Abstract
Background:
There is a renewed interest in adrenal function during severe sepsis. Most studies have used total serum cortisol levels; however, only free serum cortisol is biologically active. The aim of this study was to determine the validity of salivary cortisol levels as a surrogate for free serum cortisol levels during septic shock.
Methods:
Fifty-seven patients with septic shock were studied to determine the correlation between total serum cortisol and salivary cortisol to free serum cortisol levels. Thirty-eight patients were included in the salivary to free serum cortisol correlation. Salivary cortisol level was tested by enzyme immunoassay. Serum total cortisol, free cortisol, and cortisol-binding globulin (CBG) levels were determined by liquid chromatography-mass spectrometry, equilibrium analysis, and radioimmunoassay, respectively.
Results:
The mean ± SD age was 56.6 ± 18.5 years. Fifty-seven percent were women. APACHE (Acute Physiology and Chronic Health Evaluation) II score median was 26, Simplified Acute Physiology Score II median was 61, and Sequential Organ Failure Assessment median was 13. The correlation between salivary and free serum cortisol levels was 0.79 (95% CI, 0.63-0.89; P < .0001). The correlation between free serum cortisol and total serum cortisol levels was 0.86 (95% CI, 0.78-0.92; P < .0001). The mean ± SD free serum cortisol level was 2.27 ± 1.64 μg/dL. The mean ± SD salivary cortisol level was 2.60 ± 2.69 μg/dL. The mean ± SD total serum cortisol level was 21.56 ± 8.71 μg/dL. The mean ± SD CBG level was 23.54 ± 8.33 mg/dL.
Conclusions:
Salivary cortisol level can be used as a surrogate of free serum cortisol level in patients with septic shock with very good correlation. Salivary cortisol testing is noninvasive, easy to perform, and can be conducted daily.
Trial registry:
ClinicalTrials.gov; No.: NCT00523198; URL: www.clinicaltrials.gov
Balance between inflammation and antiinflammation is essential for survival during severe infections. Initially, studies performed during the late 1980s and 1990s using high doses of corticosteroids for short periods of time in severe sepsis and septic shock did not improve survival; even more, it increased the risk of dying.1‐4 More recently, a new concept of “relative” adrenal insufficiency in sepsis or critical illness-related corticosteroid insufficiency has been coined, and there has been a renewed interest in adrenal function during sepsis. Research studies using smaller doses of corticosteroids for longer period of time (5-7 days) were tested.5,6 In 2002, Annane et al7 performed a randomized controlled trial using hydrocortisone and fludrocortisone in patients with severe sepsis. They found that patients with less than expected response to adrenocorticotropic hormone (ACTH) stimulation test had better survival when corticosteroid therapy was used. A subsequent study (Corticosteroid Therapy of Septic Shock) failed to demonstrate improvement in survival; however, patients were not treated with fludrocortisone as in the Annane study.6 All these studies have used total serum cortisol levels to evaluate adrenal function, even though it is well known that only the unbound or free cortisol is biologically active. Free or unbound serum cortisol may be higher than expected during sepsis because of decrease in cortisol-binding globulin (CBG) and albumin. Eighty percent to 90% of serum cortisol is bound to CBG, approximately 5% to 10% is bound to albumin, and only 5% is unbound.8 CBG and albumin levels can decrease up to 50% within 24 h in severe infections and trauma.9,10 Hepatic biosynthesis of CBG is reduced during acute inflammation, and degradation of CBG occurs at sites of inflammation. CBG is rapidly cleaved by neutrophil elastase, allowing steroid release directly to the binding site to control the destructive potential of activated neutrophils.8
The laboratory method to measure free serum cortisol is technically demanding and not feasible for routine use. Salivary cortisol measurement is noninvasive, needs only a small volume of saliva, reflects the biologically active serum unbound cortisol level, is unaffected by variations in CBG, and is independent of salivary flow rate.11 The rate of appearance of cortisol in the saliva following an increase in the blood level is very rapid, < 5 min.12 Studies in healthy volunteers and for the evaluation of patients with hypothalamus-pituitary-adrenal axis disease have showed an excellent correlation between free or unbound serum and salivary cortisol levels, although the relationship is nonlinear and correlation is more closely related at lower levels (when total serum concentration is < 16.3 μg/dL).13‐15 We evaluated the correlation between salivary cortisol and total serum cortisol with free serum cortisol levels in adult medical intensive care patients with septic shock.
Materials and Methods
Patients
Adult (≥ 18 years of age) medical intensive care patients with septic shock, as per Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society international sepsis definitions conference in 1997 and 2001, were eligible for the study.16,17 Septic shock was defined as sepsis with acute circulatory failure characterized by persistent arterial hypotension unexplained by other causes. Hypotension was defined by a systolic arterial pressure < 90 mm Hg, a mean arterial pressure < 60, or a reduction in systolic BP > 40 mm Hg from baseline, despite adequate volume resuscitation, in the absence of other causes for hypotension, associated with organ dysfunction. Only patients in shock requiring vasopressors for suspected sepsis were enrolled.
Blood and saliva samples were obtained simultaneously, between 8:00 am and 10:00 am. The salivary samples were initially collected using two different methods and sent to ensure that the sample collected was enough for analysis (using eye sponges or oral suction with tracheal tubing connected to a trap). After the initial results, we found that eye sponges did not provide enough saliva for analysis. The rest of the salivary samples were obtained using the suction to a trap method. For study purposes, 20 mL of blood was collected. The blood was centrifuged for 10 min at 3,000 rpm. The serum was extracted and stored. Both samples were frozen at −70°C. The samples were sent in batches until the calculated sample size was reached. The initial batch was from patients 1 to 7, second batch from 8 to 15, third batch from 16 to 40, and fourth batch from 41 to 60. As per protocol design, salivary samples that were contaminated with blood (nasal or oral evidence of bleeding), patients who were recently treated with corticosteroids, and patients with withdrawal orders at the moment of evaluation were excluded.
The salivary cortisol was measured by enzyme immunoassay sent to reference laboratory (Salimetrics Laboratory; State College, Pennsylvania). The serum total cortisol, free serum cortisol, and CBG levels were determined by liquid chromatography-mass spectrometry, equilibrium analysis, and radioimmunoassay, respectively (Quest Laboratories; San Juan Capistrano, California). The patients were enrolled from February 2006 to June 2008. There were no significant changes in the management of the patients during this period. The enrollment was not consecutive, since as stated previously the samples were sent in batches to reach the calculated sample size. The primary intensive care team decided the management of all the patients independent of the research team.
The committee of protection of human subjects approved the study (IRB 06-0413). Written consent was obtained from the patient or next of kin.
Data Collection
Data collection included general demographics; hospital stay prior to septic shock diagnosis; comorbidities such as malignancy or AIDS; surgical or medical admission; APACHE (Acute Physiology and Chronic Health Evaluation) II score, Simplified Acute Physiology Score (SAPS) II, and Sequential Organ Failure Assessment (SOFA) score; source of infection if known; culture results; vasopressors types and dosage; hemogram; chemistries; total protein levels; arterial blood gas measurements; CBG levels; free and total serum cortisol levels; salivary cortisol levels; ventilator settings; and mortality (in-hospital or 28-day mortality, whichever was first).
Statistical Analysis
Hamrahian et al18 described that the correlation between free serum and total serum cortisol levels was 55% to 60% in critically ill patients. The correlation between free serum cortisol and salivary cortisol levels varies from patients with excess cortisol concentration, such as Cushing disease and psychiatric patients.15 Most studies available on salivary cortisol are in healthy volunteers or noncritically ill subjects with excess production of cortisol, and in these patients the correlation between salivary cortisol and free serum cortisol levels is 80% to 90%.19
Sample size was calculated using single correlations. The first correlation between free serum cortisol to total serum cortisol levels of 0.55 and the second correlation between free serum cortisol and salivary cortisol levels of 0.80 using a two-sided z test with a significance level of 0.05 and achieved an 0.81 power. A sample size of 37 was obtained.
Data Analysis
Simple regression and correlation were used to determine and predict the degree of association between salivary cortisol to free serum cortisol and free serum cortisol to total serum cortisol levels. Comparison mean and SD between free serum cortisol and salivary cortisol were analyzed using paired t test. Multivariable regression analysis was performed for variables with P value < .05. Mortality (28-day or in-hospital, whichever was first) was analyzed using logistic regression as secondary outcome. A P value < .05 was considered significant. All sample sizes and analyses were performed using a statistical software package (NCSS 2007, PASS 2005; NCSS; Kaysville, Utah).
Results
Of the 60 patients with septic shock who gave consent, three patients were excluded because of blood cortisol levels too high to quantify, leaving 57 patients in the free serum cortisol and total serum cortisol analysis. From the 57 patients, only 38 salivary samples (38 patients) were available for analysis (19 patients were excluded, three initial samples did not provide any saliva using the sponges, and 16 were eliminated because of insufficient saliva or blood contamination). We found no significant difference in demographics, severity of disease scores, and survival between both groups analyzed, the 57 in the free serum to total serum cortisol group and the 38 in the salivary cortisol to free serum cortisol group.
General group characteristics were as follows: mean ± SD age 56.6 ± 18.5 years (range 18-97 years), 30 female patients (57%), APACHE II score median 26 (range 8-42), SAPS II score median 61 (range 13-97), and SOFA score median 13 (range 3-20); 63% had APACHE II score ≥ 25, 81% SAPS II score ≥ 45, and 40% SOFA score ≥ 14. Seventy-two percent were mechanically ventilated at the moment of inclusion. Most of the patients were admitted from ED (75%) and enrolled within 48 h of ICU admission (75%). The rest of the patients were enrolled up to 16 days of septic shock. Thirty-five percent of the patients had pneumonia, 19% had urinary tract infection, 31% had bacteremia, one had meningitis, and one had peritonitis; the rest of the patients had skin infection and/or abscess. Cultures were positive in 40% of the patients. Albumin level was ≤ 2.5 g/dL in 50 of 57 patients (88%). See Table 1 for details. The characteristics of the subgroup of patients included in the salivary cortisol and free serum cortisol analysis are presented in e-Appendix 1.
Table 1.
—Patient Characteristics
| Characteristics | N = 57 | Range |
| Patient characteristics | ||
| Age, y | 56.6 (18.5) | 18-97 |
| Gender, No. (%) | … | |
| Women | 30 (53) | |
| Men | 27 (47) | |
| Admission category, No. (%) | … | |
| Medical admission | 50 (88) | |
| Emergency surgery | 5 (9) | |
| Elective surgery | 2 (3) | |
| Mortality, No. (%) | 23 (40) | … |
| Severity of illness | ||
| Temperature, °C | 37.2 (1.8) | 32.9-41.1 |
| Heart rate, beats/min | 109 (29) | 50-170 |
| Mean arterial pressure, mm Hg | 56 (7) | 38-68 |
| APACHE II score | 26 (8) | 8-42 |
| SAPS II score | 60 (18) | 13-97 |
| SOFA score | 13 (0.4) | 3-20 |
| Creatinine, mg/dL | 2.9 (2.1) | 0.5-9.5 |
| BUN, mg/dL | 38.9 (24.7) | 3-101 |
| Serum bicarbonate, mg/dL | 18.9 (4.8) | 7-31 |
| Albumin, g/dL | 2 (0.5) | 0.9-3.1 |
| Hemoglobin, g/dL | 9.2 (1.9) | 4.4-13.6 |
| Leukocytes, × 103/μL | 17,201 (12,818) | 2,300-69,000 |
| Platelet count, × 103/μL | 184,842 (126,092) | 22,000-570,000 |
| Aspartate aminotransferase, mg/dL | 163 (379) | 12-2,600 |
| Alanine aminotransferase, mg/dL | 98 (222) | 11-1,491 |
| Pao2/Fio2, mm Hg | 214 (116) | 50-512 |
| Vasopressors | ||
| Dopamine, μg/kg/min | 13.5 (5.6) | 8-20 |
| Norepinephrine, μg/min | 23.1 (47.7) | 1-150 |
| Phenylephrine, μg/min | 57.5 (47.7) | 1-100 |
| Vasopressin, unit/min | 0.04 (0.001) | 0.04-0.07 |
Data are presented as mean ± SD unless otherwise noted. APACHE = Acute Physiology and Chronic Health Evaluation; SAPS = Simplified Acute Physiology Score; SOFA = Sequential Organ Failure Assessment.
Norepinephrine was used alone (67%) or combined with another vasopressor (mainly vasopressin) in 84% of the patients. Dopamine was used alone in five patients (9%). None of the four patients requiring three vasopressors or receiving phenylephrine alone survived. Vasopressin was only used in combination with norepinephrine.
Cortisol Results
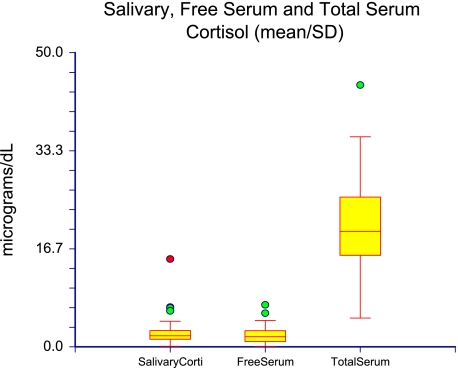
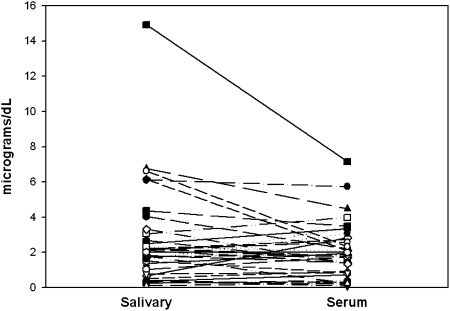
The total serum cortisol level was 21.56 ± 8.71 μg/dL (mean ± SD). CBG level was 23.54 ± 8.33 mg/dL, free serum cortisol level was 2.27 ± 1.64 μg/dL, and salivary cortisol level was 2.60 ± 2.69 μg/dL (Fig 1, Table 2). There was no significant difference between free serum cortisol and salivary cortisol levels (P = .28). Figure 2 shows the absolute value between salivary cortisol to free serum cortisol levels in μg/dL for each patient. See e-Appendix 2 for cortisol data from the 57 patients.
Figure 1.
Salivary cortisol, free serum cortisol, and total serum cortisol levels in μg/dL (mean ± SD). Corti = cortisol.
Table 2.
—Cortisol and Cortisol-Binding Globulin
| Substance Measured | μg/dL Mean (SD) | Range | nmol/L Mean (SD) | Range | μg/dL Median (25th-75th Percentile) | nmol/L Median (25th-75th Percentile) |
| Salivary cortisol | 2.6 (2.69) | 0.11-14.93 | 71.73 (74.21) | 3.03-411.89 | 2.02 (1.29-2.77) | 55.73 (35.59-76.42) |
| Free serum cortisol | 2.27 (1.64) | 0.09-7.15 | 62.62 (46.07) | 2.48-197.25 | 1.86 (0.90-2.75) | 51.32 (24.83-75.87) |
| Total serum cortisol | 21.56 (8.71) | 4.9-44.5 | 594.80 (240.29) | 135.18-1227.67 | 19.75 (15.57-25.45) | 544.9 (429.58-702.16) |
| Cortisol-binding globulina | 23.54 (8.33)a | 9-50 | … | … | 23 (17.75-29.25)b | … |
N = 38 patients.
Data given in mg/dL mean (SD).
Data given in mg/dL median (25th-75th percentile).
Figure 2.
Salivary cortisol and free serum cortisol levels for each individual patient in μg/dL.
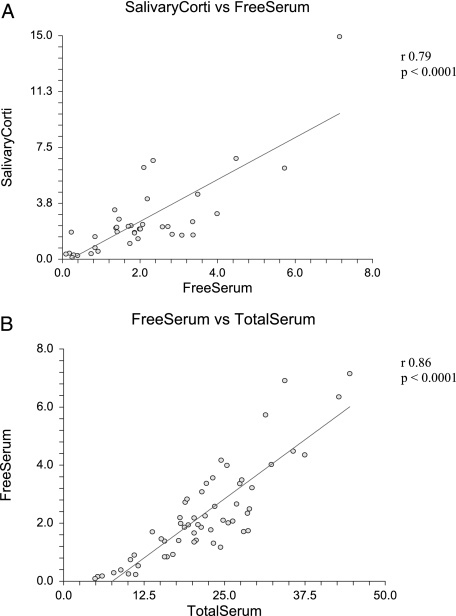
The Pearson correlation coefficient for salivary cortisol to free serum cortisol levels was 0.79 (r2, 0.63; 95% CI, 0.63-0.89) because the data were skewed, Spearman rank correlation coefficient was performed with similar results (0.70; 95% CI, 0.45-0.83), all with P value of < .0001. The coefficient of determination suggests that 63% of variation in salivary cortisol is accounted by variation of free serum cortisol (Fig 3A).
Figure 3.
A, Correlation between salivary cortisol and free serum cortisol in μg/dL (r = 0.79, P < .0001). B, Correlation between free serum cortisol and total serum cortisol in μg/dL (r = 0.86, P < .0001). See Figure 1 legend for expansion of the abbreviation.
The regression equation for free serum cortisol is
The data were transformed using square root to correct for normality and constant residual variance without significant changes in the results.
The Pearson correlation coefficient for free serum cortisol to total serum cortisol level was 0.86 (r2, 0.74; 95% CI, 0.78-0.92) because the data were skewed, Spearman rank correlation coefficient was performed with similar results (0.83; 95% CI, 0.72-0.89), all with P value < .0001. The coefficient of determination suggests that 74% of variation in total serum cortisol level is accounted for by variation of free serum cortisol level (Fig 3B).
The regression equation for free serum cortisol is
The data were transformed using square root to correct for normality and constant residual variance without significant changes in the results.
Only three variables correlate with low free serum cortisol concentration by multiple regression analysis: CBG level, total serum cortisol level, and patients who received etomidate any time prior to ICU admission (data not shown). Regression analysis did not show any correlation with other variables, such as age, gender, ICU severity scores, temperature, mean arterial pressure, WBC count, type of vasopressor, liver function tests, mechanical ventilation, Pao2/Fio2 ratio, source of infection, positive cultures, or survival. Multiple regression analysis did not show any correlation with low salivary cortisol level and any of the variables.
The in-hospital or 28-day mortality was 40%. Logistic regression analysis was performed to determine the odds ratio between cortisol levels and survival. The patients who survive had lower free serum cortisol concentration (OR, 0.45; 95% CI, 0.27-0.075; P = .002). The same was seen with salivary cortisol—better survival at lower levels (OR, 0.55; 95% CI, 0.34-0.91; P = .02). Lower total serum cortisol level also correlated with higher probability of survival (OR, 0.85; 95% CI, 0.77-0.94).
Discussion
We evaluated the correlation between salivary cortisol and total serum cortisol to free serum cortisol levels in patients with medical septic shock. We found the correlation between salivary cortisol and total serum cortisol levels to be very good (80%). In the majority of the cases, salivary cortisol can replace free serum cortisol and total serum cortisol measurements to determine adrenal function in adult medical intensive care patients with septic shock. Arafah et al20 studied critically ill patients using salivary cortisol levels to assess adrenal function. CBG and total serum cortisol concentrations were similar to the patients; however, free serum cortisol and salivary cortisol concentrations were significantly lower in the patients. We believe these differences are because their study subjects came from different ICUs and only 12 were patients with sepsis; many of their patients were not intubated.
We confirmed the finding by Arafah et al20 that albumin concentration does not alter the salivary cortisol concentration or free serum cortisol level, improving the usefulness of salivary cortisol measurements to study septic patients with suspected critical care adrenal insufficiency (data not shown). It should be noticed that, as stated previously, 88% of our patients have albumin concentration < 2.5 g/dL.
A prior study performed by Coolens in 198721 suggested that unbound plasma cortisol can be calculated using total serum cortisol and CBG measurements. Ho et al22 found an excellent correlation between measured free serum cortisol and calculated free serum cortisol using Coolens formula in patients with sepsis and septic shock. This was not the case in our study. The correlation between free serum cortisol and cortisol using Coolens method was very poor (0.004; P = .65; data not shown). Coolens formula has multiple assumptions, such as normal albumin, and only included healthy volunteers. We cannot explain this discrepancy, even after reviewing our results and recalculations. Our patients’ age ranges were wider than reported by Ho et al22 (mean 58 ± 20 years and 64 ± 2 years, respectively), but APACHE II scores and mortality were very similar (24 ± 7 and 40%, 25 ± 8 and 37%, respectively). However, we do not know from what type of ICU the patients were enrolled or any other characteristics.
It has been suggested that free serum cortisol should replace total serum cortisol measurement to evaluate adrenal function in critically ill patients with hypoproteinemia.18 This is very important, since 88% of our patients have serum albumin concentration < 2.5g/dL that we believe reflects a common pathophysiologic abnormality seen in patients with severe sepsis/septic shock. Albumin has a greater carrying capacity but lower affinity for cortisol than CBG. Both albumin and CBG are low in critically ill patients, explaining why a patient in the ICU may have normal free serum cortisol concentration despite low total serum cortisol concentration. In the Hamrahian et al18 study, only 11 patients had sepsis. Our subjects better reflect the type of patients seen in a medical ICU with severe sepsis, in whom corticosteroids may be beneficial.
In our study (as well as prior reported studies in patients with septic shock), serum CBG concentration was lower than observed in healthy individuals (mean 23.5 ± 8.3 mg/dL).9,21 The lower CBG concentration in sepsis is multifactorial because of decreased liver synthesis, proteolytic cleavage by neutrophil elastase, and less common genetic mutations.8,9,23 Also during sepsis, CBG has low binding activity for serum cortisol.24 All these mechanisms may explain the increase of free serum cortisol available to interact with its receptors. It has been suggested that after CBG reaches saturation point the increase in serum free cortisol concentrations is reflected in salivary cortisol in a few minutes (< 5 min), even when total serum cortisol is still low.25 Salivary cortisol determination omits the need to measure CBG to calculate the free cortisol level, and it is less altered by changes in protein concentration.
Contrary to prior studies, we did not find salivary cortisol concentrations one-third lower than free serum cortisol concentration (hypothesized because of the conversion of cortisol to cortisone in the salivary glands). In our study the mean salivary cortisol was higher than the mean free serum cortisol, without reaching statistical significance.12,19 Prior salivary cortisol studies were performed in healthy volunteers and not ICU patients with septic shock in whom normal adrenal function and cortisol concentrations are still unknown. It has been postulated that salivary cortisol determination is more useful, especially after ACTH stimulation test, since it reflects immediate changes in the unbound serum cortisol without being affected by the CBG or albumin concentration.25
It has been suggested that critically ill patients with serum free cortisol concentration ≤ 2 μg/dL are at risk for adrenal insufficiency.18 Using this criterion, 53% (30 of 57) of the patients by free serum cortisol and 47% (18 of 38) by salivary cortisol concentration probably have adrenal insufficiency. Annane et al26 found that 60% (24 of 40) of patients with sepsis with free serum concentration ≤ 2 μg/dL had adrenal insufficiency after overnight metyrapone stimulation test, reflecting a high prevalence of adrenal insufficiency in patients with septic shock.
Salivary cortisol was mildly elevated in some patients compared with free serum cortisol. In one case, the salivary cortisol was very high (14.93 μg/dL), twice the free serum cortisol concentration. We could not identify any significant difference between this case and the rest of the study group. The relationship between salivary cortisol and serum total cortisol concentration is nonlinear, with a rapid increase in salivary concentration once the serum CBG is saturated. CBG binds cortisol with high affinity but low capacity, whereas albumin has low affinity but an almost infinite capacity. CBG is saturated at a serum concentration of 16 to 18 mg/dL. When the concentration exceeds CBG saturation, the free fraction increases.14 The transfer from serum to saliva occurs by free diffusion of unbound cortisol through the acinar cells of the salivary glands.11 In healthy volunteers, up to 15% of salivary cortisol is bound to CBG, a normal component of parotid saliva.27 However, we do not know whether this holds true in patients with sepsis. Moreover, little is known about the parotid gland conversion from cortisol to cortisone due to the presence of 11β-hydroxysteroid dehydrogenase 2 in salivary glands. This does not affect the correlation of salivary cortisol and free serum cortisol in healthy volunteers,13,28 but during sepsis changes in CBG or 11β-hydroxysteroid dehydrogenase 2 may explain the mild increase in concentration of free salivary steroids compared with serum free levels.
One limitation of our study was obtaining enough saliva for testing prior to fluid resuscitation in patients with sepsis. Although the reference laboratory has reported results with as little as 0.1 mL of saliva, we realized after sending the initial batches that at least 1 mL of saliva was necessary to obtain results. We do not know if this was due to the storage of the sample. However, prior studies stored their samples for further analysis without any mention of lack of material. Nevertheless, it is controversial how early corticosteroids should be replaced.
We did not evaluate the response to ACTH stimulation since we were mainly interested in establishing the relationship between salivary and free cortisol levels to allow further studies in adrenal function during severe sepsis. This was not an interventional study; it is not known if a randomized controlled study replacing cortisol using salivary samples will change patients’ outcomes, but there are no studies, to our knowledge, using free serum cortisol either. We believe this question can be answered now since salivary cortisol can replace free serum cortisol measurement in most cases, and the correlation is very good.
We cannot draw any conclusion about the observation that patients with lower total serum, free serum, and salivary cortisol levels have lower mortality, since some of these patients received corticosteroid by the ICU team after the samples were obtained. The alarming mortality observed in our study is well recognized in septic shock and supports the urgent need for funding and further research to revert the course of this highly lethal syndrome.
In summary, we have demonstrated that salivary cortisol can be used as a surrogate of free serum cortisol in patients with septic shock with very good correlation. This is the first study to our knowledge that measures salivary cortisol and free serum cortisol levels in intensive care patients with septic shock. We have demonstrated that salivary cortisol measurements are feasible and may help to resolve some of the controversy about whether the treatment of adrenal insufficiency will improve the outcomes in ICU patients with septic shock. Salivary cortisol measurement is noninvasive, easy to use, and can be performed daily; the result can be obtained in few hours. Total serum cortisol concentrations can be misleading and should be abandoned. There is enough evidence to use free serum cortisol concentration, the biologically active hormone, instead of total serum cortisol measurements, and we postulate that this is one reason why studies replacing corticosteroids in sepsis have been inconclusive.
Supplementary Material
Acknowledgments
Author contributions: Dr Estrada-Y-Martin contributed to study design, data collection and analysis, and writing of the manuscript.
Dr Orlander: contributed to study design and review of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendices can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/5/1216/DC1.
Abbreviations
- ACTH
adrenocorticotropic hormone
- APACHE
Acute Physiology and Chronic Health Evaluation
- CBG
cortisol-binding globulin
- SAPS
Simplified Acute Physiology Score
- SOFA
Sequential Organ Failure Assessment
Footnotes
Funding/Support: This study was supported by the National Institutes of Health [Grant #5UL1RR024148].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317(11):653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 2.The Veterans Administration Systemic Sepsis Cooperative Study Group Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317(11):659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 3.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26(4):645–650. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311(18):1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 5.Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27(4):723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Sprung CL, Annane D, Keh D, et al. CORTICUS Study Group Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 8.Hammond GL, Smith CL, Underhill DA. Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J Steroid Biochem Mol Biol. 1991;40(4-6):755–762. doi: 10.1016/0960-0760(91)90300-t. [DOI] [PubMed] [Google Scholar]
- 9.Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med. 2001;27(10):1584–1591. doi: 10.1007/s001340101073. [DOI] [PubMed] [Google Scholar]
- 10.Torpy DJ, Ho JT. Value of free cortisol measurement in systemic infection. Horm Metab Res. 2007;39(6):439–444. doi: 10.1055/s-2007-980200. [DOI] [PubMed] [Google Scholar]
- 11.Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29(10):1752–1756. [PubMed] [Google Scholar]
- 12.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20(pt 6):329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 13.Riad-Fahmy D, Read GF, Walker RF, Griffiths K. Steroids in saliva for assessing endocrine function. Endocr Rev. 1982;3(4):367–395. doi: 10.1210/edrv-3-4-367. [DOI] [PubMed] [Google Scholar]
- 14.Aardal E, Holm AC. Cortisol in saliva—reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33(12):927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 15.Castro M, Elias PC, Martinelli CE, Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000;33(10):1171–1175. doi: 10.1590/s0100-879x2000001000006. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 17.Muckart DJ, Bhagwanjee S. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med. 1997;25(11):1789–1795. doi: 10.1097/00003246-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 19.Umeda T, Hiramatsu R, Iwaoka T, Shimada T, Miura F, Sato T. Use of saliva for monitoring unbound free cortisol levels in serum. Clin Chim Acta. 1981;110(2-3):245–253. doi: 10.1016/0009-8981(81)90353-3. [DOI] [PubMed] [Google Scholar]
- 20.Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92(8):2965–2971. doi: 10.1210/jc.2007-0181. [DOI] [PubMed] [Google Scholar]
- 21.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26(2):197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 22.Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91(1):105–114. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
- 23.Lin HY, Muller YA, Hammond GL. Molecular and structural basis of steroid hormone binding and release from corticosteroid-binding globulin. Mol Cell Endocrinol. 2010;316(1):3–12. doi: 10.1016/j.mce.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Pugeat M, Bonneton A, Perrot D, et al. Decreased immunoreactivity and binding activity of corticosteroid-binding globulin in serum in septic shock. Clin Chem. 1989;35(8):1675–1679. [PubMed] [Google Scholar]
- 25.Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic—pituitary—adrenal axis activity. Clin Endocrinol (Oxf) 2005;63(3):336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 26.Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174(12):1319–1326. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 27.Chu FW, Ekins RP. Detection of corticosteroid binding globulin in parotid fluids: evidence for the presence of both protein-bound and non-protein-bound (free) steroids in uncontaminated saliva. Acta Endocrinol (Copenh) 1988;119(1):56–60. doi: 10.1530/acta.0.1190056. [DOI] [PubMed] [Google Scholar]
- 28.Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95(11):4951–4958. doi: 10.1210/jc.2010-1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.