Abstract
Background:
Recent literature suggests that obese critically ill patients do not have worse outcomes than patients who are normal weight. However, outcomes in extreme obesity (BMI ≥ 40 kg/m2) are unclear. We sought to determine the association between extreme obesity and ICU outcomes.
Methods:
We analyzed data from a multicenter international observational study of ICU nutrition practices that occurred in 355 ICUs in 33 countries from 2007 to 2009. Included patients were mechanically ventilated adults ≥ 18 years old who remained in the ICU for > 72 h. Using generalized estimating equations and Cox proportional hazard modeling with clustering by ICU and adjusting for potential confounders, we compared extremely obese to normal-weight patients in terms of duration of mechanical ventilation (DMV), ICU length of stay (LOS), hospital LOS, and 60-day mortality.
Results:
Of the 8,813 patients included in this analysis, 3,490 were normal weight (BMI 18.5-24.9 kg/m2), 348 had BMI 40 to 49.9 kg/m2, 118 had BMI 50 to 59.9 kg/m2, and 58 had BMI ≥ 60 kg/m2. Unadjusted analyses suggested that extremely obese critically ill patients have improved mortality (OR for death, 0.77; 95% CI, 0.62-0.94), but this association was not significant after adjustment for confounders. However, an adjusted analysis of survivors found that extremely obese patients have a longer DMV and ICU LOS, with the most obese patients (BMI ≥ 60 kg/m2) also having longer hospital LOS.
Conclusions:
During critical illness, extreme obesity is not associated with a worse survival advantage compared with normal weight. However, among survivors, BMI ≥ 40 kg/m2 is associated with longer time on mechanical ventilation and in the ICU. These results may have prognostic implications for extremely obese critically ill patients.
Obesity, defined as a BMI between 30 and 39.9 kg/m2, and extreme or severe obesity, defined as a BMI ≥ 40 kg/m2, are increasingly prevalent in the United States.1 In 2007 to 2008, 32.2% of American men and 35.5% of American women were obese.2 During the same time period, 4.2% of men and 7.2% of women were extremely obese. Overweight, obesity, and extreme obesity are associated with increased all-cause mortality among the general population, in both genders.1
However, in critically ill patients, recent evidence suggests that obese and extremely obese patients do not have increased ICU or hospital lengths of stay (LOS)3‐5 and may even have lower mortality rates than normal-weight patients.6‐8 Other studies found that obese and extremely obese patients may have longer ICU and hospital LOS, duration of mechanical ventilation (DMV), and increased mortality.9‐16 A recent meta-analysis reported that extremely obese patients with a BMI ≥ 40 kg/m2 had decreased hospital mortality compared with normal-weight patients, but this did not reach statistical significance (relative risk, 0.83; 95% CI, 0.66-1.04).17 Additionally, a recent study reported that mortality in critically ill patients who have a BMI ≥ 35 kg/m2 was improved among those who received adequate nutrition (energy and protein).18
These prior studies have included relatively small numbers of severely obese patients; thus, sample size has been insufficient to examine outcomes further subgrouped by BMI (eg, BMI 40-49.9 kg/m2, 50-59.9 kg/m2, and ≥ 60 kg/m2). To evaluate extreme obesity and ICU outcomes, including mortality, we used data obtained from an observational study of ICU nutrition practices to evaluate extremely obese patients in aggregate (BMI ≥ 40 kg/m2) and further stratified by BMI.
Materials and Methods
We examined data from a large multicenter international observational study of ICU nutrition practices that took place in 355 ICUs in 33 countries during three study periods from 2007 to 2009. Details of data collection have been published previously.18 Eligible patients were adults ≥ 18 years old, mechanically ventilated within 48 h of ICU admission, who remained in the ICU for > 72 h. Site characteristics and patients’ baseline demographic, physiologic, severity of illness, and nutrition assessment data were collected at study enrollment. Admission diagnoses were obtained from the medical record. Additional information, including amount of nutrition received, was collected daily for a maximum of 12 days or until death or ICU discharge. Patients were followed in the hospital for up to 60 days to assess ICU and hospital outcomes. This research was given exempt status by our human subjects committee because it used already collected de-identified data.
We classified patient BMIs based on the following National Heart, Lung, and Blood Institute guidelines: underweight < 18.5 kg/m2, normal weight 18.5 to 24.9 kg/m2, overweight 25 to 29.9 kg/m2, obese 30 to 39.9 kg/m2, and extremely obese ≥ 40 kg/m2.19 We further subdivided extreme obesity into three groups: 40 to 49.9 kg/m2, 50 to 59.9 kg/m2, and ≥ 60 kg/m2. These three groups were the focus of our study. Data on weight and height as gathered during the ICU nutrition survey were determined by local ICU practice, and the survey asked for the weight closest to ICU admission. We excluded underweight patients in our analyses since our focus was on extreme obesity.
Clinical outcomes included 60-day hospital mortality, and among 60-day survivors, DMV, ICU LOS, and hospital LOS. Hospital and ICU LOS began at ICU admission. Patients discharged from hospital within 60 days of ICU admission were considered 60-day hospital survivors. Time on ventilation was from the latest of ICU admission or intubation time (always within 48 h) to extubation time. Extubation (or ICU discharge) was only considered to have occurred when patients remained off the ventilator (or out of ICU) for ≥ 48 h. Reintubations (or ICU readmissions) occurring after 48 h were not measured. The 7% of survivors who remained ventilated at their ICU discharge were censored at ICU discharge. Participants were also censored when follow-up ended 60 days after ICU admission.
A priori, we considered that age, gender, APACHE (Acute Physiology and Chronic Health Evaluation) II score,20 admission diagnosis (cardiovascular, respiratory, GI, neurologic, sepsis, trauma, or other), admission category (medical, elective surgical, emergent surgical), ICU type (open vs closed), hospital type (teaching vs nonteaching), geographic region (Canada, Australia/New Zealand, United States, Europe/South Africa, Asia, Latin America), and nutritional adequacy (calories actually received as a percentage of those prescribed during the first 12 ICU days before permanent progression to exclusive oral feeding) might potentially confound the relationship between obesity and ICU outcomes. These covariates were compared between BMI groups using a mixed model clustering by ICU for continuous variables (age, APACHE II score, and nutritional adequacy) and the Rao-Scott χ2 test also clustered by ICU for categorical variables. We also compared these covariates between BMI groups after controlling for region to assess associations independent of regional differences in practice patterns and patient characteristics.
Raw unadjusted 60-day hospital mortality rates are presented by BMI group. Among 60-day hospital survivors, unadjusted clinical outcomes (DMV, ICU LOS, and hospital LOS) are presented by BMI group as quartiles and survival curves based on Kaplan-Meier (product limit) estimates. We restricted our primary analysis of DMV, ICU LOS, and hospital LOS to 60-day survivors, since these are not meaningful clinical outcomes among decedents. However, we performed a sensitivity analysis from a resource use perspective by including decedents. We also performed a sensitivity analysis by including only US patients. All ORs, hazard ratios (HRs), P values, and confidence limits account for potential within ICU dependence arising from the two-stage clustered sampling scheme of the survey. Logistic generalized estimating equations clustered by ICU were used to estimate ORs comparing mortality in each overweight BMI group to the normal-weight group.21 Similarly, the Cox proportional hazards model with robust standard errors accounting for ICU clustering was used to estimate HRs for the time-to-event outcomes.22,23 HRs below unity indicate slower rate of extubation, ICU discharge, or hospital discharge, and thus equate to longer durations (worse outcome) in the overweight groups. Each model was run first without adjusting for covariates, then adjusting for all covariates excluding nutritional adequacy, and finally adjusting for all covariates including 12-day nutritional adequacy. Based on prior knowledge and empirical considerations, we included the product term between age and APACHE II score, as well as the product term between admission diagnosis and admission category in all models. These were the only statistically significant second-order product (interaction) terms when modeling mortality. The sample size for the time-to-event analyses precluded consideration of further interaction terms. Loess smoothers and restricted cubic splines were used to assess linearity between the outcomes and continuous covariates (age, APACHE II score, and nutritional adequacy). It was determined that age and APACHE II score could be adequately modeled by single linear terms. However, nutritional adequacy demonstrated a nonlinear relationship with some of the outcomes and was therefore modeled as a restricted (natural) cubic spline with knots at the fifth, 27.5th, 50th, 72.5th, and 95th percentiles as suggested by Harrell and others.24 The Hosmer-Lemeshow test did not indicate fit problems with the logistic mortality models,25 and the methods of Lin and colleagues26 based on cumulative sums of martingale residuals supported the proportional hazards assumption of the time-to-event models. P values were based on two-sided Wald tests without adjustment for multiplicity. The entire analysis was performed using SAS, version 9.2 (SAS Inc; Cary, North Carolina).
Results
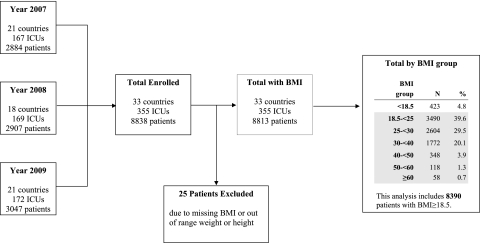
A total of 8,838 patients were included in the international nutrition survey over the three study periods. The current analysis excludes 25 patients missing BMI information and 423 underweight patients, leaving 3,490 normal-weight patients, 2,604 overweight patients, 1,772 obese patients, and 524 extremely obese patients, with 348 in BMI group 40 to 49.9 kg/m2, 118 in BMI group 50 to 59.9 kg/m2, and 58 patients with BMI ≥ 60 kg/m2 (Fig 1).
Figure 1.
Flow diagram of study participants.
Compared with normal-weight patients, extremely obese patients were significantly more likely to be younger, female, or have a respiratory diagnosis, whereas the BMI ≥ 60 kg/m2 group was more likely to have a lower APACHE II score and a medical admission category (Table 1). Extremely obese patients were predominately from the United States and were more likely to be admitted to open ICUs. Patients with BMI ≥ 60 kg/m2 were more likely to be admitted to nonteaching hospitals (Table 1). BMI was not associated with admission category, hospital type, or ICU type after controlling for geographic region (data not shown).
Table 1.
—Patient and Site Characteristics by BMI Group
| Characteristic | Normal Weight, BMI 18.5-24.9 (n = 3,490) | Overweight, BMI 25-29.9 (n = 2,604) | Obese, BMI 30-39.9 (n = 1,772) | Extreme Obesity, BMI 40-49.9 (n = 348) | Extreme Obesity, BMI 50-59.9 (n = 118) | Extreme Obesity, BMI ≥ 60 (n = 58) |
| Age,a y | 58.6 ± 18.9 | 60.2 ± 17.4 | 60.8 ± 15.2 | 57.0 ± 14.1 | 55.5 ± 12.3 | 50.6 ± 11.1 |
| P = .0001 | P = .0001 | P = .04 | P = .02 | P < .0001 | ||
| Gender,b % male | 61.0 | 66.4 | 57.7 | 46.3 | 39.0 | 48.3 |
| P < .0001 | P = .03 | P < .0001 | P < .0001 | P = .05 | ||
| APACHE II scorea | 22.2 ± 8.0 | 22.4 ± 8.1 | 22.9 ± 8.0 | 22.9 ± 8.7 | 22.3 ± 8.0 | 20.6 ± 7.7 |
| P = .86 | P = .02 | P = .95 | P = .71 | P = .01 | ||
| Admit diagnosis,b % | ||||||
| Cardiovascular | 15.0 | 18.6 | 20.0 | 19.5 | 14.4 | 6.9 |
| Respiratory | 26.7 | 23.2 | 26.0 | 33.6 | 37.3 | 55.2 |
| Gastrointestinal | 15.5 | 15.2 | 14.5 | 13.2 | 12.7 | 1.7 |
| Neurologic | 14.5 | 13.3 | 10.8 | 6.0 | 5.1 | 0 |
| Sepsis | 8.5 | 8.2 | 10.3 | 9.8 | 12.7 | 15.5 |
| Trauma | 10.9 | 12.1 | 8.8 | 7.5 | 5.9 | 6.9 |
| Other | 8.9 | 9.5 | 9.7 | 10.3 | 11.9 | 13.8 |
| P = .001 | P < .0001 | P = .001 | P = .03 | P < .0001 | ||
| Admit category,b % | ||||||
| Medical | 62.4 | 60.0 | 61.9 | 68.4 | 71.2 | 77.6 |
| Elective surgical | 12.9 | 13.9 | 14.8 | 10.3 | 9.3 | 12.1 |
| Emergent surgical | 24.7 | 26.1 | 23.4 | 21.3 | 19.5 | 10.3 |
| P = .20 | P = .24 | P = .10 | P = .14 | P = .046 | ||
| % Caloric prescription receiveda,c | 58.5 ± 29.6 | 54.2 ± 28.6 | 53.8 ± 29.4 | 55.4 ± 27.9 | 54.7 ± 29.1 | 53.5 ± 32.8 |
| P < .0001 | P < .0001 | P = .58 | P = .80 | P = .37 | ||
| Geographic region,b % | ||||||
| Canada | 21.2 | 24.5 | 25.9 | 26.7 | 32.2 | 19.0 |
| Australia/New Zealand | 14.1 | 16.9 | 13.9 | 12.6 | 11.0 | 15.5 |
| United States | 24.5 | 29.6 | 42.3 | 51.4 | 50.9 | 62.1 |
| Europe/South Africa | 16.2 | 14.9 | 11.7 | 5.5 | 5.1 | 1.7 |
| Latin America | 6.9 | 6.6 | 3.4 | 2.3 | 0.9 | 0 |
| Asia | 17.1 | 7.6 | 2.8 | 1.4 | 0 | 1.7 |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | P < .0001 | ||
| Hospital type,b % | ||||||
| Teaching | 78.8 | 78.5 | 73.9 | 73.6 | 69.5 | 60.3 |
| Nonteaching | 21.2 | 21.5 | 26.1 | 26.4 | 30.5 | 39.7 |
| P = .79 | P = .01 | P = .10 | P = .06 | P = .003 | ||
| ICU type,b % | ||||||
| Open | 23.0 | 24.2 | 28.9 | 35.3 | 33.0 | 32.8 |
| Closed | 75.5 | 73.8 | 69.1 | 61.8 | 61.9 | 67.2 |
| Other | 1.5 | 2.0 | 2.0 | 2.9 | 5.1 | 0 |
| P = .23 | P = .0007 | P < .0001 | P = .002 | P = .19 |
BMI in kg/m according to NHLBI classification of overweight and obesity by BMI. NHLBI classification includes extreme obesity as BMI ≥ 40 kg/m. We have separated extreme obesity into three groups based on BMI. APACHE = Acute Physiology and Chronic Health Evaluation; NHLBI = National Heart, Lung, and Blood Institute.
Presented as raw mean ± SD. P values compare each group to normal group using a linear mixed effects model with random ICU effects to account for potential within-ICU dependence.
Raw percentages are reported, but P values use the Rao-Scott χ method to account for ICU clustering. Asia and Latin America were combined for P value calculation for geographic region, neurologic diagnosis was combined with other for P value calculation for admission diagnosis, and other was combined with open for P value calculation of ICU type.
Mean of calories received from enteral nutrition, parenteral nutrition, and propofol for first 12 d of ICU stay expressed as a percentage of calories prescribed. Days without enteral nutrition or parenteral nutrition were included and counted as 0%. Days after death, ICU discharge, or permanent progression to exclusive oral intake were excluded. P values control for number of days used in calculation.
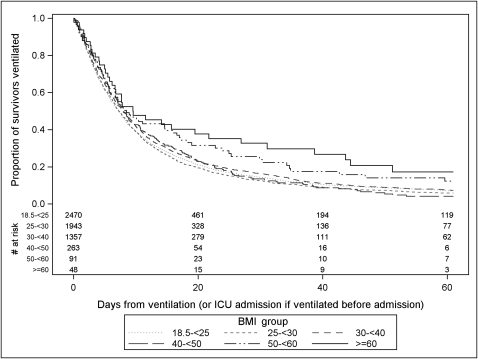
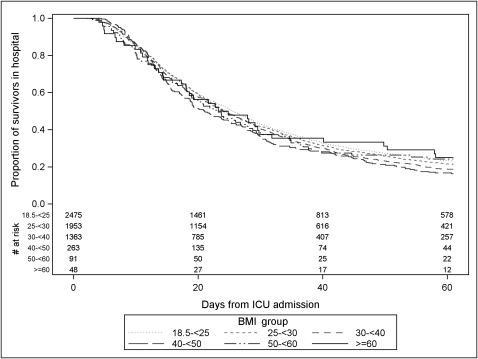
When comparing the outcomes of extremely obese patients, subdivided by BMI, to those of normal-weight patients, unadjusted analyses found patients with a BMI ≥ 60 kg/m2 had a significantly lower 60-day mortality rate compared with normal-weight patients (17.2% mortality vs 28.9% mortality, P = .04) (Table 2). Compared with surviving normal-weight patients, surviving extremely obese patients had a longer DMV, which was statistically significant in the BMI 50 to 59.9 kg/m2 and the BMI ≥ 60 kg/m2 groups (Fig 2, Table 2). There were no significant differences in unadjusted ICU LOS for any of the surviving extremely obese groups compared with normal-weight survivors; however, the survivors with BMI 40 to 49.9 kg/m2 had a significantly shorter hospital LOS compared with normal-weight survivors (Fig 3, Table 2).
Table 2.
—Unadjusted Description of Clinical Outcomes by BMI Group
| Outcome | Normal Weight, BMI 18.5-24.9 (n = 3,490) | Overweight, BMI 25-29.9 (n = 2,604) | Obese, BMI 30-39.9 (n = 1,772) | Extreme Obesity, BMI ≥ 40 (n = 524) | Extreme Obesity, BMI 40-49.9 (n = 348) | Extreme Obesity, BMI 50-59.9 (n = 118) | Extreme Obesity, BMI ≥ 60 (n = 58) |
| 60-d Mortality,a % | 28.9 | 24.9 | 23.1 | 23.3 | 24.4 | 22.9 | 17.2 |
| P = .002 | P < .0001 | P = .01 | P = .09 | P = .12 | P = .04 | ||
| No. survivors | 2,475 | 1,953 | 1,363 | 402 | 263 | 91 | 48 |
| Duration of mechanical ventilation,b median d (IQR) | 7.3 (3.0-17.0) | 7.3 (3.1-15.8) | 7.9 (3.3-18.6) | 8.2 (3.8-21.7) | 8.0 (3.7-18.9) | 8.5 (3.9-29.9) | 9.6 (4.2-43.7) |
| P = .27 | P = .12 | P = .03 | P = .61 | P = .02 | P = .02 | ||
| ICU LOS,b median d (IQR) | 10.7 (5.9-21.7) | 11.0 (6.2-19.8) | 11.5 (6.4-22.1) | 11.1 (6.5-24.1) | 10.9 (6.7-21.5) | 10.4 (5.9-26.3) | 13.3 (6.3-29.7) |
| P = .49 | P = .50 | P = .49 | P = .72 | P = .27 | P = .13 | ||
| Hospital LOS,b median d (IQR) | 25.5 (13.7-56.1) | 24.2 (13.8-51.7) | 24.0 (13.5-46.6) | 21.9 (12.8-47.0) | 21.0 (12.8-44.8) | 22.9 (12.5-57.0) | 24.1 (12.5-Undefinedc) |
| P = .27 | P = .006 | P = .03 | P = .007 | P = .71 | P = .97 |
BMI in kg/m according to NHLBI classification of overweight and obesity by BMI. NHLBI classification includes extreme obesity as BMI ≥ 40 kg/m. We have separated extreme obesity into three groups based on BMI. IQR = interquartile range; LOS = length of stay. See Table 1 legend for expansion of the other abbreviations.
Raw percentages are reported, but P values comparing each BMI group to the normal group are based on generalized estimating equations clustering by ICU.
All duration analyses are based on 60-d survivors only and are censored 60 d after ICU admission. Duration of mechanical ventilation and hospital LOS exclude time prior to ICU admission. Median (IQR) days are based on unadjusted Kaplan-Meier (product-limit) estimates. Duration P values use the score test with robust standard errors clustering by ICU.
Upper quartile undefined because 25% of patients with BMI ≥ 60 remained in hospital at day 60.
Figure 2.
Unadjusted Kaplan-Meier curve comparing the proportion of survivors remaining ventilated over time by BMI group. The number at risk is the number of patients still ventilated and in the ICU. Patients were censored if they were discharged from ICU while ventilated (7%) or remained ventilated 60 days after ICU admission (5%).
Figure 3.
Unadjusted Kaplan-Meier curve comparing the proportion of 60-day hospital survivors remaining in hospital over time by BMI group. The number at risk indicates the number of patients remaining in the hospital at each time by group. Patients remaining in hospital were censored at 60 days when follow-up ended.
Table 3 reports modeling results comparing the clinical outcomes of each BMI group to the normal-weight group. After adjustment, when combining all extremely obese patients into one group (BMI ≥ 40 kg/m2), adjusted analyses did not show significantly improved morality (OR, 0.87; 95% CI, 0.69-1.09) but among survivors did indicate a slower time to extubation (HR, 0.80; 95% CI, 0.70-0.92; P = .0013), slower time to ICU discharge (HR, 0.82; 95% CI, 0.72-0.93; P = .0016), and a trend toward a slower time to hospital discharge (HR, 0.91; 95% CI, 0.80-1.04; P = .17). After adjustment, none of the three individual extremely obese groups demonstrated significantly reduced mortality. Among 60-day survivors, the time to extubation was significantly slower in the two highest BMI groups (Fig 2). After adjusting for all covariates, including nutritional adequacy, the HR of the time to extubation was 0.44 (95% CI, 0.28-0.71) in the BMI ≥ 60 kg/m2 group (Table 3). Time to ICU discharge demonstrated a similar association with BMI. Adjusted time to hospital discharge was only significantly slower in the BMI ≥ 60 kg/m2 group (fully adjusted HR = 0.64; 95% CI, 0.46-0.88).
Table 3.
—Model-Based Comparisons of Clinical Outcomes Between Normal and High BMI Groups
| Outcome | Normal Weight, BMI 18.5-24.9 (n = 3,490) | Overweight, BMI 25-29.9 (n = 2,604) | Obese, BMI 30-39.9 (n = 1,772) | Extreme Obesity, BMI ≥ 40 (n = 524) | Extreme Obesity, BMI 40-49.9 (n = 348) | Extreme Obesity, BMI 50-59.9 (n = 118) | Extreme Obesity, BMI ≥ 60 (n = 58) |
| Death at 60 d, OR (95% CI) | Reference | 0.84 (0.74-0.94)a | 0.76 (0.67-0.86)b | 0.77 (0.62-0.94)a | 0.81 (0.64-1.04)c | 0.74 (0.51-1.08) | 0.51 (0.27-0.96)a |
| 0.81 (0.71-0.91)b | 0.74 (0.64-0.84)b | 0.87 (0.69-1.09) | 0.86 (0.66-1.12) | 0.86 (0.58-1.26) | 0.73 (0.38-1.38) | ||
| 0.80 (0.71-0.90)b | 0.73 (0.64-0.84)b | 0.87 (0.69-1.09) | 0.86 (0.66-1.12) | 0.87 (0.59-1.29) | 0.73 (0.38-1.40) | ||
| No. survivors | 2,475 | 1,953 | 1,363 | 402 | 263 | 91 | 48 |
| Time to extubation, HR (95% CI) | Reference | 1.04 (0.97-1.11) | 0.94 (0.88-1.02) | 0.88 (0.78-0.99)a | 0.97 (0.85-1.10) | 0.77 (0.62-0.97)a | 0.66 (0.46-0.94)a |
| 1.02 (0.95-1.10) | 0.91 (0.84-0.99)a | 0.85 (0.75-0.97)a | 0.92 (0.83-1.05) | 0.74 (0.59-0.94)a | 0.54 (0.36-0.80)a | ||
| 0.97 (0.90-1.05) | 0.85 (0.78-0.93)b | 0.80 (0.70-0.92)a | 0.89 (0.76-1.04) | 0.70 (0.56-0.89)a | 0.44 (0.28-0.71)b | ||
| Time to ICU discharge, HR (95% CI) | Reference | 1.03 (0.96-1.10) | 0.98 (0.91-1.05) | 0.96 (0.86-1.07) | 1.02 (0.91-1.15) | 0.88 (0.71-1.10) | 0.79 (0.58-1.07) |
| 1.00 (0.93-1.08) | 0.92 (0.85-0.99)a | 0.86 (0.77-0.97)a | 0.92 (0.81-1.04) | 0.79 (0.63-1.00)a | 0.60 (0.42-0.84)a | ||
| 0.95 (0.88-1.03) | 0.86 (0.79-0.94)b | 0.82(0.72-0.93)a | 0.89 (0.77-1.02)c | 0.75 (0.60-0.92)a | 0.54 (0.37-0.77)b | ||
| Time to hospital discharge, HR (95% CI) | Reference | 1.04 (0.97-1.12) | 1.11 (1.03-1.20)a | 1.14 (1.01-1.29)c | 1.21 (1.05-1.39)a | 1.05 (0.81-1.36) | 0.99 (0.70-1.41) |
| 1.02 (0.95-1.10) | 1.00 (0.93-1.08) | 0.92 (0.80-1.05) | 0.97 (0.83-1.12) | 0.84 (0.63-1.11) | 0.63 (0.44-0.89)a | ||
| 0.98 (0.91-1.05) | 0.96 (0.89-1.04) | 0.91 (0.80-1.04) | 0.96 (0.83-1.12) | 0.84 (0.65-1.09) | 0.64 (0.46-0.88)a |
BMI in kg/m according to NHLBI classification of overweight and obesity by BMI. In each cell, the first estimate is unadjusted for covariates. The second estimate is adjusted for the following covariates: year, age, gender, APACHE II score, initial diagnosis by admission category (medical, elective surgical, emergency surgical), geographic region, hospital type (teaching vs nonteaching), ICU type (closed vs open), and the product of age by APACHE II score. The third estimate adjusted the percent of nutritional prescription received in addition to the other covariates. Because of missing covariates, the second and third models exclude 55 (0.7%) and 138 (1.6%) patients. OR < 1 indicates reduced mortality compared with reference group. HR < 1 indicates reduced rate of event, which implies longer durations (worse outcome). Boldface type indicates statistical significance. HR = hazard ratio. See Figure 1 legend for expansion of other abbreviations.
P ≤ .05
P ≤ .001
.05 < P ≤ .10
All of the trends remained consistent or slightly amplified in the subgroup of US-only patients (data not shown). Furthermore, the association between BMI and duration of ventilation, ICU, and hospital stay persisted if decedents were included in addition to 60-day survivors (data not shown).
Discussion
Although prior studies have included outcomes of severely obese critically ill trauma and medical patients,4,27 to our knowledge, our study includes the largest cohort to date of extremely obese critically ill patients. In addition to its size, other strengths of this research include its geographic and population diversity, which increases its generalizability. We found that when stratifying extremely obese patients (BMI 40-49.9 kg/m2, 50-59.9 kg/m2, and ≥ 60 kg/m2), there was an apparent trend of reduced 60-day mortality with increasing BMI, although this trend did not remain statistically significant after adjusting for potential confounders. When examining indices of morbidity among extremely obese patients who survived, after controlling for confounders we found that extremely obese patients have significantly longer times to extubation and ICU discharge with no significant difference in time to hospital discharge except in the BMI ≥ 60 kg/m2 group.
The mortality rate in the general population has been shown to be J-shaped, with increased mortality rates in underweight people, lowest mortality rates in patients with a BMI in the low to mid-20 range, and increasing mortality rates in overweight, obese, and extremely obese patients.1 In our study of critically ill patients, however, lower unadjusted mortality rates were found in overweight, obese, and extremely obese patients compared with normal-weight patients (which did not remain significant in the extremely obese group after controlling for potential confounders). Health-care providers might assume that extremely obese patients have higher mortality and morbidity due to presumed difficulties of caring for such patients, including positioning, transport, skin care, intravascular access, diagnostic imaging, and ventilator weaning. Although our results do show that extremely obese patients have longer durations of ventilation and ICU lengths of stay, their survival is at least as good as normal-weight patients.
Explanations for lack of mortality increase in extremely obese patients, when such an increase might be expected,28 are unclear. There may be a protective effect of extreme obesity in critical illness, although the etiology of this has not been fully elucidated. A recent study found that obese patients with acute lung injury have lower levels of several proinflammatory cytokines (IL-6, IL-8, and surfactant protein D).29 The lack of reduced mortality in obesity may therefore be due to an altered inflammatory response. An alternate hypothesis is that extremely obese patients may have a lower threshold for ICU admission compared with normal-weight patients, meaning the disease severity is less than perceived. For example, an obese patient with an infection, compared with a normal-weight patient, may have an increased heart rate due to their obesity, making them appear sicker and provoking an ICU admission when actually they are tachycardic from their obesity rather than their disease process. This possibility is supported by our data showing a lower APACHE II score in extremely obese patients with a BMI ≥ 60 kg/m2.
Reasons for increased DMV and ICU LOS in critically ill extremely obese patients who survive their ICU stay are, however, perhaps more understandable. The increased morbidity in the extremely obese population may be due to factors that may complicate or prolong mechanical ventilation and ICU LOS but do not necessarily increase mortality. For example, general ICU care, including turning and mobility, are often more difficult in extremely obese patients. Imaging modalities may also be limited (eg, weight limit for CT scan and MRI), which may restrict diagnostic and treatment capabilities. Additionally, potential lung derecruitment due to heavy chest wall, protuberant abdomen in the recumbent position, and provider reluctance to extubate an extremely obese patient who may have a difficult airway30 may contribute to higher morbidity. Extremely obese patients are more likely to be hypoxemic than people of normal weight,31 which, combined with problems positioning patients for intubation and difficult anatomy, may make securing an airway challenging.30 Another possibility is that difficulty in obtaining venous access in extremely obese patients and the reluctance of providers to remove venous catheters in the setting of infectious signs or symptoms may increase morbidity. Indeed, one study found that BMI ≥ 40 kg/m2 was an independent risk factor for catheter-related (OR, 2.2; 95% CI, 1.5-3.4) and other bloodstream infections (OR, 3.2; 95% CI, 1.9-5.3).32 Finally, it is possible that other differences in provided care between obese and nonobese patients may explain our results.7,33
Extremely obese patients were more likely to be younger and female, which is consistent with prior studies.3 They were also more likely to have respiratory admission diagnoses. Many intensivists, especially in the United States, may assume that critically ill extremely obese patients are transferred to teaching hospitals where there may be more available resources (such as ancillary services to assist in intravenous access or CT scanners that can accommodate larger patients), but this does not appear to be the case, as we found that extremely obese patients were more likely to be cared for in open ICUs at nonteaching hospitals.
Comparing our findings in the extreme obesity groups with the results of prior studies is difficult because, to our knowledge, prior research has not examined extremely obese critically ill patients by subdividing BMI as we have in this study. The literature to date with patients with BMI ≥ 40 kg/m2 examined as a cohort is quite variable, with some studies showing no difference in ICU outcomes and mortality,4,5 others reporting improved mortality,6‐8 and others finding longer ICU LOS, increased DMV, and increased mortality.14‐16 One metaanalysis from 2009 aggregating 23 studies found extremely obese patients as a group had similar hospital mortality (relative risk, 0.83; 95% CI, 0.66-1.04), a trend toward longer ICU and hospital stays, and no difference in DMV compared with patients of normal weight.17 A recent study found that patients who had 2009 influenza A(H1N1) who were obese had a longer DMV, ICU LOS, and hospital LOS without a difference in mortality.34
There are several limitations to our study. First, the data are observational and were most often recorded by clinical personnel at sites participating in the international ICU nutrition study, thus allowing for potential inaccuracy of some data (such as admission diagnosis) because they were obtained from the medical record rather than from strict definitions. Second, as mentioned above, even though we are able to report on a larger number of extremely obese patients than prior studies, the BMI ≥ 60 kg/m2 group remains relatively small and reflects the low proportion of these patients cared for in ICUs around the world. The small sample size limits the power and precision of our estimates for this most extreme group. Third, there is not a severity of illness assessment tool specifically for obese patients, and any existing assessment tool, including APACHE II, may not accurately reflect mortality risk in extremely obese patients because there may be “hidden” factors that are not accounted for in the assessment tool. Fourth, we were not able to adjust for comorbid illnesses because these data were not recorded during the survey. Fifth, measurement of BMI may be altered by IV fluid administration33 or may not accurately reflect obesity syndromes compared with other measurements, such as waist circumference, but these additional data were not collected during the survey. Sixth, because these data were gathered as part of an international survey, we were not able to protocolize weaning or sedation practices and other processes of care in participating ICUs, and it is possible that such variation may have biased our results. Additionally, it can be very difficult to assess critical illness in extremely obese patients (such as accuracy of noninvasive blood pressure measurements35 or interpretations of chest radiographs), and we may therefore be overestimating severity of illness in these patients, which would affect our results.36
In conclusion, our study demonstrates that extremely obese critically ill patients survive at least at often as normal-weight patients. Among survivors, however, the most extremely obese have a longer DMV, ICU LOS, and hospital LOS. As the prevalence of obesity continues to rise, it will be important for critical care physicians to consider these findings when discussing prognosis and expectations with patients and families. In addition, understanding the mechanism of obesity and critical illness will have clinical and research implications helping to improve the care of critically ill patients.
Acknowledgments
Author contributions: Dr Stapleton is the guarantor of the entire manuscript.
Dr Martino: contributed to study design, results interpretation, drafting the manuscript, and manuscript review.
Dr Stapleton: contributed to study design, results interpretation, and manuscript review.
Ms Wang: contributed to study design, statistical analysis, results interpretation, and manuscript review.
Mr Day: contributed to study design, statistical analysis, results interpretation, and manuscript review.
Ms Cahill: contributed to study design, database, study supervision, results interpretation, and manuscript review.
Dr Dixon: contributed to study design, results interpretation, and manuscript review.
Dr Suratt: contributed to study design, results interpretation, and manuscript review.
Dr Heyland: contributed to study design, database, study supervision, results interpretation, and manuscript review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Ms Cahill currently holds a Canadian Institutes for Health Research (CIHR) Fellowship in Knowledge Translation. Drs Martino, Stapleton, Dixon, Suratt, and Heyland; Ms Wang; and Mr Day have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in designing the study, collecting and analyzing the data, or preparing the manuscript.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- DMV
duration of mechanical ventilation
- HR
hazard ratio
- LOS
length of stay
Footnotes
Funding/Support: This study was supported by the National Institutes of Health [Grant R01-HL084200].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Ray DE, Matchett SC, Baker K, Wasser T, Young MJ. The effect of body mass index on patient outcomes in a medical ICU. Chest. 2005;127(6):2125–2131. doi: 10.1378/chest.127.6.2125. [DOI] [PubMed] [Google Scholar]
- 4.Frat JP, Gissot V, Ragot S, et al. Association des Réanimateurs du Centre-Ouest (ARCO) study group Impact of obesity in mechanically ventilated patients: a prospective study. Intensive Care Med. 2008;34(11):1991–1998. doi: 10.1007/s00134-008-1245-y. [DOI] [PubMed] [Google Scholar]
- 5.Sakr Y, Madl C, Filipescu D, et al. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34(11):1999–2009. doi: 10.1007/s00134-008-1243-0. [DOI] [PubMed] [Google Scholar]
- 6.Aldawood A, Arabi Y, Dabbagh O. Association of obesity with increased mortality in the critically ill patient. Anaesth Intensive Care. 2006;34(5):629–633. doi: 10.1177/0310057X0603400501. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien JM, Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34(3):738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrouste-Orgeas M, Troché G, Azoulay E, et al. Body mass index. An additional prognostic factor in ICU patients. Intensive Care Med. 2004;30(3):437–443. doi: 10.1007/s00134-003-2095-2. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest. 2003;123(4):1202–1207. doi: 10.1378/chest.123.4.1202. [DOI] [PubMed] [Google Scholar]
- 10.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 11.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134(5):974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris AE, Stapleton RD, Rubenfeld GD, Hudson LD, Caldwell E, Steinberg KP. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131(2):342–348. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 13.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring) 2008;16(3):515–521. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 14.Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005;20(3):147–154. doi: 10.1177/0885066605275314. [DOI] [PubMed] [Google Scholar]
- 15.El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120(6):1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 16.Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC. Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit Care Med. 2004;32(4):998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 17.Hogue CW, Jr, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–1170. doi: 10.1007/s00134-009-1424-5. [DOI] [PubMed] [Google Scholar]
- 18.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 19.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 21.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 22.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 23.Binder DA. Fitting Cox’s proportional hazards models from survey data. Biometrika. 1992;79(1):139–147. [Google Scholar]
- 24.Harrell FE. Regression Modeling Strategies: With Application to Linear Models, Logistic Regression and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 25.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: Wiley; 2000. [Google Scholar]
- 26.Lin D, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 27.Diaz JJ, Jr, Norris PR, Collier BR, et al. Morbid obesity is not a risk factor for mortality in critically ill trauma patients. J Trauma. 2009;66(1):226–231. doi: 10.1097/TA.0b013e31815eb776. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien JM, Jr, Aberegg SK, Ali NA, Diette GB, Lemeshow S. Results from the national sepsis practice survey: predictions about mortality and morbidity and recommendations for limitation of care orders. Crit Care. 2009;13(3):R96. doi: 10.1186/cc7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT. NHLBI Acute Respiratory Distress Syndrome Network The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138(3):568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walz JM, Zayaruzny M, Heard SO. Airway management in critical illness. Chest. 2007;131(2):608–620. doi: 10.1378/chest.06-2120. [DOI] [PubMed] [Google Scholar]
- 31.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318(5):293–297. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Dossett LA, Dageforde LA, Swenson BR, et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009;10(2):137–142. doi: 10.1089/sur.2008.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien JM, Jr, Welsh CH, Fish RH, Ancukiewicz M, Kramer AM. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med. 2004;140(5):338–345. doi: 10.7326/0003-4819-140-5-200403020-00009. [DOI] [PubMed] [Google Scholar]
- 34.Díaz E, Rodríguez A, Martin-Loeches I, et al. H1N1 SEMICYUC Working Group Impact of obesity in patients infected with 2009 influenza A(H1N1) Chest. 2011;139(2):382–386. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell MH, Waks AU, Schroth PC, Karam M, Dornfeld LP. Error in blood-pressure measurement due to incorrect cuff size in obese patients. Lancet. 1982;2(8288):33–36. doi: 10.1016/s0140-6736(82)91163-1. [DOI] [PubMed] [Google Scholar]
- 36.Honiden S, McArdle JR. Obesity in the intensive care unit. Clin Chest Med. 2009;30(3):581–599. doi: 10.1016/j.ccm.2009.05.007. [DOI] [PubMed] [Google Scholar]