1 silencing (Fig. 3D).
MMP-9 deficiency and PPAR stimulation modulate HIV-1 Tat-induced alterations of cav-1 expression in brain microvessels in an animal model
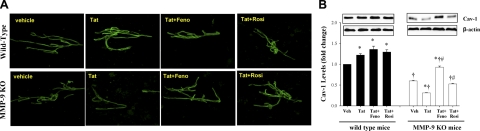
To further examine the relationship between MMP-9 and cav-1 expression at the BBB level, mice deficient in MMP-9 were injected with the HIV-1-specific protein Tat (20 μg) into the cerebral vasculature. Exposure to Tat can mimic several vascular effects of HIV-1; therefore, it was employed to substitute for HIV-1-induced effects in mice that are not susceptible to HIV-1 infection.
As illustrated in Fig. 4, injection of Tat markedly upregulated cav-1 levels in brain microvessels of wild-type mice. Stimulation of PPARα by administration of fenofibrate (100 mg/kg) or stimulation of PPARγ by rosiglitazone (10 mg/kg) 1×/d for 7 d did not affect Tat-mediated effects on cav-1 levels in wild-type mice. MMP-9 deficiency dramatically altered the pattern of Tat-induced alterations of cav-1 expression. First, the baseline expression of cav-1 in brain microvessels was lower as compared with wild-type animals. Then, injection with Tat resulted in a decrease in cav-1 expression. Treatment of MMP-9-deficient mice with fenofibrate or rosiglitazone attenuated these effects. Fenofibrate appeared to be especially effective in protection against decreased cav-1 levels in Tat-injected MMP-9-deficient mice.
Figure 4.
HIV-1 Tat-induced alterations of cav-1 expression are modulated by MMP-9 deficiency and PPAR stimulation. MMP-9-deficient mice (males, 4-mo-old) and matched FVB/NJ control mice were treated 1×d for 7 d with PPARα agonist fenofibrate (Feno; 100 mg/kg), PPARγ agonist rosiglitazone (Rosi; 10 mg/kg), or vehicle (Veh), administered i.p. Then, mice were exposed for 24 h to HIV-1-specific Tat protein (20 μg/mouse) or vehicle (saline) administered via the internal carotid artery. Cav-1 expression was evaluated by immunofluorescent staining (A) and Western blotting (B) in isolated brain microvessels. Images and blots are representative data from 3 independent experiments; quantified results from Western blots are depicted as bar graphs. Results are means ± se. *P < 0.05 vs. vehicle-treated control; †P < 0.05 vs. corresponding wild-type group; #P < 0.05 vs. Tat group.
Transcriptional regulation of MMP-9 by HIV-1 involves ERK1/2 and Akt-mediated activation of redox-stimulated transcription factors
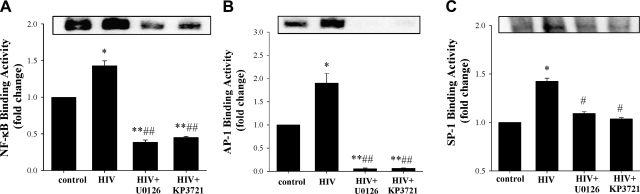
MMP-9 is regulated primarily at the transcription levels. The promoter region of MMP-9 has binding sites for NF-κB, AP-1, and SP-1; therefore, we next focused on the effects of exposure to HIV-1 on activation of these transcription factors. In these experiments, hCMECs were cocultured with HIV-1-infected monocytes for 3 h, i.e., for a longer time period than that used to examine ERK1/2 and Akt phosphorylation. As indicated in Fig. 5, exposure to HIV-1-infected monocytes markedly increased DNA binding activity of AP-1, SP-1, and NF-κB. Notably, inhibition of the MEK/ERK1/2 or Akt pathway by U0126 or KP3721, respectively, effectively protected against these effects. Control assays (data not shown) were performed with unlabeled or mutated probes to confirm the specificity of these reactions.
Figure 5.
Inhibition of ERK1/2 and Akt protects against HIV-1-induced activation of AP-1, NF-κB, and SP-1. Confluent hCMECs were exposed to HIV-1-infected monocytes for 3 h in the presence or absence of U0126 or KP3721 as in Fig. 1. DNA binding activity of NF-κB (A), AP-1 (B), and SP-1 (C) was determined by EMSA. Results are means ± se of 4 separate experiments. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. HIV group.
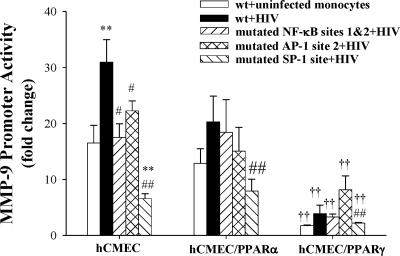
In the final series of experiments, we evaluated MMP-9 promoter activity in hCMECs transfected with MMP-9 promoter constructs with mutated NF-κB, AP-1, or SP-1 binding sites and cocultured with HIV-1 infected monocytes for 24 h. The most significant protection against HIV-1-induced MMP-9 promoter activity was achieved by mutation of SP-1 binding site (g1940t_g1941t; Fig. 6). MMP-9 promoter has 2 binding sites for AP-1 and NF-κB. Mutation of AP-1 site 1 (c1460t_a1461g) did not affect HIV-1-induced MMP-9 promoter activity (data not shown). On the other hand, mutations of AP-1 site 2 (c1918t_a1919g) successfully protected against MMP-9 promoter up-regulation (Fig. 6). Individual mutation of NF-κB site 1 or site 2 (g1389c and g1664c, respectively) did not influence HIV-1-stimulated MMP-9 promoter activity (data not shown). However, mutation of both NF-κB binding sites efficiently reduced HIV-1-induced activation of MMP-9 promoter (Fig. 6). Similar analyses with mutated MMP-9 promoter constructs were also performed in hCMEC clones overexpressing PPARα or PPARγ. Overexpression of PPARα and, in particular, PPARγ lowered the baseline activity of MMP-9 promoter and protected against HIV-1-mediated induction, indicating the importance of alterations of the redox status in regulation of MMP-9 expression. Due to lower baseline activity of MMP-9 promoter and less robust HIV-1 effects, mutations of AP-1 and NF-κB sites did not alter HIV-1-induced stimulation of MMP-9 promoter in hCMEC/PPARα or hCMEC/PPARγ cells. However, mutation of the SP-1 site markedly decreased HIV-1-stimulated MMP-9 promoter activity in these clones, confirming the critical role of SP-1 in HIV-1-induced transcriptional regulation of MMP-9 expression.
Figure 6.
Transcriptional regulation of MMP-9 promoter activity. Confluent hCMEC, hCMEC/PPARα or hCMEC/PPARγ cells were transfected with a wild type (wt) MMP-9 promoter construct or with constructs with mutated binding sites of NF-κB, AP-1, and SP-1. Then, cultures were exposed to normal or HIV-1-infected monocytes for 24 h. **P < 0.01 vs. uninfected control; ††P < 0.01 vs. corresponding hCMEC group; #P < 0.05, ##P < 0.01 vs. wt-HIV-1 group.
DISCUSSION
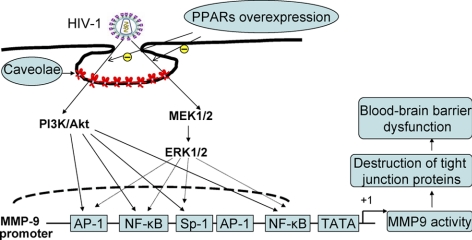
It is widely accepted that HIV-1 can enter the CNS via transfer across the BBB in early stages of the infection during the peak viremia period that occurs around ∼10–14 d postinfection. The virus can reside in the CNS for a prolonged period of time and contribute to the development of neurological complications at later stages of infection (2). BBB disruption was demonstrated in HIV-1-infected patients (4, 6). Although the exact mechanisms of this process remain unclear, they appear to include increased tissue oxidative stress and up-regulation of MMPs (12, 18). In line with these hypotheses, the results of the present study demonstrate that coculture of brain endothelial cells with HIV-1-infected monocytes resulted in stimulation of redox-responsive ERK1/2 and Akt signaling, leading to activation of redox-responsive transcription factors and induction of MMP-9 promoter and enzyme activities. In addition, our results indicate that overexpression of PPARα or PPARγ in brain endothelial cells can attenuate this sequence of events (Fig. 7).
Figure 7.
Schematic diagram of the signaling pathways involved in PPARα- and PPARγ-mediated protection against HIV-1-induced MMP-9 overexpression. PPAR overexpression in hCMECs protected against HIV-1-induced MMP activity via caveolae-associated ERK and Akt signaling. Among activated transcription factors, SP-1 appears to be the critical factor involved in HIV-1-induced MMP-9 expression.
Regulatory and signaling mechanisms evaluated in the present study are closely associated with caveolae, a major subset of lipid rafts in endothelial cells. Indeed, ERK1/2 and other components of the Ras-MAPK pathway (e.g., Ras or Raf1) reside in caveolae (35). This close interaction between ERK1/2 and caveolae was demonstrated in the present study. For example, while exposure to HIV-1-infected monocytes activated ERK1/2, silencing of cav-1 significantly attenuated these effects (Fig. 2). These results are in agreement with literature data indicating that membrane cholesterol depletion, the procedure that disrupts caveolae and lipid rafts, also attenuated ERK1/2 activation (35). Similarly, overexpression of cav-1 mutant that lacked a functional scaffolding domain aborted ERK1/2 activation (36). The results of the present study also show that HIV-1-induced phosphorylation of Akt is regulated by cav-1, further demonstrating the importance of caveolae in HIV-1-induced endothelial dysfunction. Similar protective effects against Akt phosphorylation by cav-1 silencing or disruption of lipid rafts were reported in the literature for different cell systems and types of stimuli (37–41).
The ERK1/2 and Akt pathways are stimulated in the response to increased oxidative stress (42). Thus activation of these signaling cascades observed in the present study is consistent with alterations of the redox status observed in HIV-1-infected patients and in cells exposed to HIV-1 (43). Nevertheless, it should be noted that we exposed brain endothelial cells to HIV-1-monocytes; therefore, we cannot fully differentiate between viral and a variety of nonviral factors produced by infected cells that may also influence the signaling pathways in brain endothelial cells. For example, inflammatory cytokines produced by HIV-1-infected cells are potent activators of the redox-related pathways.
One of the novel elements of the present study was to demonstrate that cocultures with HIV-1-infected monocytes stimulated activation of NF-κB, SP-1, and AP-1 transcription factors in hCMECs. While similar effects were described in other cell systems in response to HIV-1 (44), we demonstrate that pharmacological inhibition of ERK1/2 and Akt was highly protective in blocking these effects, indicating the role of caveolae-associated signaling in activation of redox-regulated transcription factors.
Transcriptional regulation of MMP-9 expression was a subject of several studies (45). The results of the present study are consistent with recent reports that indicated the essential role of SP-1 in MMP-9 gene expression (Fig. 7 and refs. 46, 47). While SP-1 closely interacts with AP-1 (46), novel results of our study indicate that AP-1 site 2, but not site 1, is involved in HIV-1-induced MMP-9 expression. In addition, it was highly unexpected that mutation of individual NF-κB binding sites did not affect HIV-1-stimulated MMP-9 promoter activity, and only mutation of both sites was required to inhibit MMP-9 promoter. To support this finding, it was recently shown that low levels of NF-κB were not sufficient for MMP-9 induction and inhibition of NF-κB did not effectively interfere with MMP-9 production (48).
Literature reports on the interaction between MMPs and caveolae are primarily focused on the regulation of metastatic properties of tumor cells via caveolae-associated regulation of proteolytic activity of MMPs (49, 50). Evidence indicates that specific MMPs, such as MMP-2 and its activator membrane-type 1 MMP (MT-1 MMP or MMP-14), are localized in caveolae in several cell types (51, 52), including human microvascular endothelial cells derived from dermal vessels (53). Consistent with such locations, both MMP-2 and MT-1 MMP were shown to be closely influenced by cav-1 levels. For example, silencing of cav-1 decreased MT1-MMP-dependent degradation of the gelatin matrix (54, 55). It was also shown that interactions between MT1-MMP and cav-1 required cav-1 phosphorylation on tyrosine 14 (51). In contrast, MMP-9 protein expression does not appear to be localized in caveolae and MMP-9 positive immunostaining is uniformly distributed throughout endothelial cells (53). Nevertheless, novel results of the present study provide evidence that MMP-9 expression is controlled by caveolae and caveolae-associated pathways. In fact, silencing of cav-1 markedly decreased HIV-1-induced MMP-9 promoter activity (Fig. 3). We propose that these regulatory mechanisms are secondary to the influence of cav-1 on the ERK1/2 and Akt signaling and expression of redox-regulated transcription factors. Indeed, inhibition of ERK1/2 or Akt signaling protected against HIV-1-induced binding activity of SP-1, AP-1, or NF-κB. The interaction between cav-1 and MMP-9 was also observed in MMP-9-deficient mice, which exhibited lower basal levels of cav-1 as compared with wild-type controls.
PPARs are ligand-activated transcription factors that are considered to be critical rescue molecules that can down-regulate activation of redox-responsive transcription factors and exert anti-inflammatory effects (24). Our earlier studies indicated that overexpression of PPARα or PPARγ in brain endothelial cells attenuated HIV-1-mediated dysregulation of TJs, partly by inhibition of MMP activity (19), and protected against HIV-1-induced proinflammatory responses (24). Similar protection was achieved when comparing the effects of PPAR stimulation in normal hCMECs by specific agonists with those achieved by ectopic overexpression of PPARs in hCMEC/PPARα or hCMEC/PPARγ cells, i.e., cells that were used also in the present study (19, 24). Thus, it appears that the levels of endogenous PPAR ligands are sufficient to activate these transcription factors in cells overexpressing PPARα or PPARγ proteins. In addition, viral proteins may stimulate PPARs as it was recently shown for the hepatitis B virus (56). In the present study, overexpression of PPARα or PPARγ effectively attenuated both baseline phosphorylation of ERK1/2 and Akt as well as HIV-1-induced activation of these kinases. These effects likely reflect the overall improvement of the cellular redox status and are in line with the results that demonstrated that stimulation of PPARγ by rosiglitazone suppressed phosphorylation of ERK1/2 in adipocytes (57). Inhibition of ERK activation by rosiglitazone was also linked to protection against TNF-α- and interferon-γ-induced inflammatory responses in human endothelial cells (58).
Overexpression of PPARα and, especially, PPARγ was highly effective in protection against HIV-1-induced activation of MMP-9 promoter. These effects were further potentiated by mutation of the SP-1 binding site in the MMP-9 promoter. Silencing of cav-1 enhanced the protective effects of PPARα, further demonstrating the dependency of PPARs and/or MMP-9 expression on functional caveolae. Indeed, treatment of MMP-9-deficient mice with fenofibrate or rosiglitazone restored cav-1 levels in brain microvessels. The protective effects of rosiglitazone on MMP-9 expression were also demonstrated in RAW264.7 cells stimulated with lipopolysaccharide (59).
In summary, exposure to HIV-1-infected monocytes induced MMP-9 promoter and enzymatic activity in brain endothelial cells via caveolae-associated ERK1/2 and Akt signaling. Activation of these pathways resulted in activation of SP-1, AP-1, and NF-κB, i.e., transcription factors that regulate MMP-9 expression. HIV-1-induced signaling and MMP-9 promoter activity were markedly attenuated by overexpression of PPARα or PPARγ as well by silencing of cav-1, indicating a close relationship between HIV-1-induced cerebrovascular toxicity, redox-regulated mechanisms, and functional caveolae.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants MH63022, MH072567, DA027569, ES07380, and AHA09POST2060217. Tat was produced and provided by support from the National Center for Research Resources (grant P20 RR15592).
REFERENCES
- 1. Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010) Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 [DOI] [PubMed] [Google Scholar]
- 2. McGee B., Smith N., Aweeka F. (2006) HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin. Trials 7, 142–153 [DOI] [PubMed] [Google Scholar]
- 3. Andersson L. M., Hagberg L., Fuchs D., Svennerholm B., Gisslen M. (2001) Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals–correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J. Neurovirol. 7, 542–547 [DOI] [PubMed] [Google Scholar]
- 4. Avison M. J., Nath A., Greene-Avison R., Schmitt F. A., Greenberg R. N., Berger J. R. (2004) Neuroimaging correlates of HIV-associated BBB compromise. J. Neuroimmunol. 157, 140–146 [DOI] [PubMed] [Google Scholar]
- 5. Boven L. A., Middel J., Verhoef J., De Groot C. J., Nottet H. S. (2000) Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol. Appl. Neurobiol. 26, 356–360 [DOI] [PubMed] [Google Scholar]
- 6. Dallasta L. M., Pisarov L. A., Esplen J. E., Werley J. V., Moses A. V., Nelson J. A., Achim C. L. (1999) Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 155, 1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Persidsky Y., Zheng J., Miller D., Gendelman H. E. (2000) Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 68, 413–422 [PubMed] [Google Scholar]
- 8. Buttner A., Mehraein P., Weis S. (1996) Vascular changes in the cerebral cortex in HIV-1 infection. II. An immunohistochemical and lectinhistochemical investigation. Acta Neuropathol. 92, 35–41 [DOI] [PubMed] [Google Scholar]
- 9. Lukes A., Mun-Bryce S., Lukes M., Rosenberg G. A. (1999) Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol. Neurobiol. 19, 267–284 [DOI] [PubMed] [Google Scholar]
- 10. McCoig C., Castrejon M. M., Saavedra-Lozano J., Castano E., Baez C., Lanier E. R., Saez-Llorens X., Ramilo O. (2004) Cerebrospinal fluid and plasma concentrations of proinflammatory mediators in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 23, 114–118 [DOI] [PubMed] [Google Scholar]
- 11. Sporer B., Paul R., Koedel U., Grimm R., Wick M., Goebel F. D., Pfister H. W. (1998) Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J. Infect. Dis. 178, 854–857 [DOI] [PubMed] [Google Scholar]
- 12. Conant K., McArthur J. C., Griffin D. E., Sjulson L., Wahl L. M., Irani D. N. (1999) Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann. Neurol. 46, 391–398 [DOI] [PubMed] [Google Scholar]
- 13. Liuzzi G. M., Mastroianni C. M., Santacroce M. P., Fanelli M., D'Agostino C., Vullo V., Riccio P. (2000) Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J. Neurovirol. 6, 156–163 [DOI] [PubMed] [Google Scholar]
- 14. Persidsky Y., Limoges J., Rasmussen J., Zheng J., Gearing A., Gendelman H. E. (2001) Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J. Neuroimmunol. 114, 57–68 [DOI] [PubMed] [Google Scholar]
- 15. Ghorpade A., Persidskaia R., Suryadevara R., Che M., Liu X. J., Persidsky Y., Gendelman H. E. (2001) Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 75, 6572–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciborowski P., Enose Y., Mack A., Fladseth M., Gendelman H. E. (2004) Diminished matrix metalloproteinase 9 secretion in human immunodeficiency virus-infected mononuclear phagocytes: modulation of innate immunity and implications for neurological disease. J. Neuroimmunol. 157, 11–16 [DOI] [PubMed] [Google Scholar]
- 17. Louboutin J. P., Agrawal L., Reyes B. A., Van Bockstaele E. J., Strayer D. S. (2010) HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J. Neuropathol. Exp. Neurol. 69, 801–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sporer B., Koedel U., Paul R., Kohleisen B., Erfle V., Fontana A., Pfister H. W. (2000) Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: role of matrix metalloproteinase-9. J. Neuroimmunol. 102, 125–130 [DOI] [PubMed] [Google Scholar]
- 19. Huang W., Eum S. Y., Andras I. E., Hennig B., Toborek M. (2009) PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 23, 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gil L., Martinez G., Gonzalez I., Tarinas A., Alvarez A., Giuliani A., Molina R., Tapanes R., Perez J., Leon O. S. (2003) Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol. Res. 47, 217–224 [DOI] [PubMed] [Google Scholar]
- 21. Melendez M. M., McNurlan M. A., Mynarcik D. C., Khan S., Gelato M. C. (2008) Endothelial adhesion molecules are associated with inflammation in subjects with HIV disease. Clin. Infect. Dis. 46, 775–780 [DOI] [PubMed] [Google Scholar]
- 22. Suresh D. R., Annam V., Pratibha K., Prasad B. V. (2009) Total antioxidant capacity–a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J. Biomed. Sci. 16, 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kline E. R., Kleinhenz D. J., Liang B., Dikalov S., Guidot D. M., Hart C. M., Jones D. P., Sutliff R. L. (2008) Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am. J. Physiol. Heart Circ. Physiol. 294, H2792–H2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang W., Rha G. B., Han M. J., Eum S. Y., Andras I. E., Zhong Y., Hennig B., Toborek M. (2008) PPARalpha and PPARgamma effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J. Neurochem. 107, 497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramirez S. H., Heilman D., Morsey B., Potula R., Haorah J., Persidsky Y. (2008) Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J. Immunol. 180, 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanley T. M., Blay Puryear W., Gummuluru S., Viglianti G. A. (2010) PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog. 6, e1000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weksler B. B., Subileau E. A., Perriere N., Charneau P., Holloway K., Leveque M., Tricoire-Leignel H., Nicotra A., Bourdoulous S., Turowski P., Male D. K., Roux F., Greenwood J., Romero I. A., Couraud P. O. (2005) Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 [DOI] [PubMed] [Google Scholar]
- 28. Pascale J. M., Isaacs M. D., Contreras P., Gomez B., Lozano L., Austin E., De Martin M. C., Gregory R. L., McLaughlin G. L., Amador A. (1997) Immunological markers of disease progression in patients infected with the human immunodeficiency virus. Clin. Diagn. Lab. Immunol. 4, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reddy M. M., McKinley G., Englard A., Grieco M. H. (1989) Effect of azidothymidine (AZT) on P24 antigen levels in patients with AIDS-related complex and AIDS. J. Clin. Lab. Anal. 3, 199–201 [DOI] [PubMed] [Google Scholar]
- 30. Zhong Y., Smart E. J., Weksler B., Couraud P. O., Hennig B., Toborek M. (2008) Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J. Neurosci. 28, 7788–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang N., Huang J., Greshock J., Liang S., Barchetti A., Hasegawa K., Kim S., Giannakakis A., Li C., O'Brien-Jenkins A., Katsaros D., Butzow R., Coukos G., Zhang L. (2008) Transcriptional regulation of PIK3CA oncogene by NF-kappaB in ovarian cancer microenvironment. PLoS One 3, e1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang W., Rha G. B., Chen L., Seelbach M. J., Zhang B., Andras I. E., Bruemmer D., Hennig B., Toborek M. (2010) Inhibition of telomerase activity alters tight junction protein expression and induces transendothelial migration of HIV-1-infected cells, Am. J. Physiol. Heart Circ. Physiol. 298, H1136–H1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Byun H. J., Hong I. K., Kim E., Jin Y. J., Jeoung D. I., Hahn J. H., Kim Y. M., Park S. H., Lee H. (2006) A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J. Biol. Chem. 281, 34833–34847 [DOI] [PubMed] [Google Scholar]
- 34. Park S. K., Hwang Y. S., Park K. K., Park H. J., Seo J. Y., Chung W. Y. (2009) Kalopanaxsaponin A inhibits PMA-induced invasion by reducing matrix metalloproteinase-9 via PI3K/Akt- and PKCdelta-mediated signaling in MCF-7 human breast cancer cells. Carcinogenesis 30, 1225–1233 [DOI] [PubMed] [Google Scholar]
- 35. Yao Z., Seger R. (2009) The ERK signaling cascade–views from different subcellular compartments. Biofactors 35, 407–416 [DOI] [PubMed] [Google Scholar]
- 36. Vihanto M. M., Vindis C., Djonov V., Cerretti D. P., Huynh-Do U. (2006) Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase. J. Cell Sci. 119, 2299–2309 [DOI] [PubMed] [Google Scholar]
- 37. Tahir S. A., Park S., Thompson T. C. (2009) Caveolin-1 regulates VEGF-stimulated angiogenic activities in prostate cancer and endothelial cells. Cancer Biol. Ther. 8, 2286–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang B., Peng F., Wu D., Ingram A. J., Gao B., Krepinsky J. C. (2007) Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell. Signal. 19, 1690–1700 [DOI] [PubMed] [Google Scholar]
- 39. Arcaro A., Aubert M., Espinosa del Hierro M. E., Khanzada U. K., Angelidou S., Tetley T. D., Bittermann A. G., Frame M. C., Seckl M. J. (2007) Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell. Signal. 19, 1081–1092 [DOI] [PubMed] [Google Scholar]
- 40. Lim E. J., Smart E. J., Toborek M., Hennig B. (2007) The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 293, H3340–H3347 [DOI] [PubMed] [Google Scholar]
- 41. Sedding D. G., Hermsen J., Seay U., Eickelberg O., Kummer W., Schwencke C., Strasser R. H., Tillmanns H., Braun-Dullaeus R. C. (2005) Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ. Res. 96, 635–642 [DOI] [PubMed] [Google Scholar]
- 42. Kim E. K., Choi E. J. (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 1802, 396–405 [DOI] [PubMed] [Google Scholar]
- 43. Toborek M., Andras I. E., Rashid C. S., Zhong Y., Nakagawa S. (2011) Endothelial cell biology and HIV-1 infection. In The Neurology of AIDS, 3rd Ed. (Gendelman H. E., Everall I. P. eds) pp. 207–219, Oxford University Press, Oxford, UK: In press [Google Scholar]
- 44. Srivastava A. K., Qin X., Wedhas N., Arnush M., Linkhart T. A., Chadwick R. B., Kumar A. (2007) Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J. Biol. Chem. 282, 35113–35124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. (2003) Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253, 269–285 [DOI] [PubMed] [Google Scholar]
- 46. Murthy S., Ryan A., He C., Mallampalli R. K., Carter A. B. (2010) Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 285, 25062–25073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reddy V. S., Prabhu S. D., Mummidi S., Valente A. J., Venkatesan B., Shanmugam P., Delafontaine P., Chandrasekar B. (2010) Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-kappaB activation. Am. J. Physiol. Heart Circ. Physiol. 299, H1242–H1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schroer N., Pahne J., Walch B., Wickenhauser C., Smola S. (2011) Molecular pathobiology of human cervical high-grade lesions: paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Cancer Res. 71, 87–97 [DOI] [PubMed] [Google Scholar]
- 49. Felicetti F., Parolini I., Bottero L., Fecchi K., Errico M. C., Raggi C., Biffoni M., Spadaro F., Lisanti M. P., Sargiacomo M., Care A. (2009) Caveolin-1 tumor-promoting role in human melanoma. Int. J. Cancer 125, 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cokakli M., Erdal E., Nart D., Yilmaz F., Sagol O., Kilic M., Karademir S., Atabey N. (2009) Differential expression of caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasion. BMC Cancer 9, 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labrecque L., Nyalendo C., Langlois S., Durocher Y., Roghi C., Murphy G., Gingras D., Beliveau R. (2004) Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J. Biol. Chem. 279, 52132–52140 [DOI] [PubMed] [Google Scholar]
- 52. Cho W. J., Chow A. K., Schulz R., Daniel E. E. (2007) Matrix metalloproteinase-2, caveolins, focal adhesion kinase and c-Kit in cells of the mouse myocardium. J. Cell. Mol. Med. 11, 1069–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Puyraimond A., Fridman R., Lemesle M., Arbeille B., Menashi S. (2001) MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp. Cell Res. 262, 28–36 [DOI] [PubMed] [Google Scholar]
- 54. Yamaguchi H., Takeo Y., Yoshida S., Kouchi Z., Nakamura Y., Fukami K. (2009) Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69, 8594–8602 [DOI] [PubMed] [Google Scholar]
- 55. Kim H. N., Chung H. S. (2008) Caveolin-1 inhibits membrane-type 1 matrix metalloproteinase activity. BMB Rep. 41, 858–862 [DOI] [PubMed] [Google Scholar]
- 56. Dubuquoy L., Louvet A., Hollebecque A., Mathurin P., Dharancy S. (2009) Peroxisome proliferator-activated receptors in HBV-related infection. PPAR Res. 2009, 145124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim J., Han D. C., Kim J. M., Lee S. Y., Kim S. J., Woo J. R., Lee J. W., Jung S. K., Yoon K. S., Cheon H. G., Kim S. S., Hong S. H., Kwon B. M. (2009) PPAR gamma partial agonist, KR-62776, inhibits adipocyte differentiation via activation of ERK. Cell. Mol. Life Sci. 66, 1766–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lombardi A., Cantini G., Piscitelli E., Gelmini S., Francalanci M., Mello T., Ceni E., Varano G., Forti G., Rotondi M., Galli A., Serio M., Luconi M. (2008) A new mechanism involving ERK contributes to rosiglitazone inhibition of tumor necrosis factor-alpha and interferon-gamma inflammatory effects in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 718–724 [DOI] [PubMed] [Google Scholar]
- 59. Mendes Sdos S., Candi A., Vansteenbrugge M., Pignon M. R., Bult H., Boudjeltia K. Z., Munaut C., Raes M. (2009) Microarray analyses of the effects of NF-kappaB or PI3K pathway inhibitors on the LPS-induced gene expression profile in RAW264.7 cells: synergistic effects of rapamycin on LPS-induced MMP9-overexpression, Cell. Signal. 21, 1109–1122 [DOI] [PubMed] [Google Scholar]