Abstract
Transient receptor potential vanilloid 6 (TRPV6) channels play an important role in Ca2+ absorption in the intestines. Both phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] and cytoplasmic ATP have been proposed to be important for maintaining TRPV6 activity. To evaluate whether PtdIns(4,5)P2 and ATP affect channel activity directly or indirectly, we have used a dual approach, examining channel activity in excised patches and planar lipid bilayers. In excised inside-out patch-clamp measurements, ATP reactivated the human TRPV6 channels after current rundown only in the presence of Mg2+. The effect of MgATP was inhibited by 3 structurally different compounds that inhibit type III phosphatidylinositol 4-kinases (PI4Ks). PtdIns(4,5)P2 also activated TRPV6 in excised patches, while its precursor PtdIns(4)P had only minimal effect. These data demonstrate that MgATP provides substrate for lipid kinases, allowing the resynthesis of PtdIns(4,5)P2. To determine whether PtdIns(4,5)P2 is a direct activator of TRPV6, we purified and reconstituted the channel protein in planar lipid bilayers. The reconstituted channel showed high activity in the presence of PtdIns(4,5)P2, while PtdIns(4)P induced only minimal activity. Our data establish PtdIns(4,5)P2 as a direct activator of TRPV6 and demonstrate that intracellular ATP regulates the channel indirectly as a substrate for type III PI4Ks.—Zakharian, E., Cao, C., Rohacs, T. Intracellular ATP supports TRPV6 activity via lipid kinases and the generation of PtdIns(4,5)P2.
Keywords: transient receptor potential channel, phosphoinositides
Members of the transient receptor potential (TRP) ion channel family play roles in a wide variety of physiological (1) and pathophysiological processes (2). Mutations of TRP channels also cause several different human diseases (3). TRP vanilloid 6 (TRPV6) is a Ca2+-selective ion channel, expressed mainly in the duodenum, where it serves as a Ca2+ uptake mechanism in the apical membrane of epithelial cells. TRPV6 expression is controlled by the active form of vitamin D; once expressed, it is constitutively active, but its activity is limited by Ca2+-induced inactivation (4). TRPV5, a close homologue of TRPV6, is expressed mainly in the kidneys. Both these channels constitute the initial entry step in the transcellular Ca2+ transport process of epithelial cells. Genetic deletion of TRPV5 or TRPV6 in mice causes disturbances in extracellular Ca2+ homeostasis (5, 6). In the human lineage, the rate of TRPV6 evolution is accelerated (7). An ancestral form has been identified that is more active than the derived channel subtype (8, 9). The presence of the ancient form was shown to be a risk factor for renal calcium stone formation in humans (8).
Phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], commonly referred to as PIP2, is a membrane phospholipid with many biological functions. Phospholipase C (PLC) enzymes hydrolyze PtdIns(4,5)P2 to form the two classic second messengers inositol 1,4,5-trisphosphate and diacylglycerol (10). On receptor stimulation, phosphoinositide 3-kinases (PI3Ks) further phosphorylate PtdIns(4,5)P2 to form PtdIns(3,4,5)P3, which plays roles mainly in signaling by various growth factors. PtdIns(4,5)P2 is a common regulator of many ion channels, including inwardly rectifying K+ channels, voltage-gated KCNQ K+ channels, and voltage-gated Ca2+ channels (for reviews, see refs. 11–13). In most cases, PtdIns(4,5)P2 is required for channel activity, and reduction of the concentration of the lipid by activation of PLC inhibits channel activity. The general assumption is that PtdIns(4,5)P2 activates ion channels via direct interactions between the negatively charged lipid headgroup and positively charged amino acid residues in the cytoplasmic domains of the channel protein.
TRP channel regulation is very diverse, but most are modulated by membrane phosphoinositides (14, 15), particularly PtdIns(4,5)P2. For several TRP channels, PtdIns(4,5)P2 is an absolute requirement for activity. We have shown previously that, in excised patches, TRPV6 channel activity runs down and the rate of rundown is accelerated by polylysine, which is known to bind anionic lipids. The channels could be reactivated by the water-soluble diC8 analog of PtdIns(4,5)P2 but not its precursor PtdIns(4)P (16). We also demonstrated that Ca2+ influx through TRPV6 activates a Ca2+-sensitive phospholipase C (PLC) isoform, and the resulting decrease in PtdIns(4,5)P2 levels limits channel activity, contributing to Ca2+-induced inactivation (16, 17). This Ca2+-dependent inactivation mechanism is similar to that proposed for TRPM8 (18, 19), TRPV1 (20–22), and TRPV2 (23). Calcium-calmodulin has also been shown to be involved in Ca2+-induced inactivation of TRPV6 (24, 25).
Recently, it was shown that providing ATP or other nucleotides in the patch pipette prevents rundown of TRPV6 activity in whole-cell patch-clamp experiments (26). ATP was also shown to bind to the ankyrin repeat domain (ARD) of TRPV6, and thus it was concluded that intracellular ATP is a direct regulator of TRPV6 (26). These conclusions were based mainly on whole-cell patch-clamp experiments, where the presence of much of the cellular machinery makes it difficult to draw a firm conclusion that an intracellular molecule is a direct regulator of ion channels (27). Here we have performed experiments to evaluate the role of intracellular nucleotides in the regulation of TRPV6. We have used two techniques, excised inside-out patches and planar lipid bilayers, where the presence of the cellular machinery is less prevalent or nonexistent. We found that in excised patches, MgATP, but not NaATP, reactivated TRPV6 channels after rundown, and other nucleotides were ineffective. Furthermore, the effect of MgATP was prevented by 3 structurally different inhibitors of type III phosphatidylinositol 4-kinases (PI4Ks). We conclude that MgATP supports TRPV6 activity by providing substrate for lipid kinases to resynthesize PtdIns(4,5)P2. Furthermore, we find that PtdIns(4,5)P2 dependence of TRPV6 was retained in the purified TRPV6 protein incorporated into planar lipid bilayers, firmly establishing this lipid as a direct regulator of TRPV6 channels.
MATERIALS AND METHODS
Xenopus oocyte preparation and electrophysiology
Oocytes were extracted from Xenopus laevis frogs using collagenase digestion and maintained in a solution containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES (pH 7.4). Expression of the human TRPV6 was achieved by microinjection of a linearized cRNA construct in a pGEMSH (16) vector using a nanoliter-injector system (Warner Instruments, Hamden, CT, USA). Excised inside-out macropatch experiments were performed as described earlier (16) using borosilicate glass pipettes (World Precision Instruments, Sarasota, FL, USA) of 0.8–1.2 MΩ resistance with an electrode solution containing 96 mM LiCl, 1 mM EGTA, and 5 mM HEPES (pH 7.4). After we established gigaohm resistance seals and excision, currents were measured in the inside-out configuration using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) with a ramp protocol from −100 to +100 mV (0.25 mV/ms), immediately preceded by a 100-ms step to −100 mV. The protocol was applied every second, and holding potential was 0 mV. Data were analyzed with pClamp 9.0 software (Molecular Devices). The perfusion solution contained 96 mM KCl, 5 mM EGTA, and 10 mM HEPES, pH adjusted to 7.4. DiC8 and DiC16 PtdIns(4,5)P2 and PtdIns(4)P were purchased from Cayman Chemicals (Ann Arbor, MI, USA), whereas the powder forms of the natural long acyl-chain PtdIns(4,5)P2 and PtdIns(4)P, purified from porcine brain, were purchased from Avanti Polar Lipids (Birmingham, AL, USA). The acyl-chain composition of these compounds is a mixture, with the arachydonyl-stearyl (AASt) form being the most prevalent. Long acyl-chain PtdIns(4,5)P2 and PtdIns(4)P were sonicated, when they were dissolved as stock solution in water, before they were portioned into aliquots and stored at −70°C and also before experiments in the working solutions. DiC8 phosphoinositides were dissolved in aqueous buffers or water, portioned into aliquots, stored at −70°C, and diluted in the working solution on the day of the experiments. LY294002, wortmannin (Sigma-Aldrich, St. Louis, MO, USA), and PIK93 (B-Bridge International, Cupertino, CA, USA) were dissolved in DMSO, portioned into aliquots, and stored at −20°C. Wortmannin and PIK93 were used with a final DMSO concentration of 0.1%. For LY294002, the stock concentration was 100 mM, and thus the final DMSO concentration in working solutions was 0.3%; the same concentration of DMSO was used in control measurements. For the solutions containing various nucleotides (Sigma-Aldrich), free magnesium concentrations were calculated by the Maxchelator program (http://maxchelator.stanford.edu/).
Since in excised patches the current amplitudes between patches are inherently variable, due to the different number of channels in each patch, data summaries are presented in a normalized format. In most cases, current amplitude was normalized to that induced by diC8 PtdIns(4,5)P2, which generally gave less variable results than normalizing to current levels after excision, likely because the endogenous PtdIns(4,5)P2 concentration varies between patches. In some experiments (Figs. 3 and 6), diC8 PtdIns(4,5)P2 was either not applied or not applied in every measurement; therefore, we normalized to the current amplitude at the time of excision in those experiments. The current level immediately after excision was, in most cases, ∼10–20% higher than that observed in the cell attached configuration, probably due to the relief from inhibition by some cytoplasmic compounds. The current increase after excision was quite variable, ranging from an immediate decrease to a severalfold increase. Thus, we found that normalizing to current level immediately after excision gave less variable results than normalizing to cell-attached current levels.
Figure 3.
Effects of wortmannin on the current recovery induced by MgATP. A–C) Representative traces; measurements were performed as described in Fig. 1. Applications of 2 mM MgATP, 35 μM wortmannin, 35 nM wortmannin, and 25 μM DiC8 PtdIns(4,5)P2 are shown by the horizontal bars. D) Data summary, normalized to the current levels immediately after establishing the inside-out configuration. *P < 0.05.
Figure 6.
Natural PtdIns(4,5)P2 but not PtdIns(4)P activates TRPV6 in excised patches. A) Representative trace; measurements were performed as described in Fig. 1; applications of 10 μM natural (arachydonyl-stearyl) PtdIns(4,5)P2, and PtdIns(4)P are indicated by horizontal bars. B) Data summary, normalized to the current level after excision. C, D) Representative traces for measurements where the patches were pretreated with 10 μM natural PtdIns(4)P, and MgATP-induced current recovery was examined in the absence (C) and presence (D) of 300 μM LY294002. E) Data summary. *P < 0.05.
Means ± se are presented in the figures; we quantified the current amplitudes at −100 mV. Statistical significance was calculated with Student's t test, or when the data significantly deviated from normal distribution, with the Mann-Whitney test for unpaired samples or the Wilcoxon test for paired samples, as appropriate. Values of P < 0.05 were considered significant.
Expression and purification of TRPV6
HEK-293 cells transiently expressing TRPV6 tagged with the Myc epitope on its N terminus were grown to 70–80% confluence, washed, and collected with cold PBS. Cells were harvested and resuspended in NCB buffer containing 500 mM NaCl, 50 mM NaH2PO4, 20 mM HEPES, 2 mM Na-orthovanadate, and 10% glycerol (pH 7.5), with addition of 1 mM of the protease inhibitor PMSF and 5 mM β-mercaptoethanol. Then, the cells were lysed by freeze-thawing method and centrifuged at low speed to remove cell debris and DNA. The supernatant was further centrifuged at 40,000 g for 2.5 h, and the pellet was resuspended in NCB buffer with addition of a protease inhibitor cocktail (Roche, Indianapolis, IN, USA), 20 μg/ml DNase, 20 μg/ml RNase, 0.1% Nonidet P40 (Roche), and 0.5% dodecyl-maltoside (DDM; CalBiochem, San Diego, CA, USA). The suspension was incubated overnight at 4°C on a shaker with gentle agitation and then centrifuged for 1 h at 40,000 g. Further, the TRPV6 protein was purified with immunoprecipitation method using G-protein magnetic beads (GE Healthcare, Piscataway, NJ, USA) conjugated with the Myc antibodies. Protein was eluted from the beads with Myc peptide (50 μg/ml; Sigma-Aldrich). All steps of purification were performed at 4°C.
Planar lipid bilayer experiments
Planar lipid bilayers were formed from a solution of synthetic 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine (POPE; Avanti Polar Lipids) in a 3:1 ratio in n-decane (Sigma-Aldrich). The solution was used to paint a bilayer in an aperture of ∼150 μm diameter in a Delrin cup (Warner Instruments) between symmetric aqueous bathing solutions of 150 mM KCl, 0.02 mM MgCl2, and 20 mM HEPES (pH 7.4), at 22°C. All salts were ultrapure (Sigma-Aldrich). Bilayer capacitances were in the range of 50–75 pF. After the bilayers were formed, 0.2 μl of the TRPV6 micellar solution (0.02 μg/ml) was added to the cis compartment with gentle stirring. Unitary currents were recorded with an integrating patch-clamp amplifier (Axopatch 200B; Molecular Devices). The trans solution (voltage command side) was connected to the CV 201A head-stage input, and the cis solution was held at virtual ground via a pair of matched Ag-AgCl electrodes. Currents through the voltage-clamped bilayers (background conductance <3 pS) were filtered at the amplifier output (low pass, −3 dB at 10 kHz, 8-pole Bessel response). Data were secondarily filtered at 100 Hz through an 8-pole Bessel filter (950 TAF; Frequency Devices, Ottawa, IL, USA) and digitized at 1 kHz using an analog-to-digital converter (Digidata 1322A; Molecular Devices) controlled by pClamp9 software (Molecular Devices). Single-channel conductance events, open probabilities and other parameters were identified and analyzed using Clampfit9 software (Molecular Devices).
RESULTS
We have examined the effects of ATP on the human TRPV6 channels expressed in Xenopus oocytes in excised inside-out patches (Fig. 1). Monovalent currents were measured as described earlier (16) using a ramp protocol from −100 to +100 mV, and data are plotted at −100 and +100 mV. TRPV6 currents showed marked inward rectification, and channel activity gradually decreased after establishing the inside-out configuration, a phenomenon we refer to as rundown. For PtdIns(4,5)P2-sensitive channels, this phenomenon is thought to be mediated by the gradual loss of PtdIns(4,5)P2 from the patch membrane, presumably by dephosphorylation (28). Figure 1A shows that ATP, added to the superfusate, reactivated TRPV6 channels when provided together with Mg2+. On removal of MgATP, channel activity increased transiently at −100 mV, due to the removal of block by Mg2+, and then ran down again. Currents at +100 mV were completely blocked by Mg2+, consistent with earlier reports on the importance of Mg2+ for the inward rectification of these channels (29). MgATP was provided as 2 mM MgCl2 and 2 mM NaATP; the free Mg2+ concentration was calculated to be 300 μM by the Maxchelator program. The second application of MgATP stimulated TRPV6 activity; even though in some experiments it was less effective than the first application (see, for example, Fig. 2). ATP did not reactivate TRPV6 in the absence of Mg2+ (Fig. 1B), and in the same patches the subsequent application of ATP together with Mg2+ induced significant current activation, showing that the lack of activation by NaATP was not due to the unresponsiveness of the patch (Fig. 1B). MgATP activated the channels with relatively slow kinetics, suggesting an indirect effect. By comparison, the water soluble DiC8 analog of PtdIns(4,5)P2 activated TRPV6 essentially instantaneously, and the effect was quickly reversible (Fig. 1A, B), consistent with our earlier data (16). Figure 1D shows a representative trace in which the channels were reactivated by MgATP and then Mg2+ was washed out. On removal of Mg2+, channel activity ran down after a transient increase, despite the presence of ATP.
Figure 1.
ATP reactivates TRPV6 channels in excised patches in the presence of Mg2+. Excised inside-out macropatch measurements were performed as described in Materials and Methods, using a ramp protocol from −100 mV to +100 mV, 1×/s. Current (I) traces at −100 and +100 mV are shown. A, B) Representative traces showing the effects of ATP with and without Mg2+ on channel activity; dashed lines show zero current levels. Applications of 25 μM DiC8 PtdIns(4,5)P2, 2 mM MgATP (2 mM Mg2+ and 2 mM NaATP; A), and 2 mM NaATP (B) are shown by horizontal bars. Free Mg2+ concentration in the MgATP solution was estimated to be 300 μM by the Maxchelator program. Arrows indicate establishment of the inside-out (i/o) configuration. C) Summary of data at −100 mV. Current amplitudes for MgATP were measured immediately after washout of MgATP, when the Mg2+ block was relieved and then normalized to the amplitude of the current evoked by the first application of DiC8 PtdIns(4,5)P2. The effect of MgATP was statistically significant when compared with NaATP with a paired test. The effect of MgATP in patches pretreated with NaATP was also statistically significantly smaller than the effect of the second application of MgATP in control patches (P<0.005). ***P < 0.005. D) Representative trace of n = 6 measurements when NaATP was applied immediately after MgATP.
Figure 2.
ADP, GTP, and AMP-PCP do not reactivate TRPV6 in excised patches. A–D) Representative traces for various nucleotides as indicated; measurements were performed as described in Fig. 1. Concentrations of all nucleotides were 2 mM, applied together with 2 mM MgCl2. Free Mg2+ concentrations calculated by the Maxchelator program were 300 μM for MgATP, 878 μM for MgADP, and 316 μM for MgGTP. We could not find binding constants for AMP-PCP; given the quite similar overall structure to ATP, we assume the free Mg2+ concentration was comparable to that in the MgATP solutions. E) Summary data, normalized to the current evoked by PtdIns(4,5)P2. MgATP was applied after the nucleotide tested as a positive control in all experiments. Right columns show summary for experiments where control solution was applied instead of the nucleotides for the same time period, before the application of MgATP. *P < 0.05; ***P < 0.005.
Interestingly, even though MgATP clearly activated TRPV6 after NaATP (Fig. 1B), this effect was smaller than when MgATP was applied a second time after the washout of the first pulse of MgATP (Fig. 1A). We have not further investigated this difference, since it could be due to a number of different effects, for example, loss of lipid kinases from the patch induced by NaATP, and it cannot account for the reported activating effect of ATP on TRPV6.
We also examined the effects of several other nucleotides that were reported to affect TRPV6 activity in whole-cell patch-clamp experiments (26). Figure 2 shows that ADP, GTP, and the nonhydrolyzable ATP analog AMP-PCP (all of them 2 mM with 2 mM Mg2+) did not reactivate TRPV6 after current rundown. MgATP applied in the same patches after any of these compounds stimulated TRPV6 activity.
The Mg2+ dependence of ATP activation of currents suggests that the hydrolysis of ATP is necessary for its effect on TRPV6. As shown earlier (16) and in Figs. 1 and 2, PtdIns(4,5)P2 reproducibly reactivates TRPV6 in excised patches. PtdIns(4,5)P2 is generated by lipid kinases in two sequential phosphorylation steps from PtdIns and PtdIns(4)P. The first step is catalyzed by PI4Ks, whereas the second is by phosphatidylinositol 4-phosphate 5-kinases (PIP5Ks; ref. 30). None of these enzymes have specific inhibitors, but type III PI4Ks can be inhibited by several different PI3K inhibitors, when these drugs are used at high concentrations (31). In the next set of experiments, we tested the involvement of type III PI4Ks. Figure 3A, B shows that pretreatment with 35 μM wortmannin inhibited the effect of MgATP on TRPV6. When MgATP was applied for the second time after the removal of wortmannin, the effect of MgATP partially recovered. Wortmannin had no effect at 35 nM, where it selectively inhibits PI3Ks (Fig. 3C).
Wortmannin is known to be unstable in aqueous solutions and also light sensitive (30); thus, it is possible that the partial effect of this compound is due to its lack of stability. Another often used compound is LY294002, which is known to be more stable (32) and also inhibits type III PI4Ks at high concentrations, (100–300 μM; ref. 32). Figure 4A, B shows that 300 μM LY294002 almost completely eliminated the effect of MgATP, when compared with vehicle-treated patches. At 10 μM, where it selectively inhibits PI3Ks, LY294002 had no effect (Fig. 4C). When we applied MgATP for the second time after the removal of LY294002, we observed a significant current recovery (Fig. 4B, D). Neither wortmannin nor LY294002 significantly affected the subsequent responses to DiC8 PtdIns(4,5)P2 when compared with the first application (Figs. 3 and 4).
Figure 4.
Effects of LY294002 on the current recovery induced by MgATP. A–C) Representative traces, measurements were performed as described in Fig. 1. Applications of 2 mM MgATP, 300 μM LY294002, 10 μM LY294002, and 25 μM DiC8 PtdIns(4,5)P2 are shown by horizontal bars. D) Data summary, normalized to the current levels evoked by DiC8 PtdIns(4,5)P2. *P < 0.05; ***P < 0.005.
Another recently described dual PI3K and PI4K inhibitor is PIK93 (33). This compound has been reported to differentiate between the type IIIα and type IIIβ PI4K enzymes. The IC50 of PIK93 for type IIIβ PI4Ks was reported to be 19 nM, and it inhibits PI3Ks at similar concentrations (33). Type IIIα PI4Ks, on the other hand, require >10 μM to be inhibited by 50% (34). Figure 5 shows that high concentrations of PIK93 (30 and 300 μM) essentially eliminated the effect of MgATP on TRPV6; 300 nM caused a partial inhibition, whereas 30 nM had no effect. PIK93 had no significant effect on the current amplitude evoked by the subsequent PtdIns(4,5)P2 application at any of the concentrations tested. These data suggest that the major PI4K isoform involved in the effect of MgATP is the type IIIα (see also Discussion).
Figure 5.
Effects of PIK93 on the current recovery induced by MgATP. A–D) Representative traces, measurements were performed as described in Fig. 1. Applications of various concentrations of PIK93, 2 mM MgATP, and 25 μM DiC8 PtdIns(4,5)P2 are shown by horizontal bars. E) Data summary for different concentrations of PIK93, from 4 different experiments, normalized to the relative amplitude of the MgATP-induced current.
We also examined the effect of pretreating the patch membrane with a bacterial PI-PLC enzyme that selectively hydrolyzes phosphatidylinositol, the substrate for PI4Ks, the precursor for PtdIns(4)P (35). PI-PLC significantly inhibited the effect of MgATP, as expected if MgATP acts through PI4Ks, while subsequent responses to DiC8 PtdIns(4,5)P2 were not affected (Supplemental Fig. S1).
PI4Ks catalyze the formation of PtdIns(4)P from PtdIns. Is PtdIns(4)P the effector molecule on TRPV6, or does it need to be further phosphorylated by PIP5Ks to generate PtdIns(4,5)P2? Unfortunately, PIP5Ks do not have known inhibitors; thus, we cannot address this question with pharmacological tools. In our previous study (16), we showed that the synthetic DiC8 PtdIns(4,5)P2 but not DiC8 PtdIns(4)P activated TRPV6 in excised patches. Here we have performed additional experiments with natural long acyl-chain PtdIns(4,5)P2 and PtdIns(4)P, purified from porcine brain. Figure 6 shows that the natural PtdIns(4)P (10 μM) only induced minimal TRPV6 activity in excised patches after rundown, while the subsequent application of the same concentration of natural PtdIns(4,5)P2 induced a marked activation. The long acyl-chain natural PtdIns(4,5)P2 acted much slower than DiC8 PtdIns(4,5)P2, and its effect was essentially irreversible, consistent with findings on other ion channels (28). The minimal effect of PtdIns(4)P in excised patches is also compatible with our previously published results (16) showing that conversion of PtdIns(4,5)P2 into PtdIns(4)P by a rapamycin-induced translocation of a 5-phosphatase enzyme markedly inhibits TRPV6 activity in whole-cell patch-clamp experiments. We also found that dephosphorylating PtdIns(4,5)P2 at the 5-phosphate with a voltage-sensitive phosphatase CiVSP (36) inhibited TRPV6 activity in intact Xenopus oocytes (unpublished results).
Given the minimal effect of PtdIns(4)P, we used this compound to further support the idea that the inhibitors we used act through PI4Ks. PtdIns(4)P is the product of PI4Ks; thus, it should overcome the effect of the inhibition of this enzyme. As shown in Fig. 6C–E, we pretreated the patches with the natural long chain PtdIns(4)P after current rundown, and tested the effect of LY294002 on the MgATP-induced current recovery. As expected, MgATP induced a significant current recovery after PtdIns(4)P pretreatment, even in the presence of 300 μM LY294002, which in our previous experiments (Fig. 4) almost completely eliminated the effect of MgATP.
Our data so far establish that MgATP affects TRPV6 activity by providing substrate for lipid kinases to synthesize PtdIns(4,5)P2. Is PtdIns(4,5)P2 the final effector, or are there other factors downstream of PtdIns(4,5)P2? To answer this question, we have purified the full-length TRPV6 channel protein (Fig. 7) and reconstituted it into planar lipid bilayers. The purified TRPV6 protein derived in DDM micelles was incorporated into lipid micelles consisting of a mixture of POPC/POPE (3:1, v/v) and then into planar lipid bilayers of the same lipid composition between aqueous solutions of 150 mM KCl and 0.02 mM MgCl2 in 20 mM HEPES (pH 7.4). Similar to TRPM8 channels (37), the presence of Mg2+ in the experimental solution was required to sustain normal channel activity of TRPV6 with optimal concentration of 0.02 mM. Higher concentrations of Mg2+ (≥0.5 mM) evoked an inhibition of TRPV6 currents.
Figure 7.
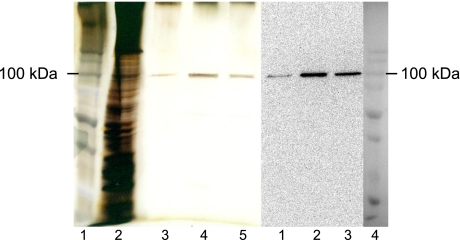
Purification of the full-length TRPV6 protein. TRPV6 protein was purified as described in Materials and Methods. Left panel: lane 1, molecular mass standards; lane 2, cell lysate silver stain; lanes 3–5, purified TRPV6 silver stain. Right panel: lanes 1–3, Western blot with anti-myc antibody, purified TRPV6; lane 4, molecular mass standards.
Figure 8 shows that no channel activity was observed when TRPV6 alone was incorporated in lipid bilayers (Fig. 8, n=26). Subsequent application of 5 μM of diC8 PtdIns(4,5)P2 or the long acyl-chain dipalmitoyl diC16 PtdIns(4,5)P2 resulted in full opening of the channels [P0=0.93±0.05 in the presence of diC8 PtdIns(4,5)P2, n=16, 16,684 events analyzed; P0=0.85±0.14 in the presence of diC16 PtdIns(4,5)P2, n=10, 6722 events analyzed; Fig. 8A). Polylysine is often used to sequester phosphoinositides in excised inside-out patches and inhibit PtdIns(4,5)P2-sensitive ion channels (28). We found that in planar lipid bilayers application of poly-lysine (3 μg/ml) to the cytoplasmic side of the channel completely abolished TRPV6 activity, while no effect was observed with application to the external side of TRPV6 (Fig. 8A, B; n=6).
Figure 8.
PtdIns(4,5)P2 supports TRPV6 channel activity in planar lipid bilayers. A) Representative single-channel current recordings of TRPV6 channels incorporated in planar lipid bilayers. Experiments were performed as described in Materials and Methods; clamping potential was −100 mV. Top and bottom traces consist of 3 segments with additions of components as indicated by horizontal bars: 5 μM of diC8 PtdIns(4,5)P2 (top trace), diC16 PtdIns(4,5)P2 (bottom trace), or 3 μg/ml polylysine. Dash at right indicates closed state of the channel. B) Summary of inhibition of TRPV6 channel activity with polylysine (3 μg/ml); open probability of TRPV6 is plotted. Data were obtained at −100 mV; n = 6. C) Current/voltage (I/V) relationship of TRPV6: channels were incorporated in planar lipid bilayers in the presence of 5 μM diC8 PI(4,5)P2. D) Open probability of TRPV6 channels operating in inward and outward directions measured at −100 mV and +100 mV. Data were analyzed from a total of 16 experiments.
TRPV6 channels are inward rectifiers when expressed in cells. We found that TRPV6 demonstrated different open probability and conductance for current flowing in outward and inward directions in planar lipid bilayers, characteristic of TRPV6 inward rectification. Single-channel current-voltage relationships and open probabilities are presented in Fig. 8C, D. Channels were obtained in the presence of 5 μM diC8 PtdIns(4,5)P2. Inward currents exhibited mean slope conductance values of 51.2 ± 8 pS and P0 of ∼0.93 at −100 mV (n=16; 9362 events analyzed), and outward currents were observed with a main conductance level of 30.4 ± 3 pS and P0 of ∼0.277 (at 100 mV; n=16; 2401 events analyzed). The observed value of the mean conductance of the inward current (51 pS at −100 mV, 22°C) is similar to that previously reported (38). We also detected smaller conductance states that have been observed randomly during protein incorporation into the bilayers. These conductance states might represent intermediate folding states of the protein. The orientation of the channels incorporated in the lipid bilayers was determined by inward rectification inherent to TRPV6.
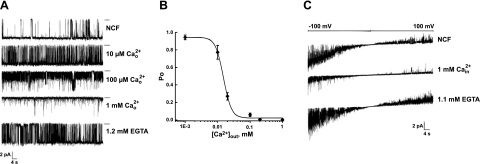
TRPV6 is a Ca2+-selective ion channel, but it conducts monovalent cations in the absence of calcium, and monovalent currents are inhibited by Ca2+ (39). Figure 9 shows that both extracellular and intracellular Ca2+ inhibits monovalent currents through TRPV6 channels in planar lipid bilayers. Channels were obtained in the presence of 10 μM diC8 PtdIns(4,5)P2 in the nominally Ca2+-free solution. Addition of Ca2+ to the outside of TRPV6 resulted in concentration-dependent inhibition of channel activity (Fig. 9A, B). Inhibition was also observed when Ca2+ was applied to the internal side of TRPV6 (Fig. 9C). In both cases, the blockage was reversible, and channel activity was restored after the application of excess EGTA (Fig. 9A, C). Overall, 9 experiments were conducted, and open probability values obtained for each Ca2+ concentration were derived from the analysis of 19,556 events.
Figure 9.
Ca2+ block of TRPV6 in planar lipid bilayers. Representative current traces obtained in the presence of 10 μM of diC8 PtdIns(4,5)P2. A) Top trace was obtained in nominally Ca2+-free (NCF) solution. Additional concentrations of Ca2+ as indicated were subsequently added to the external side of the channel. B) Concentration response relationship of the effect of external Ca2+ at −100 mV on TRPV6 currents. C) Block of TRPV6 activity by internal Ca2+. Representative current traces of TRPV6 obtained during a −100- to +100-mV ramp protocol recording.
Figure 6 shows that TRPV6 has a marked selectivity for PtdIns(4,5)P2 over PtdIns(4)P (16). We asked whether this selectivity is retained in the purified reconstituted TRPV6. Figure 10 shows that 25 μM DiC8 PtdIns(4)P and 25 μM DiC16 PtdIns(4)P induced only minimal channel activity (n=3/form), even if channels were kept in the presence of PtdIns(4)P isoforms for several hours. Subsequent application of 5 μM DiC8 or DiC16 PtdIns(4,5)P2 resulted in activation of TRPV6 to a fully opened state.
Figure 10.
PtdIns(4,5)P2 but not PtdIns(4)P supports TRPV6 activity in planar lipid bilayers. A) TRPV6 channels were incorporated into planar lipid bilayers. Only minimal channel activity was observed in the presence of 25 μM of DiC8 or DiC16 chain PtdIns(4)P. Subsequent addition of either form of PtdIns(4,5)P2 (5 μM) fully activated TRPV6 channels. B) Summary of open probability values of TRPV6 channels obtained in the presence of the various phosphoinositides.
DISCUSSION
The present study addresses the regulation of TRPV6 by intracellular nucleotides and phosphoinositides. We found that TRPV6 currents in excised inside-out macropatches run down completely in a bath solution devoid of MgATP. MgATP restored TRPV6 activity, but ATP in the absence of Mg2+ had no effect. Both protein kinases and lipid kinases require Mg2+ to utilize ATP (40). Thus, these data suggest that a phosphoryl transfer reaction is involved in the effect of ATP.
PtdIns(4,5)P2 is required for the activity of many different ion channels, including TRPV6. This lipid is generated by the sequential phosphorylation of PtdIns into PtdIns(4)P by PI4Ks and the further phosphorylation of PtdIns(4)P by PIP5Ks (31). While none of these enzymes have any specific inhibitors, type III PI4K enzymes can be inhibited by several PI3K inhibitors when these drugs are used at high concentrations. We have used 3 of these compounds in the current study, wortmannin, LY294002, and PIK93. All 3 compounds inhibited the effect of MgATP at concentrations where they inhibit PI4Ks. We also show that pretreating the patch with PtdIns(4)P, the product of PI4K, alleviates the effect of LY294002. Furthermore, pretreatment of the patches with a phosphatidylinositol-specific PI-PLC enzyme significantly inhibited the effect of MgATP. These data strongly suggest that the effect of MgATP is mediated by generation of PtdIns(4,5)P2 by lipid kinases. These observations are consistent with the ∼90% inhibition of whole-cell TRPV6 currents by 10 μM wortmannin observed earlier (16).
Our data imply that the excised patches must contain 4 different types of functional phosphoinositide metabolizing enzymes: 4-phosphatases, 5-phosphatases, PI4Ks, and PIP5Ks, even several minutes after patch excision. This is quite remarkable, considering that none of these enzymes are transmembrane proteins. In fact, some of our data are compatible with the gradual loss of these enzymes after excision. In some experiments, the effect of MgATP decreased with time, with the second application being less effective than the first (see for example, Fig. 2A, E), which is compatible with the loss of lipid kinases. Current rundown also tended to be slower after the second application of MgATP in some, but not all experiments, which could be compatible with the loss of phosphatase activity (data not shown).
It is noteworthy that both LY294002 and PIK93 almost completely eliminated the effect of MgATP. Wortmannin had a partial effect, but this drug is known to be unstable in aqueous solutions and is also known to be light sensitive (30). Even though we made efforts to minimize light exposure, these experiments were not performed in the dark, and wortmannin had to be present in the perfusion system for several hours. Thus incomplete inhibition of the MgATP effect may be due to the chemical instability of wortmannin. The concentrations of PIK93 and LY294002 that eliminated the effect of MgATP inhibit type III but not type II PI4Ks (31, 34). Type III PI4K enzymes are mainly cytoplasmic and show only minimal plasma membrane localization (31). Type II PI4Ks, on the other hand, are tightly membrane bound, and a significant fraction are found in the plasma membrane, but they are insensitive to the drugs we used in this study (31). Thus, our data suggest that, despite their more abundant membrane localization, type II PI4K enzymes play no role in supplying PtdIns(4,5)P2 to TRPV6 channels in our system. Our data, on the other hand, suggest that enough of the type III PI4K enzymes must be associated with the plasma membrane in a stable manner to be able to supply PtdIns(4)P to PIP5Ks to synthesize PtdIns(4,5)P2 and support TRPV6 activity. The complete dependence of TRPV6 on type III PI4Ks is also compatible with the almost exclusive role of these enzymes in supplying the PtdIns(4,5)P2 pool that is sensitive to PLC-coupled agonists (41).
In addition to being selective for type III over type II PI4Ks, PIK93 also preferentially inhibits the type IIIβ over the type IIIα enzyme (34). The IC50 of PIK93 for type IIIβ PI4Ks was reported to be 19 nM, and it inhibits PI3Ks at similar concentration (33). Type IIIα PI4Ks, on the other hand, require >10 μM to be inhibited by 50% (34), and type II enzymes are thought to be insensitive to this compound (42). Overall, our data show that type IIIα enzymes are likely to be the dominant enzymes conveying the effect of MgATP, since 30 nM PIK93, which already substantially inhibits type IIIβ enzymes, had no effect. On the other hand, the partial inhibition by 300 nM suggests that type IIIβ enzymes may also play some role. However, it is hard to draw a definitive conclusion on the involvement of type IIIβ enzymes, since there are no data available on the pharmacological sensitivity of Xenopus enzymes. Nevertheless, PI4K enzymes are highly conserved, and the sequences of the catalytic domains of the Xenopus enzymes are almost identical to those of mammalian isoforms (42).
Several other nucleotides, including ADP, GTP, and the nonhydrolyzable ATP analog AMP-PCP, have been reported to be effective in preventing TRPV6 current rundown in whole-cell patch-clamp experiments (26). We found that MgADP, MgGTP, and AMP-PCP (all with Mg2+) did not support TRPV6 activity in excised patches. How can we explain this discrepancy? In whole-cell patch-clamp experiments, most small intracellular molecules, including nucleotides, are thought to be dialyzed out of the cell through the patch pipette. Thus with time, intracellular ATP concentrations will substantially decrease without ATP in the pipette. Since ATP is buffered inside the cell, this washout is slow, and ATP concentrations most likely do not decrease to zero, because protein kinases that have high affinity for ATP (43) may function in whole-cell patch-clamp experiments, even if ATP is not provided in the pipette solution (44). The drop in ATP under these circumstances is likely to be enough to limit the function of type III PI4Ks, which have low affinity for ATP (31), and based on our experiments, they are very important in supplying PtdIns(4,5)P2 to maintain TRPV6 activity. When ADP or AMP is supplied, it is possible that the cellular metabolic machinery can phosphorylate these nucleotides to create ATP, thereby explaining the ability of these nucleotides to prevent TRPV6 rundown in whole-cell patch-clamp experiments (26). These data so far are compatible with ours, since nucleotides in the above study were used together with Mg2+ in the whole-cell experiments.
It is more difficult to reconcile our data with the finding that GTP and UTP were also active in the whole-cell experiments, even though to a lesser extent than adenosine nucleotides (26). Theoretically, these nucleotides can provide a high energy phosphate to generate ATP, but it presumes some source of adenosine nucleotides, which are likely to diffuse out of the cell through the patch pipette. It is also hard to reconcile our data with the effectiveness of AMP-PCP in whole-cell experiments, as well as the lack of effect of supplying diC8 PtdIns(4,5)P2 through the patch pipette (26).
Intracellular ATP modulates many ion channels via various mechanisms (43) and has also been shown to bind to and possibly regulate several TRP channels other than TRPV6. TRPV1 has been shown to be potentiated by ATP in excised patches, both in the presence and absence of Mg2+. This effect was inhibited by mutation in either an N-terminal (D178N) or a C-terminal (K735R) Walker motif (45). Accordingly, another study demonstrated that the C terminus of TRPV1 binds ATP and found that the K735 residue is involved in this binding (46). More recent work (47) demonstrated that ATP binds to the N-terminal ARDs of TRPV1 and ATP included in the whole-cell patch pipette inhibited capsaicin-induced desensitization of TRPV1. The ARDs of TRPV3 and TRPV4, but not those of TRPV2, TRPV5, or TRPV6, have also been shown to bind to ATP agarose (48). Intracellular ATP provided through the whole cell patch pipette inhibited sensitization of TRPV3 currents but had no effect on TRPV2 (48). The reported lack of ATP binding to the ARD of TRPV6 (48, 49) is at odds with the findings of Al-Ansary et al. (26) who demonstrated ATP binding to this domain.
In contrast to the lack of effect of ATP in the absence of Mg2+ in excised patches, we observed a robust and highly reproducible effect of PtdIns(4,5)P2. PtdIns(4,5)P2 is a common regulator of many, if not the majority, of mammalian ion channels (11–13), including TRP channels (15). This observation is compatible with the idea that the lipid activates these channels directly. Even excised patches, however, contain many proteins in addition to the ion channel being studied (50). Thus we cannot exclude based on these data that the effect of PtdIns(4,5)P2 is mediated by another PtdIns(4,5)P2 binding protein in the patch membrane or in the associated cytoskeleton. To address this question, we purified the TRPV6 protein, incorporated it into planar lipid bilayers, and demonstrated that the activity of the reconstituted channel depends on the presence of PtdIns(4,5)P2. These data firmly establish that PtdIns(4,5)P2 is a direct activator of TRPV6. Our data add TRPV6 to the list of ion channels recently demonstrated to be activated by PtdIns(4,5)P2 in reconstituted systems (37, 51–53).
In summary, our data do not support a simple model in which binding of ATP is necessary and sufficient to activate TRPV6 channel opening. We cannot exclude direct ATP binding, which was demonstrated with biochemical techniques (26), as a modulator of TRPV6 activity, but such an effect would have to depend on the presence of cellular components that are lost on patch excision. On the other hand, our data show that PtdIns(4,5)P2 is both necessary and sufficient to support channel activity, and its effect is preserved even when the purified protein is incorporated into a lipid bilayer. Thus our data firmly establish this lipid as a direct regulator of TRPV6.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants NS-055159 and GM-093290 and by a grant from the University of Medicine and Dentistry of New Jersey Foundation (to T.R.) and by a Scientist Development grant from the American Heart Association (to E.Z). The authors thank Dr. Tamas Balla for insightful discussions and comments on the manuscript and Dr. Joshua Berlin for careful reading of and commenting on the manuscript. The clone for the human TRPV6 was generously provided by Dr. T. V. McDonald (Albert Einstein College of Medicine, New York, NY, USA).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Wu L. J., Sweet T. B., Clapham D. E. (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nilius B., Owsianik G., Voets T., Peters J. A. (2007) Transient receptor potential channels in disease. Physiol. Rev. 87, 165–217 [DOI] [PubMed] [Google Scholar]
- 3. Nilius B., Owsianik G. (2010) Transient receptor potential channelopathies. Pflügers Arch. 460, 437–450 [DOI] [PubMed] [Google Scholar]
- 4. Hoenderop J. G., Nilius B., Bindels R. J. (2005) Calcium absorption across epithelia. Physiol. Rev. 85, 373–422 [DOI] [PubMed] [Google Scholar]
- 5. Hoenderop J. G., van Leeuwen J. P., van der Eerden B. C., Kersten F. F., van der Kemp A. W., Merillat A. M., Waarsing J. H., Rossier B. C., Vallon V., Hummler E., Bindels R. J. (2003) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianco S. D., Peng J. B., Takanaga H., Suzuki Y., Crescenzi A., Kos C. H., Zhuang L., Freeman M. R., Gouveia C. H., Wu J., Luo H., Mauro T., Brown E. M., Hediger M. A. (2006) Marked disturbance of calcium homeostasis in mice with targeted disruption of the trpv6 calcium channel gene. J. Bone Miner. Res. 22, 274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akey J. M., Swanson W. J., Madeoy J., Eberle M., Shriver M. D. (2006) TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum. Mol. Genet. 15, 2106–2113 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki Y., Pasch A., Bonny O., Mohaupt M. G., Hediger M. A., Frey F. J. (2008) Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum. Mol. Genet. 17, 1613–1618 [DOI] [PubMed] [Google Scholar]
- 9. Hughes D. A., Tang K., Strotmann R., Schoneberg T., Prenen J., Nilius B., Stoneking M. (2008) Parallel selection on TRPV6 in human populations. PLoS ONE 3, e1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clapham D. E. (2007) Calcium signaling. Cell 131, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 11. Hilgemann D. W., Feng S., Nasuhoglu C. (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, RE19 [DOI] [PubMed] [Google Scholar]
- 12. Suh B. C., Hille B. (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu. Rev. Biophys. 37, 175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Logothetis D. E., Nilius B. (2007) Dynamic changes in phosphoinositide levels control ion channel activity. Pflügers Arch. 455, 1–3 [DOI] [PubMed] [Google Scholar]
- 14. Nilius B., Owsianik G., Voets T. (2008) Transient receptor potential channels meet phosphoinositides. EMBO J. 27, 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rohacs T. (2009) Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium 45, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thyagarajan B., Lukacs V., Rohacs T. (2008) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium induced inactivation of TRPV6 channels. J. Biol. Chem. 283, 14980–14987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thyagarajan B., Benn B. S., Christakos S., Rohacs T. (2009) Phospholipase C mediated regulation of TRPV6 channels: implications in active intestinal Ca2+ transport. Mol. Pharmacol. 75, 608–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohacs T., Lopes C. M. B., Michailidis I., Logothetis D. E. (2005) PI(4,5)2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 [DOI] [PubMed] [Google Scholar]
- 19. Daniels R. L., Takashima Y., McKemy D. D. (2008) The activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol-4,5-bisphosphate. J. Biol. Chem. 284, 1570–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B., Zhang C., Qin F. (2005) Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 4835–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lukacs V., Thyagarajan B., Balla A., Varnai P., Balla T., Rohacs T. (2007) Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 27, 7070–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao J., Qin F. (2009) Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS Biol. 7, e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mercado J., Gordon-Shaag A., Zagotta W. N., Gordon S. E. (2010) Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 30, 13338–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niemeyer B. A., Bergs C., Wissenbach U., Flockerzi V., Trost C. (2001) Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc. Natl. Acad. Sci. U. S. A. 98, 3600–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derler I., Hofbauer M., Kahr H., Fritsch R., Muik M., Kepplinger K., Hack M. E., Moritz S., Schindl R., Groschner K., Romanin C. (2006) Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. J. Physiol. 577, 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al Ansary D., Bogeski I., Disteldorf B. M., Becherer U., Niemeyer B. A. (2010) ATP modulates Ca2+ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J. 24, 425–435 [DOI] [PubMed] [Google Scholar]
- 27. Toth B., Csanady L. (2010) Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J. Biol. Chem. 285, 30091–30102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rohacs T., Lopes C., Mirshahi T., Jin T., Zhang H., Logothetis D. E. (2002) Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Methods Enzymol. 345, 71–92 [DOI] [PubMed] [Google Scholar]
- 29. Voets T., Janssens A., Prenen J., Droogmans G., Nilius B. (2003) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J. Gen. Physiol. 121, 245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balla T. (2001) Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr. Pharm. Des. 7, 475–507 [DOI] [PubMed] [Google Scholar]
- 31. Balla A., Balla T. (2006) Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 16, 351–361 [DOI] [PubMed] [Google Scholar]
- 32. Korzeniowski M. K., Popovic M. A., Szentpetery Z., Varnai P., Stojilkovic S. S., Balla T. (2009) Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J. Biol. Chem. 284, 21027–21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toth B., Balla A., Ma H., Knight Z. A., Shokat K. M., Balla T. (2006) Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 281, 36369–36377 [DOI] [PubMed] [Google Scholar]
- 35. Hilgemann D. W., Ball R. (1996) Regulation of cardiac Na+/Ca2+ exchange and KATP potassium channels by PIP2. Science 273, 956–959 [DOI] [PubMed] [Google Scholar]
- 36. Murata Y., Okamura Y. (2007) Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J. Physiol. 583, 875–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zakharian E., Thyagarajan B., French R. J., Pavlov E., Rohacs T. (2009) Inorganic polyphosphate modulates TRPM8 channels. PLoS ONE 4, e5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yue L., Peng J. B., Hediger M. A., Clapham D. E. (2001) CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410, 705–709 [DOI] [PubMed] [Google Scholar]
- 39. Hoenderop J. G., Vennekens R., Muller D., Prenen J., Droogmans G., Bindels R. J., Nilius B. (2001) Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J. Physiol. 537, 747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Rourke B. (1993) Ion channels as sensors of cellular energy. Mechanisms for modulation by magnesium and nucleotides. Biochem. Pharmacol. 46, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 41. Nakanishi S., Catt K. J., Balla T. (1995) A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. U. S. A. 92, 5317–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balla A., Tuymetova G., Toth B., Szentpetery Z., Zhao X., Knight Z. A., Shokat K., Steinbach P. J., Balla T. (2008) Design of drug-resistant alleles of type-III phosphatidylinositol 4-kinases using mutagenesis and molecular modeling. Biochemistry 47, 1599–1607 [DOI] [PubMed] [Google Scholar]
- 43. Hilgemann D. W. (1997) Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu. Rev. Physiol. 59, 193–220 [DOI] [PubMed] [Google Scholar]
- 44. Tominaga M., Wada M., Masu M. (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. U. S. A. 98, 6951–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwak J., Wang M. H., Hwang S. W., Kim T. Y., Lee S. Y., Oh U. (2000) Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J. Neurosci. 20, 8298–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grycova L., Lansky Z., Friedlova E., Vlachova V., Kubala M., Obsilova V., Obsil T., Teisinger J. (2007) ATP binding site on the C-terminus of the vanilloid receptor. Arch. Biochem. Biophys. 465, 389–398 [DOI] [PubMed] [Google Scholar]
- 47. Lishko P. V., Procko E., Jin X., Phelps C. B., Gaudet R. (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54, 905–918 [DOI] [PubMed] [Google Scholar]
- 48. Phelps C. B., Wang R. R., Choo S. S., Gaudet R. (2010) Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J. Biol. Chem. 285, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phelps C. B., Huang R. J., Lishko P. V., Wang R. R., Gaudet R. (2008) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47, 2476–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suchyna T. M., Markin V. S., Sachs F. (2009) Biophysics and structure of the patch and the gigaseal. Biophys. J. 97, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zakharian E., Cao C., Rohacs T. (2010) gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D'Avanzo N., Cheng W. W., Doyle D. A., Nichols C. G. (2010) Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 285, 37129–37132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leal-Pinto E., Gomez-Llorente Y., Sundaram S., Tang Q. Y., Ivanova-Nikolova T., Mahajan R., Baki L., Zhang Z., Chavez J., Ubarretxena-Belandia I., Logothetis D. E. (2010) Gating of a G protein-sensitive mammalian Kir3.1 prokaryotic Kir channel chimera in planar lipid bilayers. J. Biol. Chem. 285, 39790–39800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.