Abstract
Background:
A recently published report from the United Kingdom suggested an association between sarcoidosis and pulmonary embolism (PE). We sought to examine whether this association was present among US decedents with sarcoidosis.
Methods:
We used data from the National Center for Health Statistics to investigate the association between sarcoidosis and PE among US decedents from 1988 to 2007.
Results:
From 1988 to 2007, there were 46,450,489 deaths in the United States and 23,679 decedents with sarcoidosis mentioned on their death certificates. Among these, 602 (2.54%) had PE mentioned on their death certificates, compared with only 1.13% of the background population (P < .0001 for comparison). The association between sarcoidosis and PE was significant regardless of gender (OR, 2.07; 95% CI, 1.80-2.39; P < .0001 for men and OR, 1.76; 95% CI, 1.59-1.96; P ≤ .0001 for women) or race (OR, 1.57; 95% CI, 1.41-1.76; P < .0001 for blacks and OR, 1.87; 95% CI, 1.63-2.14; P < .0001 for whites). Among decedents with sarcoidosis, there was no difference in risk of PE between men and women (2.30% vs 2.54%, χ2 = 1.32, P = .25) or between blacks and whites (2.60% vs 2.23%, χ2 = 3.09, P = .08). The association between sarcoidosis and PE held regardless of age.
Conclusions:
Using death certificate data from 1988 to 2007, we detected an association between sarcoidosis and PE regardless of gender, race, or age. Further investigation is needed to decipher the mechanisms of this apparent association.
Sarcoidosis, a multisystem, granulomatous, inflammatory disease of unknown cause,1 affects the lung parenchyma or mediastinal lymph nodes in > 90% of patients.2 Worldwide, the highest annual incidence of sarcoidosis (5-40 cases per 100,000 population) has consistently been observed in northern European countries.3 In the United States, young women and blacks4 develop sarcoidosis at rates that greatly outpace other demographics. Blacks are more likely than other racial groups in the United States to suffer extrathoracic organ (eg, skin or eye) involvement and a chronic disease course.2,5
Although clinically insignificant disease or spontaneous remission is common,5,6 in a significant minority of patients sarcoidosis is a chronic, debilitating, and even life-threatening condition.7‐9 Among people who die of sarcoidosis, deaths are attributed to respiratory, neurologic, or cardiovascular system involvement.8,10,11
Pulmonary embolism (PE) is a potentially fatal condition associated with numerous heritable or acquired conditions. Some investigators estimate the incidence of fatal PE to be 100,000 to 200,000 per year12,13; however, estimates vary greatly depending on the case definition used. Rates based on autopsy data likely overestimate the occurrence of clinically significant PE, whereas rates derived from clinical diagnoses likely underestimate the incidence.14
Recently, investigators in the United Kingdom identified an association between sarcoidosis and PE among patients admitted to National Health Service hospitals over a 35-year period.15 We sought to determine whether this association exists in the United States by examining death records from all US decedents from 1988 to 2007.
Materials and Methods
Briefly, we used multiple cause-of-death files, compiled and manipulated annually by the National Center for Health Statistics, that were derived from all US death certificates from 1988 to 2007. The National Center for Health Statistics applies computer algorithms to the death certificate data to produce a standardized “record axis.” The record axis includes up to 20 associated causes of death, including the underlying cause of death. A full description of the methods has been published previously.16,17 More details may be found in e-Appendix 1. We included in this study files from any decedent with “sarcoidosis” in the record axis. Besides using the background population (ie, all decedents without sarcoidosis) as the comparator, to examine the possibility of ascertainment bias, we also compared the risk of PE between decedents with sarcoidosis and those with COPD. For this particular analysis, we excluded decedents coded with both sarcoidosis and COPD.
We used χ2 tests to compare proportions between groups and logistic regression to determine the risk of PE between different age strata. All data were analyzed using SAS, version 9.2 (SAS Institute; Cary, North Carolina). We were not required to obtain institutional review board approval for this study because all data contained in the database files have been deidentified and are of public record.
Results
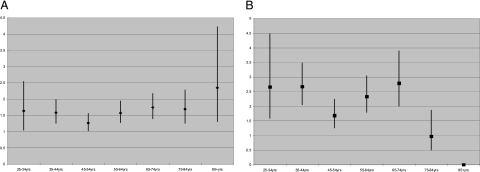
From 1988 to 2007, there were 46,450,489 deaths in the United States, and 23,679 multiple cause-of-death records contained a diagnostic code for sarcoidosis. Table 1 shows that among decedents with sarcoidosis, 602 (2.54%) had PE mentioned on their death certificates, compared with only 1.13% of the background population (P < .0001 for comparison). The association between sarcoidosis and PE was significant regardless of gender (women: OR, 1.76; 95% CI; 1.59-1.96, P ≤ .0001 and men: OR, 2.07; 95% CI, 1.80-2.39; P < .0001) or race (blacks: OR, 1.57; 95% CI, 1.41-1.76; P < .0001 and whites: OR, 1.87; 95% CI, 1.63-2.14; P < .0001) individually, or when the sample was stratified by both gender and race (Table 2). The association between sarcoidosis and PE was independent of age (Table 3). When we stratified by gender and age, the association between sarcoidosis and PE remained significant for each gender-age stratum except for men > 75 years old (Fig 1). The risk of PE among decedents with sarcoidosis was significantly greater than for decedents with COPD (2.58% vs 1.05%; χ2 = 474.5; OR, 2.50; 95% CI, 2.29-2.72; P < .0001).
Table 1.
—Numbers and Percentages of Decedents, Either in the Background Population or With Sarcoidosis, Who Had PE Mentioned on Their Death Certificates as Contributing to Death
| Background Population |
Sarcoidosis |
|||||
| Year | PE Absent | PE Present | % | PE Absent | PE Present | % |
| 1988 | 2,138,305 | 28,895 | 1.33 | 779 | 20 | 2.50 |
| 1989 | 2,120,865 | 28,749 | 1.34 | 824 | 28 | 3.29 |
| 1990 | 2,120,218 | 27,404 | 1.28 | 816 | 25 | 2.97 |
| 1991 | 2,142,204 | 26,424 | 1.22 | 866 | 24 | 2.70 |
| 1992 | 2,149,037 | 25,594 | 1.18 | 953 | 29 | 2.95 |
| 1993 | 2,242,723 | 24,867 | 1.10 | 948 | 15 | 1.56 |
| 1994 | 2,253,692 | 24,301 | 1.07 | 969 | 32 | 3.20 |
| 1995 | 2,285,923 | 25,067 | 1.08 | 1,112 | 30 | 2.63 |
| 1996 | 2,288,734 | 24,836 | 1.07 | 1,089 | 31 | 2.77 |
| 1997 | 2,288,940 | 24,228 | 1.05 | 1,058 | 19 | 1.76 |
| 1998 | 2,311,170 | 24,871 | 1.06 | 1,188 | 27 | 2.22 |
| 1999 | 2,365,025 | 25,302 | 1.06 | 1,049 | 23 | 2.15 |
| 2000 | 2,376,409 | 25,643 | 1.07 | 1,260 | 39 | 3.00 |
| 2001 | 2,388,670 | 26,378 | 1.09 | 1,345 | 32 | 2.32 |
| 2002 | 2,415,318 | 26,596 | 1.09 | 1,433 | 40 | 2.72 |
| 2003 | 2,419,565 | 27,248 | 1.11 | 1,429 | 46 | 3.12 |
| 2004 | 2,369,685 | 26,498 | 1.11 | 1,395 | 37 | 2.58 |
| 2005 | 2,423,871 | 27,323 | 1.11 | 1,478 | 42 | 2.76 |
| 2006 | 2,401,525 | 27,779 | 1.14 | 1,534 | 36 | 2.29 |
| 2007 | 2,399,052 | 27,876 | 1.15 | 1,552 | 27 | 1.71 |
| Totals | 45,900,931 | 525,879 | 1.13a | 23,077 | 602 | 2.54a |
PE = pulmonary embolism.
χ2 = 419.7; OR, 2.3; 95% CI, 2.1-2.5; P < .0001 for association between sarcoidosis and PE.
Table 2.
—Associations Between Sarcoidosis and PE for Decedents Stratified by Race and Gender
| Background Population |
Sarcoidosis |
||||||
| PE Absent | PE Present | % | PE Absent | PE Present | % | OR (95% CI) | |
| Black women | 2,617,913 | 46,375 | 1.74 | 8,349 | 236 | 2.75 | 1.60 (1.40-1.82) |
| Black men | 2,908,046 | 39,436 | 1.43 | 4,709 | 131 | 2.71 | 2.05 (1.72-2.44) |
| White women | 19,837,652 | 235,280 | 1.17 | 6,038 | 155 | 2.50 | 2.16 (1.85-2.54) |
| White men | 19,684,323 | 199,455 | 1.00 | 3,842 | 79 | 2.01 | 2.03 (1.62-2.55) |
Gender was not recorded for one subject with sarcoidosis and PE. All OR P < .0001. See Table 1 legend for expansion of abbreviation.
Table 3.
—Percentages of Decedents With Sarcoidosis and PE Stratified by Age
| Background Population |
Sarcoidosis |
||||||
| Age, y | PE Absent | PE Present | % | PE Absent | PE Present | % | OR (95% CI) |
| 15-24 | 675,922 | 4,063 | 0.60 | 122 | 1 | 0.81 | 1.36 (0.19-9.76) |
| 25-34 | 976,160 | 11,581 | 1.17 | 176 | 33 | 2.73 | 2.37 (1.67-3.34) |
| 35-44 | 1,748,754 | 26,246 | 1.48 | 3,750 | 125 | 3.21 | 2.22 (1.86-2.66) |
| 45-54 | 2,960,293 | 46,123 | 1.53 | 5,276 | 128 | 2.37 | 1.56 (1.31-1.87) |
| 55-64 | 4,957,182 | 73,876 | 1.47 | 4,810 | 140 | 2.83 | 1.95 (1.65-2.31) |
| 65-74 | 8,869,505 | 121,253 | 1.35 | 4,189 | 111 | 2.58 | 1.93 (1.61-2.34) |
| 75-84 | 13,136,018 | 148,437 | 1.13 | 2,964 | 52 | 1.57 | 1.53 (1.17-2.02) |
| ≥ 85 | 11,792,765 | 93,491 | 0.79 | 776 | 12 | 1.52 | 1.95 (1.10-3.45) |
There were 14 cases of sarcoidosis (none with PE) among decedents < 15 y. All OR P < .0001 except age 15-24 y (P = .7), age 75-84 y (P = .001), and age ≥ 85 y (P = .01). See Table 1 for expansion of abbreviation.
Figure 1.
Risk of pulmonary embolism (PE) among decedents with sarcoidosis relative to same-gender decedents in the background population. A, Female. B, Male. Bars = 95% CI; ♦ = OR point estimates for women; ■ = OR point estimates for men. All estimates statistically significant, except for 75- to 84-year-old and ≥ 85-year-old men.
Among the 23,679 decedents with sarcoidosis, the risk of PE was similar between men and women (2.30% vs 2.54%, χ2 = 1.32, P = .25) and between blacks and whites (2.60% vs 2.23%, χ2 = 3.09, P = .08). Compared with decedents 75 to 84 years old with sarcoidosis (the age stratum with the lowest risk when measured against the background), those in all but one other age strata were significantly more likely to have PE (25-34 years: OR, 1.78; 95% CI, 1.13-2.78; P = .012; 35-44 years: OR, 2.08; 95% CI, 1.48-2.92; P < .0001; 55-64 years: OR, 1.76; 95% CI, 1.26-2.46; P = .001; and 65-74 years: OR, 1.61; 95% CI, 1.14-2.27; P = .007). For decedents aged 45 to 54 years, there was a trend toward a greater risk of PE compared with decedents aged 75 to 84 years (OR, 1.37; 95% CI, 0.98-1.93; P = .06).
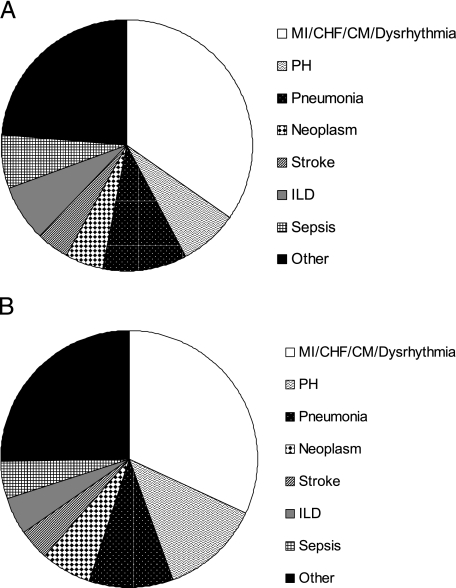
Comparing conditions mentioned on the death certificate as contributing to death among sarcoidosis decedents with and without PE yielded the following results: Those with PE were significantly more likely than decedents without PE to have pulmonary hypertension (PH) (10.6% vs 7.6%, P = .005), but significantly less likely to have any other cardiac disease, including myocardial infarction, myocardial ischemia, congestive heart failure, cardiomyopathy, cardiac dysrhythmia, or sudden cardiac death (combined total for all cardiac diseases other than PH 27.2% vs 34.7%, P = .0001), interstitial lung disease (4.2% vs 7.2%, P = .004), or sepsis (3.8% vs 6.9%, P = .003), and equally as likely to have solid organ neoplasm (5.3% vs 5.2%, P = .9), pneumonia (9.0% vs 10.6%, P = .2), or stroke (3.3% vs 4.1%, P = .3) (Fig 2).
Figure 2.
Conditions, other than sarcoidosis or PE, contributing to the death of decedents. A, Decedents with sarcoidosis and PE. B, Decedents with sarcoidosis but no PE. Dysrhythmia refers to cardiac dysrhythmia or sudden cardiac death; other refers to any other underlying cause of death. CHF = congestive heart failure; CM = cardiomyopathy; ILD = interstitial lung disease; MI = myocardial infarction/myocardial ischemia; PH = pulmonary hypertension. See Figure 1 legend for expansion of other abbreviation.
Discussion
We analyzed > 46 million records of US decedents from 1988 to 2007 and found that the risk of PE among the 23,679 people coded with sarcoidosis was more than twofold greater than the risk of PE in the background population, regardless of gender, race, or age. Among decedents with sarcoidosis, the risk of PE was independent of gender or race; men were just as likely as women to have PE, and whites were equally as likely as blacks to have PE. Any decedent with sarcoidosis, except those 45 to 55 years old, was significantly more likely to develop PE than sarcoidosis decedents 75 to 84 years old.
When we stratified by gender and age, we found the risk of PE among men ≥ 75 years was not greater than the background population. The reason for this is unknown; perhaps among the oldest-of-the-old men, PE was less likely than other entities to be considered as a diagnosis. For example, ischemic heart disease contributed to the deaths of 34% of decedents in this demographic; this suggests, as anticipated, that death certifiers were more concerned about, or convinced that, ischemic heart disease, rather than PE, was a major driver of mortality in this subgroup.
To our knowledge, only one other group has examined the risk of PE among patients with sarcoidosis in a large database. Crawshaw and colleagues15 identified 1,002 patients, < 65 years old, with an index admission to any UK National Health Service hospital with a primary diagnosis of sarcoidosis from 1963 to 1998. They then queried their database for postindex NHS hospitalizations for PE or other cardiovascular disorder. Compared with a reference population of > 526,000 people matched for age, gender, year of first admission, and district of residence, those with sarcoidosis were nearly twofold more likely to be given a diagnosis of PE. Unlike our study, they did not examine the effect of age, gender, or race on the association between sarcoidosis and PE. Unfortunately, we are not able to make any further comparisons between the results from their study and ours.
Why might people with sarcoidosis be at increased risk of developing PE? BAL fluid from patients with pulmonary sarcoidosis possesses procoagulant activity.18‐20 Alveolar macrophages from patients with sarcoidosis exhibit greater tissue factor activity than do macrophages from healthy control subjects.21 Circulating and BAL levels of fibrin degradation products (eg, D-dimers) have also been found by investigators to be elevated in patients with pulmonary sarcoidosis.22,23 Thus, sarcoidosis, via inflammatory or other biochemical mechanisms, may predispose to PE.
Another granulomatous, inflammatory condition, Crohn’s disease, has a well-established,24‐26 albeit poorly understood, association with VTE. That association is even stronger (ie, patients with Crohn’s disease are at even greater risk of PE) at the time of a Crohn’s disease flare.24 Whether this might also be true for sarcoidosis is not known; the data set we used does not allow us to determine the level of disease activity at the time the PE occurred.
Likewise, we are not able to examine smoking status (cigarette smoking being another risk factor for PE) in this data set. Although case-control studies suggest smoking is protective from a diagnosis of sarcoidosis,27,28 smoking status is not a variable in this database, so we could not be certain of the effect of smoking on the results we observed. Immobility (eg, as might be caused by progressive, severe sarcoidosis with respiratory, cardiac, or neurologic system involvement) is a known risk factor for PE. Maybe people with severe sarcoidosis develop PE simply because they become immobile; however, we would expect the same degree of immobility among people with severe COPD, and the risk of PE among decedents with sarcoidosis was significantly greater than the risk of PE among decedents with COPD.
Because sarcoidosis is a female-predominant disease, there is concern that oral contraceptive use (another well-known risk factor for PE) could confound the apparent association between the disease and PE. Clearly, that would not explain why men or older women with sarcoidosis develop PE. Nor could neoplasm account for the increased risk of PE in this study; sarcoidosis decedents with PE had the same number of neoplasms as those without PE.
Compared with decedents without PE, a greater percentage of sarcoidosis decedents with PE had PH mentioned in their death record; however, the overall percentage of sarcoidosis decedents with PH was only 7.8%. Furthermore, compared with that of the background population, the association between sarcoidosis and PE remained significant, and more than twofold greater, even after excluding decedents for whom PH was mentioned anywhere in their death record (data not shown). Sarcoidosis decedents with PE were less likely than those without PE to have certain other conditions that might have predisposed to PE, including myocardial infarction or ischemia, congestive heart failure, cardiomyopathy, cardiac dysrhythmia, sudden cardiac death, interstitial lung disease, or sepsis, and equally as likely to have pneumonia or stroke contribute to death (Fig 2).
The data set we used imposed limitations on our study: The nature of the database forced us to rely on death certifiers to correctly identify cases and then accurately code these conditions on the death certificate, and we had no way to assess the fidelity of the diagnoses for sarcoidosis or PE. Because in the United States the medical care of many patients with sarcoidosis is coordinated by pulmonologists, we suspect the pulmonologist was the physician most likely to have filled out the death certificate of decedents with sarcoidosis. This could have led to ascertainment (or overdiagnosis) bias for PE: Perhaps pulmonologists were more likely than primary care or other subspecialty physicians to contemplate the role that PE might have played in the death of someone with sarcoidosis. Even if that were true, the risk of PE among decedents with sarcoidosis was 2.5 times the risk of PE among decedents with COPD—another disease for which pulmonologists likely coordinate patients’ care. Some people with sarcoidosis, even absent VTE, have elevated D-dimer levels in peripheral circulation and BAL fluid. Overdiagnosis of PE could have occurred if an elevated D-dimer level were to be considered diagnostic for PE. Unfortunately, we have no way to determine how frequently that might have occurred in this data set.
We could not determine the status of many definite (eg, smoking status, oral contraceptive use, prior episodes of venous thromboembolic events, heritable hypercoagulable conditions) or possible (eg, sarcoidosis disease activity, acute infection) risk factors for PE. When sarcoidosis is mentioned on a death certificate, there can be little dispute that it was chronic, severe, or both: It contributed to death in some way. Because of the high incidence of spontaneous remission or clinically insignificant disease among people ever given a diagnosis of sarcoidosis in their lives, the results here should not only be viewed as strictly hypothesis generating, but also as possibly applying to only a subset of patients with sarcoidosis (ie, those with chronic and/or severe disease).
Finally, it is not accurate to consider the percentage of decedents with sarcoidosis, or the percentage of decedents with sarcoidosis and PE, as substitutes for prevalence estimates; the denominator for prevalence is people alive and at risk of the disease, not people who have already died. Our goal was not to derive prevalence estimates or to prove that sarcoidosis causes PE; rather, we aimed to further expose an apparent association that could generate hypotheses and pathophysiologic questions to be tested in prospective research.
Conclusions
In the United States from 1988 to 2007, > 2.5% of the 23,679 decedents with sarcoidosis had PE, more than twice the percentage observed in the background population. This increased risk of PE was present for both genders, blacks and whites, and for every age group. Among sarcoidosis decedents, men were equally as likely as women, and blacks were equally as likely as whites, to develop PE. What is driving the risk of PE in sarcoidosis requires further exploration; meanwhile, PE should be strongly considered as a potential explanation for worsening or potentially grave respiratory status in patients with chronic or severe sarcoidosis.
Supplementary Material
Acknowledgments
Author contributions: Dr Swigris: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Dr Olson: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Dr Huie: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Dr Fernandez-Perez: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Dr Solomon: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Mr Sprunger: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Dr Brown: contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Swigris receives funding via a National Institutes of Health K23 Career Development Award and has received money in the last 3 years for consultancy agreements with Actelion, Bayer, Genentech, and Gilead. Drs Olson, Huie, Fernandez-Perez, Solomon, and Brown and Mr Sprunger have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/5/1261/suppl/DC1.
Abbreviations
- PE
pulmonary embolism
- PH
pulmonary hypertension
Footnotes
Funding/Support: Dr Swigris is supported in part by a Career Development Award from the NIH [K23 HL092227].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Boeck C. Multiple benign sarcoid of the skin. J Cutan Genitourin Dis. 1899;17:543–550. [Google Scholar]
- 2.Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 3.Pietinalho A, Hiraga Y, Hosoda Y, Löfroos AB, Yamaguchi M, Selroos O. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis. 1995;12(1):61–67. [PubMed] [Google Scholar]
- 4.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139(1):144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki BA, Maliarik MJ, Major M, Popovich J, Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect. 1998;13(3):166–173. [PubMed] [Google Scholar]
- 7.Chappell AG, Cheung WY, Hutchings HA. Sarcoidosis: a long-term follow up study. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(2):167–173. [PubMed] [Google Scholar]
- 8.Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52(208):525–533. [PubMed] [Google Scholar]
- 9.Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130(1):29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119(2):167–172. [PubMed] [Google Scholar]
- 11.Takada K, Ina Y, Noda M, Sato T, Yamamoto M, Morishita M. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol. 1993;46(4):359–366. doi: 10.1016/0895-4356(93)90150-y. [DOI] [PubMed] [Google Scholar]
- 12.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151(5):933–938. [PubMed] [Google Scholar]
- 13.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17(4):259–270. doi: 10.1016/s0033-0620(75)80017-x. [DOI] [PubMed] [Google Scholar]
- 14.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23) suppl 1:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 15.Crawshaw AP, Wotton CJ, Yeates DG, Goldacre MJ, Ho LP. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax. 2011;66(5):447. doi: 10.1136/thx.2010.134429. [DOI] [PubMed] [Google Scholar]
- 16.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 17.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-Interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasday JD, Bachwich PR, Lynch JP, III, Sitrin RG. Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis. Exp Lung Res. 1988;14(2):261–278. doi: 10.3109/01902148809115128. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Gabazza EC, Taguchi O, et al. Protein C anticoagulant system in patients with interstitial lung disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1850–1854. doi: 10.1164/ajrccm.157.6.9709078. [DOI] [PubMed] [Google Scholar]
- 20.Nakstad B, Lyberg T, Skjønsberg OH, Boye NP. Local activation of the coagulation and fibrinolysis systems in lung disease. Thromb Res. 1990;57(6):827–838. doi: 10.1016/0049-3848(90)90150-b. [DOI] [PubMed] [Google Scholar]
- 21.Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986;133(3):437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- 22.Perez RL, Duncan A, Hunter RL, Staton GW., Jr Elevated D-dimer in the lungs and blood of patients with sarcoidosis. Chest. 1993;103(4):1100–1106. doi: 10.1378/chest.103.4.1100. [DOI] [PubMed] [Google Scholar]
- 23.Shorr AF, Hnatiuk OW. Circulating D-dimer in patients with sarcoidosis. Chest. 2000;117(4):1012–1016. doi: 10.1378/chest.117.4.1012. [DOI] [PubMed] [Google Scholar]
- 24.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657–663. doi: 10.1016/S0140-6736(09)61963-2. [DOI] [PubMed] [Google Scholar]
- 25.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53(4):542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novacek G, Weltermann A, Sobala A, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139(3):779–787. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Newman LS, Rose CS, Bresnitz EA, et al. ACCESS Research Group A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170(12):1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 28.Valeyre D, Soler P, Clerici C, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax. 1988;43(7):516–524. doi: 10.1136/thx.43.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.