Abstract
Background:
Patients with interstitial lung disease (ILD) may have features of an autoimmune disorder that do not meet the diagnostic criteria for connective tissue diseases. We determined the prevalence and characteristics of autoimmune-featured ILD (AIF-ILD) and compared these with those of idiopathic pulmonary fibrosis (IPF) and known connective tissue disease-related ILD (CTD-ILD).
Methods:
Patients with ILD who did not meet the criteria for a connective tissue disease were defined as having AIF-ILD if they had a sign or symptom suggestive of a connective tissue disease and a serologic test reflective of an autoimmune process. Clinical characteristics, high-resolution CT images, and lung biopsy specimens were analyzed and compared with those of patients with IPF and CTD-ILD. Survival was evaluated using a Kaplan-Meier curve.
Results:
Two hundred subjects completed the questionnaire and serologic testing. AIF-ILD was identified in 32%, IPF in 29%, and CTD-ILD in 19%. Gender, age, and race differed among groups (P < .01). Sixty-two percent of patients with AIF-ILD had a typical usual interstitial pneumonia (UIP) pattern on CT images. In 31 patients with AIF-ILD, lung biopsy specimens showed UIP in 81% and nonspecific interstitial pneumonia in 6%. Patients with AIF-ILD and IPF had similar survival, worse than those with CTD-ILD (P < .01). Antinuclear antibody (ANA) titers ≥ 1:1280 were associated with improved survival in patients with AIF-ILD (P = .02).
Conclusions:
Systematic evaluation of symptoms and serologic tests in ILD can identify AIF-ILD. A UIP pattern on CT images and histopathology is common in AIF-ILD. Although survival for patients with AIF-ILD is poor, ANA titers ≥ 1:1280 are associated with improved survival.
Interstitial lung disease (ILD) includes a heterogeneous group of disorders that result in diffuse parenchymal lung disease, with overlapping clinical, radiographic, and physiologic manifestations. Several rheumatologic conditions are associated with the development of ILD.1‐3 For patients with ILD, current guidelines recommend an evaluation for underlying connective tissue diseases.4 This evaluation will yield a subset of ILD patients with symptoms and autoantibodies suggestive of an autoimmune condition, who do not fulfill American College of Rheumatology (ACR) criteria for a connective tissue disease. Currently, these patients are considered to have an idiopathic interstitial pneumonia, although it has been suggested that these patients may have an undifferentiated connective tissue disease (UCTD).5 However, UCTD as described in the rheumatology literature is mild in nature6‐8 and has a low prevalence of ILD (1%).8 With these considerations, we propose a new term to describe a subset of pulmonary ILD patients with features of a connective tissue disease that do not meet ACR criteria: autoimmune-featured ILD (AIF-ILD).
We hypothesize that AIF-ILD may represent a distinct subgroup of patients with ILD with unique characteristics that differ from both idiopathic pulmonary fibrosis (IPF) and connective tissue disease-associated ILD (CTD-ILD). Patients with ILD who did not meet ACR criteria for a connective tissue disease were defined as having AIF-ILD if they had a sign or symptom suggestive of a connective tissue disease as well as a serologic test reflective of an autoimmune process. Our objective was to describe the prevalence, clinical characteristics, radiographic patterns, pathologic findings, and survival of patients with ILD and AIF-ILD. We performed a systematic analysis of symptoms and serologic tests in patients with ILD to identify cases of AIF-ILD. We reviewed the radiographic and histopathologic data to determine the prevalence of usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) in patients with AIF-ILD, and compared their clinical characteristics and survival with those patients with IPF and CTD-ILD.
Materials and Methods
Study Subjects
Patients referred to the ILD clinic at the University of Chicago were invited to participate in a prospective study designed to describe the clinical characteristics and disease progression of ILD patients. The study was approved by the institutional review board at the University of Chicago, and all enrolled patients provided informed consent (No. 14163A). Data from patients enrolled from September 2005 to September 2008 were available for analysis. The institutional review board approved the review of these patients’ medical records (No. 16555B).
As part of their initial evaluation, patients received a symptoms questionnaire and underwent comprehensive serologic testing as screening for connective tissue diseases, regardless of referring diagnosis. Patients who completed the questionnaire and had comprehensive serologic testing were included. Subjects with environmental exposures and other known causes of ILD were excluded. ILD patients who did not meet ACR criteria for a connective tissue disease were defined as having AIF-ILD if they had at least one sign or symptom suggestive of a connective tissue disease and at least one serologic test reflective of an autoimmune process, as listed in Table 1.5,7 Serologic tests were considered abnormal if the results were above the reference value, except for antinuclear antibody (ANA), for which titers ≥ 1:160 were considered positive. Study subjects who met ACR criteria for connective tissue diseases or ATS criteria for IPF served as comparator groups.9‐15
Table 1.
—Diagnostic Criteria for Autoimmune-Featured Interstitial Lung Disease
| Symptoms (One or More of the Following) | Serologic Test (One or More Positive Result of the Following) |
| Dry eyes/dry mouth | Antinuclear antibody titer ≥ 1:160 |
| Gastroesophageal reflux | Rheumatoid factor |
| Weight loss | Aldolase |
| Leg/foot swelling | Anti-Ro antibody |
| Joint pain/swelling | Anti-La antibody |
| Rash | Anti-neutrophil cytoplasmic antibody |
| Photosensitivity | Creatine kinase |
| Dysphagia | Anti-double-stranded DNA |
| Hand ulcers | Anti-Scl-70 |
| Mouth ulcers | Anti-ribonucleoprotein antibody |
| Raynaud phenomenon | Anti-Smith antibody |
| Morning stiffnessProximal muscle weakness | Anti-cyclic citrullinated peptide antibody |
| Anti-Jo-1 antibody |
Pulmonary Function Testing
Subjects underwent pulmonary function testing at their physician’s discretion. Spirometry, diffusion capacity of the lung for carbon monoxide, and lung volumes by plethysmography were obtained per ATS guidelines.16‐18
Radiographic Review
All high-resolution CT (HRCT) scans were reviewed prospectively by a senior chest radiologist (Heber MacMahon, MD, and Steven Montner, MD) without knowledge of this study. If a subpleural reticular pattern with honeycombing was the predominant finding, the HRCT scan was categorized as “typical” for UIP. If the HRCT scan lacked honeycombing and/or displayed predominantly ground glass opacities, the HRCT scan was categorized as “atypical” for UIP. In cases in which an individual had multiple HRCT scans and at least one displayed the typical UIP pattern and at least one displayed the atypical pattern, the subject was categorized as having “both.” Characterization was performed by pulmonologists with expertise in ILD (I. N., M. E. S.) as a part of clinical care.
Pathologic Review
Only surgical lung biopsies and explanted lungs were included in this analysis. All biopsy specimens were reviewed by an expert pulmonary pathologist (Aliya Husain, MD) with advanced training in the evaluation of ILD. The histopathologic patterns were classified according to American Thoracic Society (ATS)/European Respiratory Society guidelines.10 All specimens were evaluated prospectively as a part of clinical care without knowledge of this study.
Statistical Analysis
Patient characteristics, clinical symptoms, and serologic test results were reported as mean ± SD or as frequency counts and percentages. Pulmonary function tests were expressed as the percent predicted value for a given patient. The prevalence of clinical/serologic findings and radiographic patterns among the three groups was compared using Fisher exact tests. Demographic characteristics were analyzed by Fisher exact test or analysis of variance. Survivorship was obtained from medical records, telephone interviews, and the social security death index database. Survival was evaluated using a Kaplan-Meier curve and compared across groups using log-rank tests and Cox proportional hazards regression models. All data were analyzed using Stata, version 11 (Stata Corp; College Station, Texas).
Results
Study Population
Two hundred patients completed the symptoms questionnaire and had serologic testing and composed our study group of interest. Forty-two patients had known causes of ILD and were excluded. Sixty-three subjects met the criteria for AIF-ILD. Fifty-eight patients had IPF, and 37 subjects had CTD-ILD. In this study, the prevalence of AIF-ILD was 63 in 200 patients (32%), IPF 58 in 200 (29%), and CTD-ILD 37 in 200 (19%).
Demographics of AIF-ILD, IPF, and CTD-ILD Groups
Demographic and clinical characteristics of study subjects are shown in Table 2. The percentage of male subjects was 58.7% in the AIF-ILD group, 74.1% in IPF, and 24.3% in CTD-ILD (P < .01). The mean age of AIF-ILD subjects at referral to the ILD clinic was 66.0 ± 10.0 years, similar to patients with IPF and older than those with CTD-ILD (P < .01). There were no significant differences in lung function among these three groups (Table 2).
Table 2.
—Demographic and Clinical Characteristics of Study Subjects
| Variable | AIF-ILD (n = 63) | IPF (n = 58) | CTD-ILD (n = 37) | P Value |
| Sex | < .01a | |||
| Male | 37 (58.7) | 43 (74.1) | 9 (24.3) | |
| Female | 26 (41.3) | 15 (25.9) | 28 (75.7) | |
| Race | < .01 | |||
| White | 50 (79.4) | 51 (87.9) | 21 (56.8) | |
| Black | 6 (9.5) | 4 (6.9) | 15 (40.5) | |
| Hispanic/Asian/unknown | 7 (11.1) | 3 (5.2) | 1 (2.7) | |
| Mean age at referral, y | 66.0 ± 10.0 | 69.0 ± 8.2 | 54.0 ± 14.6 | < .01 |
| PFTs | ||||
| Mean FVC (% predicted) | 64.2 ± 19.1 | 68.0 ± 15.5 | 63.4 ± 21.4 | .40 |
| Mean Dlco (% predicted) | 46.8 ± 18.2 | 49.2 ± 15.7 | 52.4 ± 19.7 | .31 |
Data are presented as No. (%) or mean ± SD. Three subjects with AIF-ILD did not have PFTs at UCMC, and an additional eight could not perform Dlco; one subject with IPF did not have PFTs, and an additional three could not perform Dlco; one subject with CTD-ILD did not have PFTs, and an additional three could not perform Dlco. AIF-ILD = autoimmune-featured interstitial lung disease; CTD-ILD = connective tissue disease-associated interstitial lung disease; Dlco = diffusion capacity of the lung for carbon monoxide; IPF = idiopathic pulmonary fibrosis; PFT = pulmonary function test.
Characteristic was also statistically significant when comparing AIF-ILD with IPF subjects.
Clinical Symptoms and Serologic Tests
Symptoms reported by study subjects are shown in Table 3. The most common clinical symptoms in the AIF-ILD population were dry eyes/dry mouth (57%) and gastroesophageal reflux disease (44%). Forty-seven AIF-ILD (75%), 13 IPF (22%), and 31 CTD-ILD (84%) subjects reported multiple symptoms.
Table 3.
—Symptoms Endorsed by Study Subjects
| Symptoms | AIF-ILD (n = 63) | IPF (n = 58) | CTD-ILD (n = 37) | P Value |
| Dry eyes/dry mouth | 36 (57.1) | 9 (15.5) | 23 (62.2) | < .01a |
| GERD | 28 (44.4) | 10 (17.2) | 19 (51.4) | < .01a |
| Leg/foot swelling | 23 (36.5) | 4 (6.9) | 10 (27.0) | < .01a |
| Weight loss | 23 (36.5) | 6 (10.3) | 7 (18.9) | < .01a |
| Joint pain/swelling | 17 (27.0) | 5 (8.6) | 23 (62.2) | < .01a |
| Rash | 6 (9.5) | 3 (5.2) | 10 (27.0) | < .01 |
| Raynaud phenomenon | 6 (9.5) | 0 (0) | 19 (51.4) | < .01a |
| Sensitivity to light | 6 (9.5) | 2 (3.4) | 6 (16.2) | .10 |
| Dysphagia | 6 (9.5) | 2 (3.4) | 6 (16.2) | .10 |
| Hand ulcers | 1 (1.6) | 0 (0) | 5 (13.5) | < .01 |
| Mouth ulcers | 1 (1.6) | 1 (1.7) | 3 (8.1) | .24 |
| Morning stiffness | 1 (1.6) | 0 (0) | 5 (13.5) | < .01 |
| Proximal muscle weakness | 0 (0) | 0 (0) | 4 (10.8) | < .01 |
Data are presented as No. (%). Study subjects could endorse multiple symptoms. GERD = gastroesophageal reflux disease. See Table 2 for expansion of other abbreviations.
Symptom was statistically significant when comparing subjects with AIF-ILD to subjects with IPF.
Serologic test results are shown in Table 4. Almost all patients with AIF-ILD had a positive ANA, and 24 (40%) had an ANA titer ≥ 1:320. The distribution of ANA immunofluorescence patterns in subjects with AIF-ILD was 34 speckled, 13 homogeneous, seven speckled and homogeneous, two speckled and nucleolar, one nucleolar, and one speckled and centromere pattern. The distribution of ANA patterns in subjects with IPF was 12 speckled, 10 homogeneous, one speckled and homogeneous, and one centromere. There was no significant difference in ANA patterns between subjects with AIF-ILD and subjects with IPF.
Table 4.
—Serologic Test Results in Study Subjects
| Serologic Test | AIF-ILD (n = 63) | IPF(n = 58) | CTD-ILD (n = 37) | P Value |
| ANA | 58 (92.1) | 24 (41.4) | 33 (89.2) | < .01a |
| RF | 18 (28.6) | 4 (6.9) | 8 (21.6) | < .01a |
| Aldolase | 10 (15.9) | 4 (6.9) | 2 (5.4) | .19 |
| Anti-SSA | 4 (6.3) | 3 (5.2) | 7 (18.9) | .08 |
| ANCA | 3 (4.8) | 1 (1.7) | 0 (0) | .45 |
| CK | 2 (3.2) | 2 (3.4) | 2 (5.4) | .76 |
| Anti ds-DNA | 1 (1.6) | 2 (3.4) | 3 (8.1) | .19 |
| Anti-Scl-70 | 1 (1.6) | 0 (0) | 5 (13.5) | < .01 |
| Anti-SSB | 0 (0) | 0 (0) | 3 (8.1) | .01 |
| RNP | 0 (0) | 1 (1.7) | 4 (10.8) | < .01 |
| Anti-Smith | 0 (0) | 0 (0) | 1 (2.7) | .23 |
| ACCP | 0 (0) | 1 (1.7) | 4 (10.8) | < .01 |
| Anti-Jo-1 | 0 (0) | 0 (0) | 1 (2.7) | .23 |
Data are presented as No. (%). Study subjects could have multiple positive serologic tests. ACCP = anti-cyclic citrullinated peptide antibody; ANA = antinuclear antibody; ANCA = anti-neutrophil cytoplasmic antibody; Anti-ds-DNA = anti-double stranded DNA; Anti-SSA = anti-Ro antibody; Anti-SSB = anti-La antibody; CK = creatine kinase; RF = rheumatoid factor; RNP = anti-ribonucleoprotein antibody. See Table 2 for expansion of other abbreviations.
Serologic test was statistically significant when comparing AIF-ILD to IPF subjects.
Thirty-six subjects with AIF-ILD (57%) had one, 22 (35%) had two, and five (8%) had three or more abnormal serologic tests. For the five subjects with AIF-ILD with negative ANA titers, three had a positive rheumatoid factor, one had a positive aldolase, and one had both a positive aldolase and rheumatoid factor.
Eleven subjects with AIF-ILD had one symptom and one positive serologic test. The remaining 52 (83%) exceeded our criteria, having multiple symptoms and/or multiple positive serologic tests.
Radiographic and Lung Biopsy Results
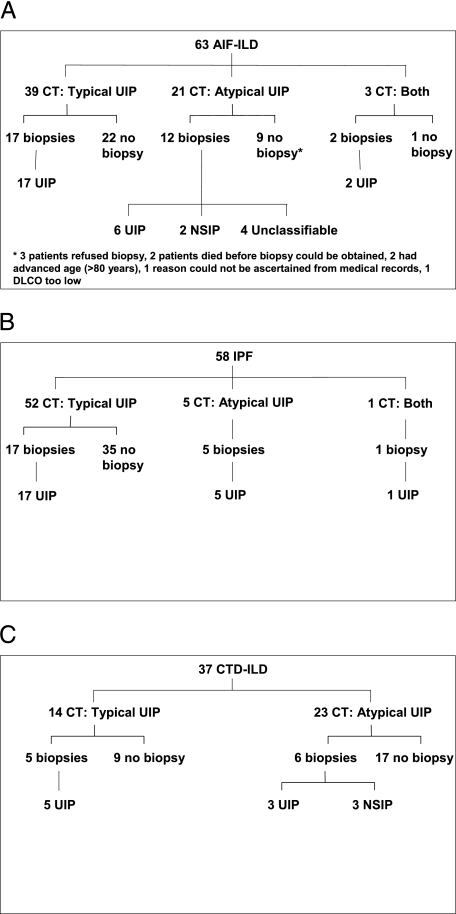
Of the 63 patients with AIF-ILD, 39 (62%) had a typical UIP pattern on HRCT scan, 21 (33%) had an atypical UIP pattern, and three (5%) had multiple HRCT scans with both patterns (Fig 1A). Seventeen patients with the typical UIP pattern on HRCT scan underwent lung biopsy, and all had UIP. Twelve patients with HRCT results that were atypical for UIP underwent lung biopsy: six had UIP, two had NSIP, and four were unclassifiable. The unclassifiable biopsy specimens displayed findings consistent with interstitial pneumonitis, but did not meet ATS/European Respiratory Society histopathologic criteria for a specific diagnosis. Two patients with both patterns on HRCT scan underwent lung biopsy; both had UIP. In the AIF-ILD group, 31 (49%) had a lung biopsy: 25 (81%) had UIP, two (6%) had NSIP, and four (13%) were unclassifiable.
Figure 1.
Flow diagram detailing the distribution of radiographic and pathologic findings in study subjects. A, AIF-ILD. B, IPF. C, CTD-ILD. AIF-ILD = autoimmune-featured interstitial lung disease; CTD-ILD = connective tissue disease-associated interstitial lung disease; Dlco = diffusion capacity of the lung for carbon monoxide; IPF = idiopathic pulmonary fibrosis; NSIP = nonspecific interstitial pneumonia; UIP = usual interstitial pneumonia.
The distribution of radiographic and histopathologic patterns for IPF and CTD-ILD group subjects are shown (Figs 1B, 1C). The radiographic patterns among the three groups were significantly different: the percentage of subjects with a typical UIP pattern on HRCT scan was 62% in AIF-ILD, 90% in IPF, and 38% in CTD-ILD (P < .01). Additional analyses are reported in e-Appendix 1.
Survival
There was a significant difference in the Kaplan-Meier survival curves. Patients with CTD-ILD had better survival than did patients with either AIF-ILD or IPF (P < .01) (Fig 2). There was no significant difference in survival between patients with AIF-ILD and those with IPF. The 1-year survival was 100% for CTD-ILD, 87% for IPF, and 82% for AIF-ILD. The 5-year survival was 95% for CTD-ILD, 48% for IPF, and 52% for AIF-ILD.
Figure 2.
Comparison of the survival curves for subjects with AIF-ILD, IPF, and CTD-ILD. Subjects with CTD-ILD had improved survival compared with either subjects with AIF-ILD or those with IPF (P < .01). PFT = pulmonary function test. See Figure 1 legend for expansion of other abbreviations.
Two variables were significantly associated with worsened survival for patients with AIF-ILD: the presence of a positive aldolase (hazard ratio [HR], 5.31; 95% CI, 1.89-14.92; P < .01) and increasing age (HR, 1.06 for each 1-year increase; 95% CI, 1.00-1.13; P = .04). Three variables were associated with improved survival in patients with AIF-ILD: a higher FVC (HR, 0.96 for each 1% increase; 95% CI, 0.93-0.99; P ≤ .01), a higher diffusion capacity of the lung for carbon monoxide (HR, 0.95 for each 1% increase; 95% CI, 0.92-0.99, P = .01), and progressively higher ANA titers (HR, 0.81 for each titer increase; 95% CI, 0.67-0.99; P = .03). Multivariate analyses could not be performed because of the low number of deaths in the AIF-ILD group.
Subjects with AIF-ILD with an ANA titer ≥ 1:1280 (n = 17) had improved survival compared with those with an ANA titer < 1:1280 (n = 46) (P = .02) (Fig 3). There was no difference in survival for subjects with AIF-ILD at lower ANA titers. For patients with IPF with a positive ANA, there was no difference in survival based on ANA titer.
Figure 3.
Comparison of the survival curves by antinuclear antibody (ANA) titer for subjects with AIF-ILD. Subjects with AIF-ILD with an ANA titer ≥ 1:1280 had improved survival compared with those with an ANA titer < 1:1280 (P = .02). See Figure 2 legend for expansion of other abbreviation.
Of the 17 subjects with AIF-ILD with an ANA titer ≥ 1:1280, eight were men and nine were women. Thirteen subjects had multiple symptoms and nine had multiple positive serologic tests. The ANA immunofluorescence patterns in this subgroup were as follows: nine speckled, four homogeneous, two speckled and nucleolar, one homogeneous and speckled, and one nucleolar. Ten subjects had a typical UIP pattern on HRCT scan, six had an atypical pattern, and one had both patterns. Their pathologic findings were eight UIP, two NSIP, one unclassifiable, and six without biopsy. This subgroup’s PFTs did not differ statistically from those of subjects with IPF or those of subjects with AIF-ILD with an ANA titer < 1:1280. Nine patients in this subgroup were treated with immunosuppressive medications.
Discussion
Our study shows that a systematic evaluation of symptoms and serologic tests in patients with ILD can identify AIF-ILD, which appears to represent a distinct subgroup of ILD, with different characteristics and outcomes from those of IPF and CTD-ILD. In this study of 200 patients with ILD, AIF-ILD was the most common subgroup, having a higher prevalence than IPF or CTD-ILD. Our analysis reveals that subjects with AIF-ILD have several distinct attributes.
The demographic profile revealed that the male to female ratio in AIF-ILD was ≈ 60:40, different from IPF (75:25) and CTD-ILD (25:75). Subjects with AIF-ILD also presented for evaluation at a mean age of 66.2 years old, younger than subjects with IPF, but older than subjects with CTD-ILD.
In this study, the predominant histopathologic finding in subjects with AIF-ILD was UIP. All individuals with the typical UIP pattern on HRCT scan displayed UIP on biopsy specimen, consistent with prior reports.19,20 One-third of the subjects with AIF-ILD had an atypical HRCT scan. Lung biopsy specimens in these subjects showed UIP three times more frequently than NSIP, in contrast to subjects with CTD-ILD with an atypical HRCT scan, in whom an equal number of biopsy specimens showed UIP and NSIP.
For study subjects without CTD-ILD, the vast majority with an atypical pattern on HRCT scan met the criteria for AIF-ILD. However, a typical UIP pattern on HRCT scan or lung biopsy specimen did not exclude AIF-ILD, because nearly one-half of subjects with AIF-ILD displayed these findings.
The survival analysis in this study shows that patients with CTD-ILD have a better prognosis than those with idiopathic interstitial pneumonias, which is consistent with previous reports21‐25 and not likely a result of lead time bias, because lung function was the same in all three groups at study entry. Although CTD-ILD is commonly associated with NSIP on lung biopsy specimen,21,26‐30 UIP is also seen.24,25 Previous studies have shown that UIP associated with connective tissue diseases has a better prognosis than IPF.24,25 In this study, the survival of patients with AIF-ILD did not differ from that of patients with IPF, and was worse than that of patients with CTD-ILD. ANA titer was not associated with improved survival in subjects with IPF, consistent with the findings of Song et al.25 However, patients with AIF-ILD with an ANA titer ≥ 1:1280 had improved survival.
The characteristics of subjects with AIF-ILD with an ANA titer ≥ 1:1280 were heterogeneous: the number of men and women was almost equal, both typical UIP and atypical patterns were observed on HRCT scan, and multiple ANA immunofluoresence patterns and several histopathologic findings were noted. The majority reported multiple symptoms and had multiple positive serologic tests. Survival was improved despite the high prevalence of UIP, suggesting that factors other than histopathologic pattern are important. One-half of this subgroup received immunosuppressive agents, which may have contributed to improved survival. Alternatively, these patients may have a different mechanism of disease development or progression. Although it would have been beneficial to identify characteristics that define this subgroup and/or predict survival, multivariate logistic regression could not be performed because of the sample size.
The criteria for AIF-ILD in this study were selected from a comprehensive list designed to evaluate patients with ILD for connective tissue diseases. Our symptoms criteria for AIF-ILD are very similar to those published by Kinder et al5 and Suda et al.31 Our serologic panel additionally includes disease-specific antibodies (Table 1). We excluded the nonspecific erythrocyte sedimentation rate and C-reactive protein.
Using similar criteria, Kinder et al5 retrospectively examined patients with idiopathic interstitial pneumonia, and identified 28 cases of possible UCTD. Eighteen patients underwent lung biopsy and 15 had NSIP. The authors concluded that patients with NSIP on lung biopsy specimen were likely to have autoimmune features. Our results support that finding, in that all subjects without CTD-ILD who had NSIP on lung biopsy specimen met the criteria for AIF-ILD. Our study demonstrates that patients with UIP on HRCT scan or lung biopsy specimen can also have autoimmune features. Kinder et al5 acknowledged that complete serologic testing was not performed in all cases, and symptoms were collected by reviewing medical records. In contrast, our evaluation was offered to all ILD patients, regardless of referring diagnosis, radiographic pattern, or histopathologic findings.
Suda et al31 reviewed 62 patients with idiopathic NSIP demonstrated on surgical lung biopsy specimens over a 19-year period, and found that 22 subjects met proposed criteria by Kinder et al5 for UCTD.31 In that study, patients with UCTD-NSIP had improved survival compared with those with idiopathic NSIP. Data were collected retrospectively, and the authors acknowledged selection and recall biases. Another limitation was that cases with other histopathologic patterns, including UIP, were excluded from analysis.
As described in the rheumatologic literature, patients with UCTD are women (95%) and younger (44-47 years old) and rarely have ILD (1%).6‐8 In contrast, our AIF-ILD cohort is predominantly male (60%) and older (66 years old) and, by definition, has ILD. Because of the disparity in these characteristics, we opted to propose a new term, AIF-ILD, to describe patients with ILD with autoimmune features. When followed over time, the subjects with AIF-ILD in our cohort had not met the ACR criteria for connective tissue diseases.
A recent commentary has proposed preliminary criteria to describe patients with ILD with autoimmune features, in which extrathoracic symptoms are permitted but not required.32 The criteria used in our study were selected independently. A systematic evaluation of signs, symptoms, and serologic tests allowed us to identify AIF-ILD, a distinct group of patients with ILD, a subset of whom had improved survival. To date, this is the largest study to systematically evaluate patients with ILD for connective tissue diseases and to identify those with autoimmune features. In our study population, AIF-ILD was more common than IPF.
There are limitations to this study. Multivariate analyses could not be performed with respect to clinical characteristics and survival, given the low number of deaths in the AIF-ILD group. ANA titers were not measured serially. A subset of patients with AIF-ILD may eventually meet the criteria for a defined connective tissue disease, although the survivors have been followed for a median of 1.7 years, without evolution. Symptoms were self-reported. Leg/foot swelling is nonspecific, and could include edema; however, all subjects with AIF-ILD with this symptom also reported at least one other symptom. None of the patients met the definition for AIF-ILD solely on the basis of having leg/foot swelling. Only a subset of patients underwent lung biopsy. For patients with idiopathic interstitial pneumonia and a typical UIP pattern on HRCT scan, previous studies19,20 suggest that a biopsy is likely to demonstrate UIP, which correlates with our results. In keeping with current guidelines, a biopsy specimen was not obtained in cases in which clinicians and radiologists were confident in the diagnosis. Lung biopsy results on all patients with AIF-ILD with an atypical HRCT scan were not obtained because of patient refusal, advanced age, and advanced disease.
We recognize that AIF-ILD may encompass a heterogeneous group of patients, and expect that our proposed criteria will be revised over time, just as many connective tissue disease criteria have been revised. Patients with AIF-ILD had different demographic characteristics compared with the IPF cohort. The clinical differences persisted, including the presence of multiple symptoms and positive serologic tests, even when subjects with AIF-ILD with an ANA titer ≥ 1:1280 were excluded. Future studies should examine which symptoms and serologic tests, alone or in combination, are necessary for identifying AIF-ILD. Additional analyses of ANA titer cutoffs, predictors of survival, and response to immunosuppressive therapy will also be useful.
Conclusions
In summary, we have shown that comprehensive evaluation of symptoms and serologic tests in patients referred to a tertiary care ILD clinic can identify a distinct, common, and novel group of ILD patients with autoimmune features, AIF-ILD. The predominant radiographic and histopathologic finding in this AIF-ILD population was UIP. Although clinical characteristics differed among patients with AIF-ILD, IPF, and CTD-ILD, survival for patients with AIF-ILD remained poor, similar to that of patients with IPF. However, the subset of patients with AIF-ILD with an ANA titer ≥ 1:1280 had improved survival, suggesting that an elevated ANA may be a marker for improved prognosis. Our findings highlight the importance of a thorough evaluation of patients with ILD.
Supplementary Material
Acknowledgments
Author contributions: Drs Noth and Strek take responsibility for the integrity of the work as a whole.
Dr Vij: contributed to the study design, data analysis, and preparation of the manuscript.
Dr Noth: contributed to the study design, senior authorship, and editing of the manuscript.
Dr Strek: contributed to the study design, senior authorship, and editing of the manuscript
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Noth has received institutional grants from Centacor, Immuneworks, Actelion, and the National Institutes of Health for conduct of clinical trials in IPF. Dr Strek has received institutional grants from InterMune and Gilead to conduct industry-sponsored clinical research trials. Dr Vij has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We gratefully acknowledge Steve White, MD, for his assistance in the preparation of this manuscript and Kristen Wroblewski, MS, for assistance with statistical analysis. We also thank Steve White, MD; D. Kyle Hogarth, MD; Nathan Sandbo, MD; Heber MacMahon, MD; Steven Montner, MD; Aliya Husain, MD; Cathy Brown, RN; Spring Maleckar-Holland, BA; and Latriese Sardin, BA, for their assistance in conducting this study.
Additional information: The e-Appendix can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/5/1292/suppl/DC1.
Abbreviations
- ACR
American College of Rheumatology
- AIF-ILD
autoimmune-featured interstitial lung disease
- ANA
antinuclear antibody
- ATS
American Thoracic Society
- CTD-ILD
connective tissue disease-related interstitial lung disease
- HR
hazard ratio
- HRCT
high-resolution CT
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- NSIP
nonspecific interstitial pneumonia
- UCTD
undifferentiated connective tissue disease
- UIP
usual interstitial pneumonia
Footnotes
Funding/Support: This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute [Grant HL080513]; the Pulmonary Fibrosis Foundation (Chicago, IL); and the Coalition for Pulmonary Fibrosis (San Jose, CA).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Lamblin C, Bergoin C, Saelens T, Wallaert B. Interstitial lung diseases in collagen vascular diseases. Eur Respir J Suppl. 2001;32:69s–80s. [PubMed] [Google Scholar]
- 2.Kim TS, Lee KS, Chung MP, et al. Nonspecific interstitial pneumonia with fibrosis: high-resolution CT and pathologic findings. AJR Am J Roentgenol. 1998;171(6):1645–1650. doi: 10.2214/ajr.171.6.9843306. [DOI] [PubMed] [Google Scholar]
- 3.Strange C, Highland KB. Interstitial lung disease in the patient who has connective tissue disease. Clin Chest Med. 2004;25(3):549–559. doi: 10.1016/j.ccm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FJ. Idiopathic interstitial pneumonias: usual interstitial pneumonia versus nonspecific interstitial pneumonia. Proc Am Thorac Soc. 2006;3(1):81–95. doi: 10.1513/pats.200511-123JH. [DOI] [PubMed] [Google Scholar]
- 5.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007;176(7):691–697. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeRoy EC, Maricq HR, Kahaleh MB. Undifferentiated connective tissue syndromes. Arthritis Rheum. 1980;23(3):341–343. doi: 10.1002/art.1780230312. [DOI] [PubMed] [Google Scholar]
- 7.Mosca M, Neri R, Bombardieri S. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin Exp Rheumatol. 1999;17(5):615–620. [PubMed] [Google Scholar]
- 8.Vaz CC, Couto M, Medeiros D, et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin Rheumatol. 2009;28(8):915–921. doi: 10.1007/s10067-009-1175-2. [DOI] [PubMed] [Google Scholar]
- 9.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 11.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 12.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 13.Smolen JS, Steiner G. Mixed connective tissue disease: to be or not to be? Arthritis Rheum. 1998;41(5):768–777. doi: 10.1002/1529-0131(199805)41:5<768::AID-ART3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Moutsopoulos HM, et al. The European Study Group on Diagnostic Criteria for Sjögren’s Syndrome Assessment of the European classification criteria for Sjögren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. Ann Rheum Dis. 1996;55(2):116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 18.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 19.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest. 2003;124(4):1215–1223. doi: 10.1378/chest.124.4.1215. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein MC. Diagnosis: to biopsy or not to biopsy: assessing the role of surgical lung biopsy in the diagnosis of idiopathic pulmonary fibrosis. Chest. 2005;128(5 suppl 1):520S–525S. doi: 10.1378/chest.128.5_suppl_1.520S. [DOI] [PubMed] [Google Scholar]
- 21.Douglas WW, Tazelaar HD, Hartman TE, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med. 2001;164(7):1182–1185. doi: 10.1164/ajrccm.164.7.2103110. [DOI] [PubMed] [Google Scholar]
- 22.Flaherty KR, Colby TV, Travis WD, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167(10):1410–1415. doi: 10.1164/rccm.200204-373OC. [DOI] [PubMed] [Google Scholar]
- 23.Wells AU, Cullinan P, Hansell DM, et al. Fibrosing alveolitis associated with systemic sclerosis has a better prognosis than lone cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 1994;149(6):1583–1590. doi: 10.1164/ajrccm.149.6.8004317. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007;175(7):705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 25.Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136(1):23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 26.Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18(2):136–147. [PubMed] [Google Scholar]
- 27.Cottin V, Donsbeck AV, Revel D, Loire R, Cordier JF. Nonspecific interstitial pneumonia. Individualization of a clinicopathologic entity in a series of 12 patients. Am J Respir Crit Care Med. 1998;158(4):1286–1293. doi: 10.1164/ajrccm.158.4.9802119. [DOI] [PubMed] [Google Scholar]
- 28.Kim DS, Yoo B, Lee JS, et al. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19(2):121–127. [PubMed] [Google Scholar]
- 29.Yamadori I, Fujita J, Bandoh S, et al. Nonspecific interstitial pneumonia as pulmonary involvement of primary Sjögren’s syndrome. Rheumatol Int. 2002;22(3):89–92. doi: 10.1007/s00296-002-0204-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoshinouchi T, Ohtsuki Y, Fujita J, et al. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol Int. 2005;26(2):121–125. doi: 10.1007/s00296-004-0527-0. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Kono M, Nakamura Y, et al. Distinct prognosis of idiopathic nonspecific interstitial pneumonia (NSIP) fulfilling criteria for undifferentiated connective tissue disease (UCTD) Respir Med. 2010;104(10):1527–1534. doi: 10.1016/j.rmed.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010;138(2):251–256. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.