Abstract
In quantitative proteomics stable isotope labeling has progressed from cultured cells toward the total incorporation of labeled atoms or amino acids into whole multicellular organisms. For instance, the recently introduced 13C6-lysine labeled SILAC mouse allows accurate comparison of protein expression directly in tissue. In this model, only lysine, but not arginine, residues are isotope labeled, as the latter may cause complications to the quantification by in vivo conversion of arginine to proline. The sole labeling of lysines discourages the use of trypsin, as not all peptides will be quantifiable. Therefore, in the initial work Lys-C was used for digestion. Here, we demonstrate that the lysine-directed protease metalloendopeptidase Lys-N is an excellent alternative. As lysine directed peptides generally yield longer and higher charged peptides, alongside the more traditional collision induced dissociation we also implemented electron transfer dissociation in a quantitative stable isotope labeling with amino acid in cell culture workflow for the first time. The utility of these two complementary approaches is highlighted by investigating the differences in protein expression between the left and right ventricle of a mouse heart. Using Lys-N and electron transfer dissociation yielded coverage to a depth of 3749 proteins, which is similar as earlier investigations into the murine heart proteome. In addition, this strategy yields quantitative information on ∼2000 proteins with a median coverage of four peptides per protein in a single strong cation exchange-liquid chromatography-MS experiment, revealing that the left and right ventricle proteomes are very similar qualitatively as well as quantitatively.
The introduction of stable isotopes into cells has significantly advanced quantitative proteomics (1–3). This technology cannot only be applied to cells in culture but also on whole organisms (reviewed by Gouw et al. (4)). This includes single cell organisms such as Escherichia coli (5) and Saccharomycis cerevisiae (6), but also higher order organisms such as Drosophila melanogaster (7) and Caenorhabditis elegans (7, 8). Even mammalian organisms such as rat (9) have been metabolically labeled with stable isotopes for proteomics experiments. The latest extension to this repertoire is the stable isotope labeling with amino acid in cell culture (SILAC)1 mouse (10), in which all lysine residues are replaced by a variant bearing six 13C-atoms. No dual labeling of arginine and lysine was used for this mouse model, to circumvent potential issues with in vivo conversion of arginine to proline (11, 12). Therefore, to quantitate all digested peptides, the analysis of the SILAC mouse favors the use of a lysine-only directed protease. In the initial demonstration of the SILAC mouse, Lys-C was used for the proteomic analysis, but an extension with other lysine directed proteases was already suggested (10). We have previously explored the usage of another lysine directed protease, Lys-N, and showed its complementarity in terms of protein sequence coverage and mapping of post-translational modifications when compared with trypsin and Lys-C (13, 14). Furthermore, Lys-N is also an excellent choice for sequencing when using electron transfer dissociation (ETD) because peptides either yield simple tandem mass spectra with merely c-type ions (15), or are rich in information (16). ETD is emerging as a complementary peptide fragmentation method that particularly performs well on higher charged (≥3+) peptides (17, 18). Although ETD proved to be very capable for peptide sequencing, until now little attention to none has been paid to its use in quantitative proteomics workflows.
The cardiac, and other muscle type, proteomes are notoriously difficult to investigate because one needs to overcome a large dynamic range of protein expression. Similar to plasma, the cardiac proteome contains a small subset of high abundant muscle proteins that obscure less abundant proteins (19). Predictions reveal that the cardiac transcriptome encodes for ∼9400 proteins in cardiac tissue (20), however the most in-depth proteomics study into a cardiac proteome has thus far revealed around half of that number (Table I) and most studies cover ∼30%. Therefore, an extension of the SILAC mouse toolbox is particularly interesting for the field of cardiac proteomics. Here, we present such an extension using Lys-N and a combination of CID and ETD to quantify proteome differences between the left (LV) and the right (RV) ventricle of the mouse heart. We covered 3749 unique proteins with on average 10 peptides per protein (median of 3–4 peptides), demonstrating that our method is a powerful addition to the SILAC mouse workflow to quantify protein expression directly in mouse tissue. The quantitative data revealed that differences between the LV and RV proteomes are more subtle than expected.
Table I. Different cardiac proteomes compared. Overview of large scale proteome analyses into the cardiac proteomes of both mouse and human tissues using both qualitative and quantitative (SILAC mouse) workflows. As shown, different sample preparations were used: SCX+RP (strong cation exchange separation of peptides followed by online reversed phase LC-MS/MS), GelC-MS (SDS-PAGE separation of proteins followed by in-gel digestion and RP-LC-MS/MS), SAX (Strong anion exchange), 2D-GE (2-dimensional gel electrophoresis, * depicts the amount of gel spots, not the amount of identified proteins by peptide identifications), n.a., not applicable.
| Species | Compartment | # Proteins identified | Art of Study | Method | Protease | Instrument | Subcellular Fractionation | Reference, Year |
|---|---|---|---|---|---|---|---|---|
| Mouse | Ventricle | 4906 | Qualitative | SCX+RP (MudPIT) | Trypsin | Linear Ion Trap (LTQ) | + (4 fractions) | (21), 2008 |
| Mouse | Whole heart | 5736 | Qualitative | SCX+RP GelCMS IMAC | Trypsin | LTQ-Orbitrap | - | (22), 2010 |
| Mouse | Whole Heart | 2626 | Quantitative Aging (SILAC mouse) | SAX+RP | Lys-C | LTQ-Orbitrap | - | (23), 2008 |
| Mouse | Ventricle | 3749 | Quantitative LV/RV (SILAC mouse) | SCX+RP CID/ETD | Lys-N | LTQ-Orbitrap-ETD | - | This study, 2011 |
| Human | Left Ventricle | 2663* | Qualitative | 2D-GE | n.a. | n.a. | - | (24), 2007 |
| Human | Left Ventricle | 3584 | Qualitative | SCX+RP | Trypsin | LTQ-Orbitrap | - | (19), 2010 |
| GelC-MS | Lys-N | Linear Ion Trap | ||||||
| Chymotrypsin | LTQ-Orbitrap-ETD |
EXPERIMENTAL PROCEDURES
Mice and Heart Tissue
One female, ∼1 year old SILAC mouse was generated as described previously (10). Two matching female wild-type mice (C57Bl/6) were obtained from Harlan (Venray, The Netherlands). From the SILAC mouse, the heart was dissected out, flushed with ice-cold phosphate-buffered saline (PBS) and immediately frozen in liquid nitrogen and stored at −80 °C until use. Prior to lysis, the myocardium as a whole (left ventricle (LV), right ventricle (RV) and septum) was carefully separated from the atria. For the wild-type mice, animals were sacrificed and the heart isolated. LV and RV were separated from the septum and the atria, prior to snap freezing in liquid nitrogen and storage at −80 °C. Animal experiments were performed in accordance with institutional guidelines for animal use in research.
Sample Preparation
Tissue samples were taken from −80 °C storage and transferred to liquid nitrogen. Subsequently the frozen tissue was pulverized in a custom made precooled (liquid nitrogen) steel mortar. The SILAC myocardium was taken up in 1 ml of lysis buffer (50 mm ammonium bicarbonate, 8 m urea, complete mini protease EDTA free inhibitor mixture (Roche, 1 tablet in 15 ml buffer) and 0.1% phosphatase inhibitor mixture (Sigma)). The wild-type LV and RV were taken up in 500 μl and 250 μl lysis buffer respectively. Samples were left at room temperature for 5 min before centrifugation in a table top eppendorf centrifuge at 14,000 rpm and 4 °C. The supernatant was carefully collected and the pellet resuspended, once more, in an equal amount of lysis buffer. After spinning, the second supernatant was collected and the pellet was resuspended again in an equal volume. Sonication was used to increase protein solubility and the third supernatant was combined with the first two. Bradford analysis was performed to establish protein concentrations. Samples were aliquoted and stored at −80 °C until further use.
Wild-type LV or RV was mixed with an equal amount (in weight) of the SILAC labeled myocardium (250 μg each). Subsequently, the mixed lysate was reduced with dithiothreitol (DTT) (45 mm, 15 min at 50 °C) and alkylated with iodoacetamide (110 mm, 15 min, room temperature in the dark). Digestion with metalloendopeptidase Lys-N (isolated from Grifola frondosa, Seikagaku Corporation, Tokyo, Japan) was performed in two steps, for 4 h (w/w 1:85) and again overnight (w/w 1:100) at room temperature. Two biological replicates were analyzed using two wild-type mice and a single SILAC labeled mouse as internal standard, yielding in total four experiments: LV1/SILAC, RV1/SILAC, LV2/SILAC, and RV2/SILAC.
Peptide Prefractionation by Strong Cation Exchange (SCX) (13,16)
Peptides from each digest (100 μg total peptides) were loaded onto two C18 cartridges using an Agilent 1100 HPLC system. The flow rate applied was 100 μl/min using water pH 2.7 as solvent. After that, peptides were eluted from the trapping cartridges with 80% acetonitrile pH 2.7 onto a PolySULFOETHYL A 200 × 2.1 mm column (PolyLC) for 10 min at the same flow rate. Separation of different peptide populations was performed using a nonlinear 65 min gradient, 0 to 10 min 100% solvent A (5 mm KH2PO4, 30% acetonitrile, pH 2.7), 10 to 15 min up to 26% solvent B (5 mm KH2PO4, 30% acetonitrile, 350 mm KCl, pH 2.7), 15 to 40 min to 35% solvent B and from 40 to 45 min to 60% solvent B. At 49 min the concentration of solvent B was 100%. The column was subsequently washed for 6 min with high salt concentration and finally equilibrated with 100% solvent A for 9 min. The flow rate applied during the SCX gradient was 200 μl/min. Fractions were collected in 1 min intervals for 40 min. After evaporation of the solvents, fractionated peptides were resuspended in 10% formic acid.
Mass Spectrometry
For MS-analysis 25–28 SCX fractions with charges between 2+ and ∼5+ were selected and analyzed on a reversed phase nano-LC-coupled LTQ-Orbitrap XL ETD (Thermo Fisher Scientific). An Agilent 1200 series HPLC system was equipped with a 20 mm Aqua C18 (Phenomenex) trapping column (packed in-house, 100 μm i.d., 5 μm particle size) and a 400 mm ReproSil-Pur 120 C18-AQ (Dr. Maisch GmbH) analytical column (packed in-house, 50 μm i.d., 3 μm particle size). Trapping was performed at 5 μl/min solvent C (0.1 m acetic acid in water) for 10 min, and elution was achieved with a gradient from 10 to 30% (v/v) solvent D (0.1 m acetic acid in 4:1 acetonitrile:water) in solvent C in 110 min, followed by a gradient of 30 to 50% (v/v) solvent D in solvent C in 30 min, followed by a gradient of 50 to 100% (v/v) solvent D in solvent C in 5 min and finally 100% solvent D for 2 min. The flow rate was passively split from 0.35 ml/min to 50 nL/min. Nano-electrospray was achieved using a distally coated fused silica emitter (360 μm o.d., 20 μm i.d., 10 μm tip i.d.; New Objective) biased to 1.7 kV. The LTQ-Orbitrap ETD was operated in the data dependent mode to automatically switch between MS and MS/MS. Survey full scan MS spectra were acquired from m/z 350 to m/z 1500 in the Orbitrap with a resolution of 60,000 at m/z 400 after accumulation to a target value of 500,000 in the linear ion trap. The two most intense ions at a threshold of above 500 were fragmented in the linear ion trap using collision-induced dissociation (CID) at a target value of 30,000 and electron-transfer dissociation with supplemental activation (ETD) at a target value of 50,000. The ETD reagent target value was set to 100,000 and the reaction time to 50 ms.
Identification and Quantitation with Proteome Discoverer
All raw data files of the individual SCX fractions of each of the four experiments (LV1/SILAC, RV1/SILAC, LV2/SILAC, and RV2/SILAC) were imported into Proteome Discoverer v2.1.201 (Beta-version) and the combined peak list for each of these four experiments was split into CID and ETD data before database searching. Subsequently, CID and ETD peak lists were searched individually against an International Protein Index (IPI, http://www.ebi.ac.uk/ipi) database containing mouse sequences and common contaminants such as bovine serum albumin and human keratins (IPI-Mouse v3.36; 51326 sequences; 23682061 residues) through a direct connection to our in-house Mascot server (Mascot v2.2.04, Matrix Science, London, UK). The following settings were used: Carbamidomethylation on cysteines as static modification; 13C6-Lysine and oxidation on methionine as variable modifications; precursor mass tolerance of 10 ppm and 0.5 Da on the fragment masses; 1 missed cleavage allowed. For ETD-data processing the precursor, the charge reduced precursor and all known neutral losses within a window of 120 Da from the charge reduced precursor were removed from the MS2 spectra. The enzyme was specified as Lys-N, and the fragment ion type was specified as either electrospray ionization (ESI)-TRAP or ETD-TRAP, depending on the type of fragment ion spectrum being searched. For both identification and quantitation, only spectra within the score limits of the 1% false discovery rate (FDR) were accepted, based on Mascot score thresholds. These were calculated independently for CID and ETD data in each dataset using the built-in FDR calculator of Proteome Discoverer, which is based on Mascot's built-in FDR calculation. The event detector and precursor ion quantifier algorithms of Proteome Discoverer were used for quantitation using a 2 ppm mass variability and a 0.2 min retention time tolerance on precursor ion pairs. Quantitation is based on the ratio of the summed areas of two matched isotope patterns (a feature) across the eluting chromatographic peak of that feature. The peptide ratios are calculated using the same number of isotopes. Protein ratios are based on the median peptide ratio. LV/RV protein ratios are based on the observation of a single feature in both LV/SILAC and RV/SILAC in the same mouse sample. At least 2 isotopic peaks were required for inclusion, as well as a minimal signal to noise level of 3. For each dataset (LV1, RV1, LV2, RV2), the ratios were corrected toward 1.0 via the median ratio. Proteins were grouped by Proteome Discoverer at the level of each mouse. Exports of peptide and protein lists from Proteome Discoverer were further analyzed using the Tableau software package (www.tableausoftware.com), allowing us to cross correlate data between the four individual data sets at unique peptide (unique combinations of sequence + modification(s)) and protein level. Spotfire (www.spotfire.tibco.com) was used for visualization. Scaffold data files (www.proteomesoftware.com) of fragmentation spectra are publicly available in the repository Tranche (https://proteomecommons.org/) using the following hash code: MwZCiqtgxaX+ZXVqP60fXfIASJCcUiPBIkUjZqx31pZtL94dEi4pIEBfN2J11BM/2FJEE4w79msmWZpaq6GuHkhH5uMAAAAAAAADiA = =, Password: M8NnUk9Yxj9AAVqtZ1xN.
RESULTS
The use of lysine directed proteases generally yields longer and potentially higher charged peptides when compared with trypsin, leading us to investigate the potential benefit of ETD and Lys-N to further increase the applicability of the SILAC mouse and other SILAC-based methods (Fig. 1A). Illustrative of this, we investigated the differential proteome of the isolated mouse LV and RV. Wild type LV and RV tissues were individually mixed with the internal standard that originated from whole heart (LV+RV+septum) 13C6-Lysine labeled SILAC mouse tissue (Fig. 1A). After digestion with Lys-N, the LV/SILAC and RV/SILAC samples were separated using SCX before analysis by reversed phase LC-MS/MS. Each selected peptide precursor was subjected to both CID and ETD sequencing. An overview of the acquired proteomics data is shown in Table II and supplemental Tables S1 and S2.
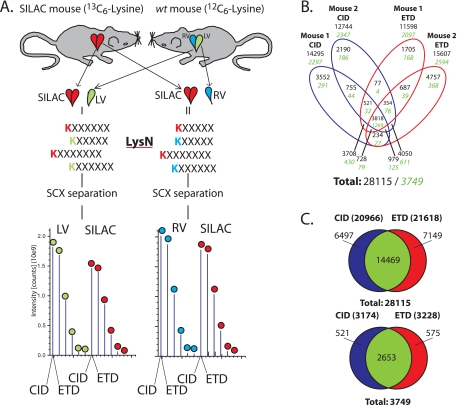
Fig. 1.
Schematic overview of the used workflow. A, Protein extracts of carefully dissected LV (green) and RV (blue) of age and sex matched wild-type mice were mixed 1:1 with whole heart (i. e. LV, RV, and septum) 13C6-Lysine SILAC mouse lysate (red) which was used as internal standard. LV+SILAC and RV+SILAC protein mixtures are individually digested with Lys-N, before peptide separation using strong cation exchange (SCX) and LC-MS/MS analysis applying both CID and ETD on each precursor peptide ion selected for tandem MS. B, Identified unique peptides (black text, unique sequence and modification combinations filtered for rank 1, FDR 1%) and proteins (green italic) in both mice (2 experiments per mouse, LV/SILAC and RV/SILAC). C, Total number of identified unique peptides (top) and proteins (bottom) by CID and ETD in the whole set of experiments.
Table II. Overview of the acquired proteomics data. Experimental details of the quantitative data presented per SCX-LC-MS/MS experiment. Protein and peptide numbers are based on the included spectra after filtering to a false discovery rate (FDR) of 1%. Numbers are either redundant (r) or nonredundant (nr).
| LV-mouse 1/SILAC | RV-mouse 1/SILAC | LV-mouse 2/SILAC | RV-mouse 2/SILAC | |
|---|---|---|---|---|
| Number of SCX fractions analyzed | 28 | 25 | 25 | 25 |
| MS/MS Spectra | 335064 | 269237 | 524573 | 453597 |
| FDR Mascot Score Threshold CID | 23.8 | 24.4 | 26.9 | 27.6 |
| FDR Mascot Score Threshold ETD | 23.6 | 23.9 | 23.7 | 25.7 |
| Identified Spectra within 1% FDR (% of total) | 42975 (13%) | 33944 (13%) | 70354 (13%) | 58716 (13%) |
| CID spectra (FDR1%) (r) | 24204 | 18617 | 33484 | 27338 |
| ETD spectra (FDR1%) (r) | 18771 | 15327 | 36870 | 31378 |
| Unique Peptides (FDR 1%) (nr) | 14357 | 11473 | 14941 | 13754 |
| Unique Peptides Identified in all 4 experiments (r) | 28115 | |||
| Unique Peptides CID (FDR 1%) (nr) | 11839 | 9430 | 10244 | 8758 |
| Unique Peptides ETD (FDR 1%) (nr) | 9128 | 7634 | 11634 | 10680 |
| Proteins Identified | 2176 | 1823 | 2324 | 2180 |
| Proteins Identified over all 4 experiments (nr) | 3749 | |||
| Quantitated Peptide Pairs (r) | 22849 | 17400 | 35928 | 30849 |
| Quantitated Proteins (nr) | 1899 | 1611 | 1971 | 1904 |
| Quantitated proteins over all 4 samples (nr) | 3107 | |||
| Average (median) quantitated peptides/protein | 12 (3) | 11 (3) | 18 (4) | 16 (3) |
| Proteins quantitated in both LV and RV | 1303 | 1457 | ||
| Proteins quantitated in each of the 4 samples | 906 | |||
Complementarity of CID and ETD
To investigate the contribution of ETD toward identification of proteins in the SILAC mouse, we first evaluated the data on a qualitative basis. We combined the data of each mouse (LV/SILAC + RV/SILAC) to evaluate the performance of CID and ETD at the single mouse level, which lead to the identification of between 11598 and 15607 unique peptides and between 2097 and 2594 unique proteins, respectively (Fig. 1B). The duplicate Mouse2/SILAC experiment generated more peptide and protein identifications which is most likely related to a slightly more extensive MS analysis, i.e. duplicates and triplicates of most intense 3+ and 2+ fractions were run (Table II). When the data of both mice were combined (Fig. 1C), CID identified 20,966 unique peptides corresponding to 3174 proteins, whereas ETD delivered 21,618 unique peptides corresponding to 3228 proteins, adding up to 3749 unique proteins and 28,115 unique peptides. The overlap between CID and ETD at the peptide level is 51% (14,469 peptides), indicative of the expected complementarity of the two peptide fragmentation techniques. Note that data-dependent acquisition was used to perform sequencing events by both fragmentation techniques on each selected precursor. Sequencing by ETD and CID both yielded ∼25% (7149 and 6497 peptides) additional unique peptides (Fig. 1C) and almost 1100 proteins being identified exclusively by either CID or ETD. Performance of CID and ETD was evaluated also at the level of peptide charge which showed the characteristics as expected from recent literature (16, 25), i.e. ETD performs better on higher charged peptides, whereas CID dominates for doubly charged peptides (supplemental Fig. S1 and Supplemental Results). As shown by us previously (15) in a qualitative approach, the use of Lys-N in combination with ETD further enhances performance within the 2+ fraction of peptides through generation of c-ion dominated, easy interpretable tandem MS-spectra (supplemental Fig. S2). More in-depth analysis of the 14469 in-common identifications between CID and ETD revealed that ETD adds a significant amount of higher scoring peptides (supplemental Fig. S3), which also adds to the amount of proteins quantified in our overall analysis.
Quantitative Data Analysis
Next, we quantified the SILAC ratios between mouse heart LV and RV by comparing each individually with the internal SILAC mouse complete ventricle standard (LV+RV+septum, Fig. 1A). The results are summarized in Table II and Fig. 2.
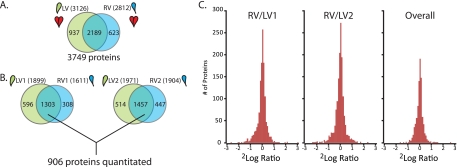
Fig. 2.
Quantitative comparison of the LV and RV proteome. A, Qualitative comparison of identified proteins in the combined LV and RV experiments (two mice). B, Overlap between proteins quantified in mouse1+SILAC (left) and mouse2+SILAC (right) and proteins quantified in each of the four runs of the whole experiment (906 proteins). C, Histogram of observed protein ratios (RV/LV) shows very few proteins with a large difference between LV and RV in both the individual experiments as well as the combined 906 proteins of mouse1 and mouse 2 (see also supplemental Figs. S3, S4, and S5).
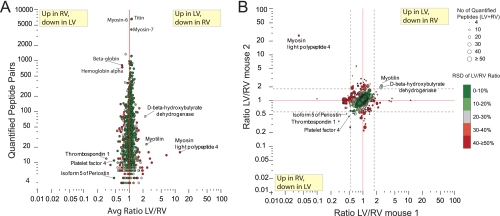
Fig. 2A depicts the overall protein identification results after summation of both biological replicates at the protein level (3749 proteins identified of which in total 3107 were quantified, ∼60% overlap between LV and RV). The level of overlap can be considered typical for the large scale analysis of complex protein mixtures. When put in perspective of prior large scale proteome studies into the heart (Table I), our strategy confirms the efficacy of Lys-N in a quantitative proteomics workflow (13). To perform RV/LV quantification in an individual mouse, only proteins quantified in both the LV/SILAC and the RV/SILAC analyses can be considered. We found 1303 (overlap of 59%) and 1457 proteins (overlap of 60%) in mouse 1 and 2, respectively (Fig. 2B). If one wants to compare across biological replicates, i.e. proteins are required to be quantitated in all four analyses, 906 proteins remain in the data set. Fig. 2C reveals that only ∼3% of the proteins have RV/LV ratios outside the twofold up- or down-change. If the ratios are averaged over both mice, the number of proteins showing a significant difference drops to below 1%. When looking more closely at the results of the individual mice, displayed in supplemental Fig. S4, the data show consistently that there are specific differences between the wild type and the SILAC mouse when the SILAC/RV ratios are compared with the SILAC/LV ratios. Proteins from the circulation (serum, red blood cells) are specifically enriched in the wild type samples. As expected variation in blood contamination is observed between the wild type and the SILAC samples. One protein seems to be more highly expressed in the SILAC mice (Nicotinamide nucleotide transhydrogenase, top right). These differences are of no concern when using the SILAC mouse as in internal standard as presented here, because the specific ventricle/SILAC ratios are consistent between the LV and RV and hence show up as unchanged in the RV/LV comparison. When inspecting the data for prominent differences between LV and RV, no proteins with large differences could be observed (Fig. 3). Fig. 3A shows the average RV/LV ratio plotted against the amount of quantified peptide pairs for each protein over all four experiments. The color-coding for the relative standard deviation on the average ratio reveals that only a handful of candidates in the lower region (less quantified spectra) are potentially differential between LV and RV, albeit with low ratios of around two-fold. Following these proteins in Fig. 3B indicates that only five proteins show consistent ratios that suggest a significant change over both mouse replicates. Ultimately, the data suggests that differences between LV and RV are very subtle and that the proteome of the LV and RV are identical at this depth level.
Fig. 3.
Detailed Quantitative comparison of LV and RV. A, Average ratio LV/RV from both mice plotted against the amount of quantitated peptide pairs shows only marginal differences between LV and RV at the lower end. Proteins with larger differences and many spectra are either caused by large relative standard deviations (RSD) on the quantitation (red data points, color coding, see legend) or slight differences in blood component contamination. B, Quantitative results from mouse 1 compared with mouse 2 show high consistency in unchanged proteins, however no proteins with a large difference in concentration between LV and RV could be observed (should scatter along y = x with large ratio), except some of the highlighted proteins with thrombospondin 1 and myotilin as most prominent representatives (consistent ratio above 1.96*sd (standard deviation) in both mice and low RSD). Lines represent the 95% confidence intervals calculated through average ratio ± 1.96 standard deviations. Colors represent relative standard deviation (RSD) on LV/RV ratio over both experiments (see legend).
DISCUSSION
The Workflow
The beneficial complementary nature of ETD has been neglected completely in large-scale quantitative proteomics experiments. Here we show for the first time that ETD adds significantly to both the identification, but more importantly also to the quantification data. Especially in the SILAC mouse the use of ETD proves valuable as the required lysine directed proteases yield longer and higher charged peptides (16, 18, 26). Our presented method also benefits from the use of Lys-N, which we demonstrate is a suitable protease for large-scale quantitative shotgun proteomics. It was not our aim to directly compare Lys-C and Lys-N here, as we showed earlier that Lys-N can yield a significant amount of complementary data when used concomitantly with trypsin and Lys-C (13). This suggests the use of Lys-C and Lys-N in one study as another potentially interesting expansion of this workflow. In addition, the success rate for ETD on Lys-N generated 2+ peptides is increased as compared with Lys-C or trypsin (supplemental Fig. S2) (15). This may prove to be valuable for site annotation when performing quantitative phosphoproteomics (16). A further expansion could be achieved by using HCD, or a decision tree based analysis that combines multiple fragmentation techniques on the potentially most suited charged species as has been recently demonstrated (27).
LV versus RV
Both ventricles have very distinct functions, the thin-walled RV directs blood low in oxygen toward the lungs. The LV free wall is much thicker because it has to achieve much higher pressures to sustain the blood flow to the rest of the body. Generally, most studies into cardiac disease focus on the deteriorating LV. Although underestimated for a long period of time, recent studies consistently demonstrate an important role for RV dysfunction in the prognosis and outcomes for a wide variety of acquired and congenital forms of cardiac dysfunction (28). Interestingly, several asymmetric pathologies exist in which one of the two ventricles is much more affected. Therefore, investigating how LV and RV differ under physiological conditions is crucial for understanding the basis of these pathologies. So far, proteomics has been used to qualitatively address the (phospho)proteome of the heart in several species (Table I). However to fully understand heart function at the molecular level, the interplay between the different parts of the heart need to be investigated with the same or even higher level of detail. To initiate this effort, we started by mapping differences between the LV and RV proteomes. The physiological differences between LV and RV are attributed to the differential anatomy. Although anatomically and physiologically very different, surprisingly both ventricles consist mainly of the same cell types, of which the cardiomyocytes represent the largest mass (∼80%) (29). The comparable cellular composition of LV and RV suggests that differences originate at the molecular and regulatory level within these cells. Even though only two mice were examined, it can be concluded that proteome differences between the LV and RV are very subtle (i.e. less than 1% of the proteome reveals consistent differences and these differences are relatively small (∼two-fold at best)). Analysis of more mice with this strategy could provide more confidence on smaller differences present in the LV and RV proteomes, however the amount of quantifiable proteins over all analyses would drop even further than the 906 reported here and the investment in terms of analysis time would probably not warrant such an approach.
The limited difference between LV and RV may come as a surprise, but also in studies on the aging of the mouse heart in general, as reported recently, overall differences observed were very subtle (23). We cannot exclude that differences in the LV and RV proteomes do exist, for instance for lower abundant proteins or at the level of post-translational modification. This latter hypothesis is underlined by a small scale study at mRNA level (30).
CONCLUSION
Here we present a powerful set of proteomic tools that complements the currently used SILAC mouse workflow with ETD fragmentation and the use of the protease Lys-N. For the first time, ETD is implemented in a quantitative large scale proteomic analysis. Using this augmented strategy provides additional and improved peptide and protein identifications and hence important additional quantitative information. Application of this technology revealed that the left and right ventricle of the mouse has negligible differences in their core-proteome. While this manuscript was in press, a comparative proteome study of the left and right ventricle of the rabbit heart revealed similar results (31).
Acknowledgments
Matthias Mann is kindly acknowledged for providing the SILAC mouse material and critically reading the manuscript.
Footnotes
* This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project CIRCULATING CELLS (grant 01C-102), and supported by the Netherlands Heart Foundation (A.S., A.J.R.H.). The Netherlands Proteomics Centre embedded in the Netherlands Genomics Initiative is kindly acknowledged for financial support (A.J.R.H., A.S., S.M.). Focus and Massa at Utrecht University is acknowledged for financial support (A.S., T.A.V.)
 This article contains supplemental Tables S1 and S2.
This article contains supplemental Tables S1 and S2.
1 The abbreviations used are:
- SILAC
- stable isototope labeling by amino acids in cell culture
- ETD
- electron transfer dissociation
- FDR
- false discovery rate
- IPI
- International Protein Index
- LV
- left ventricle
- Lys-N
- metalloendopeptidase Lys-N
- Lys-C
- endoproteinase Lys-C
- RV
- right ventricle
- SCX
- strong cation exchange.
REFERENCES
- 1. Bantscheff M., Schirle M., Sweetman G., Rick J., Kuster B. (2007) Quantitative mass spectrometry in proteomics: a critical review. Anal. Bioanal. Chem. 389, 1017–1031 [DOI] [PubMed] [Google Scholar]
- 2. Heck A. J., Krijgsveld J. (2004) Mass spectrometry-based quantitative proteomics. Expert Rev. Proteomics 1, 317–326 [DOI] [PubMed] [Google Scholar]
- 3. Ong S. E., Mann M. (2005) Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 1, 252–262 [DOI] [PubMed] [Google Scholar]
- 4. Gouw J. W., Krijgsveld J., Heck A. J. (2010) Quantitative proteomics by metabolic labeling of model organisms. Mol. Cell. Proteomics 9, 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pasa-Tolic L., Jensen P. K., Anderson G. A., Lipton M. S., Peden K. K., Martinovic S., Tolic N., Bruce J. E., Smith R. D. (1999) High throughput proteome-wide precision measurements of protein expression using mass spectrometry. J. Am. Chem. Soc. 121, 7949–7950 [Google Scholar]
- 6. de Godoy L. M., Olsen J. V., de Souza G. A., Li G., Mortensen P., Mann M. (2006) Status of complete proteome analysis by mass spectrometry: SILAC labeled yeast as a model system. Genome Biol. 7, R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krijgsveld J., Ketting R. F., Mahmoudi T., Johansen J., Artal-Sanz M., Verrijzer C. P., Plasterk R. H., Heck A. J. (2003) Metabolic labeling of C. elegans and D. melanogaster for quantitative proteomics. Nat. Biotechnol. 21, 927–931 [DOI] [PubMed] [Google Scholar]
- 8. Sury M. D., Chen J. X., Selbach M. (2010) The SILAC fly allows for accurate protein quantification in vivo. Mol. Cell. Proteomics 9, 2173–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu C. C., MacCoss M. J., Howell K. E., Matthews D. E., Yates J. R., 3rd (2004) Metabolic labeling of mammalian organisms with stable isotopes for quantitative proteomic analysis. Anal. Chem. 76, 4951–4959 [DOI] [PubMed] [Google Scholar]
- 10. Krüger M., Moser M., Ussar S., Thievessen I., Luber C. A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. (2008) SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 134, 353–364 [DOI] [PubMed] [Google Scholar]
- 11. Park S. K., Liao L., Kim J. Y., Yates J. R., 3rd. (2009) A computational approach to correct arginine-to-proline conversion in quantitative proteomics. Nat. Methods 6, 184–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Hoof D., Pinkse M. W., Oostwaard D. W., Mummery C. L., Heck A. J., Krijgsveld J. (2007) An experimental correction for arginine-to-proline conversion artifacts in SILAC-based quantitative proteomics. Nat. Methods 4, 677–678 [DOI] [PubMed] [Google Scholar]
- 13. Gauci S., Helbig A. O., Slijper M., Krijgsveld J., Heck A. J., Mohammed S. (2009) Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach. Anal. Chem. 81, 4493–4501 [DOI] [PubMed] [Google Scholar]
- 14. Helbig A. O., Gauci S., Raijmakers R., van Breukelen B., Slijper M., Mohammed S., Heck A. J. (2010) Profiling of N-acetylated protein termini provides in-depth insights into the N-terminal nature of the proteome. Mol. Cell. Proteomics 9, 928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taouatas N., Drugan M. M., Heck A. J., Mohammed S. (2008) Straightforward ladder sequencing of peptides using a Lys-N metalloendopeptidase. Nat. Methods 5, 405–407 [DOI] [PubMed] [Google Scholar]
- 16. Taouatas N., Altelaar A. F., Drugan M. M., Helbig A. O., Mohammed S., Heck A. J. (2009) Strong cation exchange-based fractionation of Lys-N-generated peptides facilitates the targeted analysis of post-translational modifications. Mol. Cell. Proteomics 8, 190–200 [DOI] [PubMed] [Google Scholar]
- 17. Mohammed S., Lorenzen K., Kerkhoven R., van Breukelen B., Vannini A., Cramer P., Heck A. J. (2008) Multiplexed proteomics mapping of yeast RNA polymerase II and III allows near-complete sequence coverage and reveals several novel phosphorylation sites. Anal. Chem. 80, 3584–3592 [DOI] [PubMed] [Google Scholar]
- 18. Swaney D. L., Wenger C. D., Coon J. J. (2010) Value of using multiple proteases for large-scale mass spectrometry-based proteomics. J. Proteome Res. 9, 1323–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aye T. T., Scholten A., Taouatas N., Varro A., Van Veen T. A., Vos M. A., Heck A. J. (2010) Proteome-wide protein concentrations in the human heart. Mol. Biosyst. 6, 1917–1927 [DOI] [PubMed] [Google Scholar]
- 20. Jongeneel C. V., Delorenzi M., Iseli C., Zhou D., Haudenschild C. D., Khrebtukova I., Kuznetsov D., Stevenson B. J., Strausberg R. L., Simpson A. J., Vasicek T. J. (2005) An atlas of human gene expression from massively parallel signature sequencing (MPSS). Genome Res. 15, 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bousette N., Kislinger T., Fong V., Isserlin R., Hewel J. A., Emil A., Gramolini A. O. (2009) Large-scale characterization and analysis of the murine cardiac proteome. J. Proteome Res. 8, 1887–1901 [DOI] [PubMed] [Google Scholar]
- 22. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausoleil S. A., Villén J., Haas W., Sowa M. E., Gygi S. P. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walther D. M., Mann M. (2011) Accurate quantification of more than 4,000 mouse tissue proteins reveals minimal proteome changes during aging. Mol. Cell Proteomics. February;10(2):M110.004523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Westbrook J. A., Wheeler J. X., Wait R., Welson S. Y., Dunn M. J. (2006) The human heart proteome: Two-dimensional maps using narrow-range immobilised pH gradients. Electrophoresis 27, 1547–1555 [DOI] [PubMed] [Google Scholar]
- 25. Swaney D. L., McAlister G. C., Coon J. J. (2008) Decision tree-driven tandem mass spectrometry for shotgun proteomics. Nat. Methods 5, 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohammed S., Heck A., Jr. (2011) Strong cation exchange (SCX) based analytical methods for the targeted analysis of protein post-translational modifications. Current Opinion Biotechnol. 2011. February;22(1) 9–16 [DOI] [PubMed] [Google Scholar]
- 27. Frese C. K., Altelaar A. F., Hennrich M. L., Nolting D., Zeller M., Griep-Raming J., Heck A. J., Mohammed S. (2011) Improved Peptide Identification by Targeted Fragmentation Using CID, HCD and ETD on an LTQ-Orbitrap Velos. J. Proteome Res. 10, 2377–2388 [DOI] [PubMed] [Google Scholar]
- 28. Sheehan F., Redington A. (2008) The right ventricle: anatomy, physiology and clinical imaging. Heart 94, 1510–1515 [DOI] [PubMed] [Google Scholar]
- 29. Redington A. N., Knight B., Oldershaw P. J., Shinebourne E. A., Rigby M. L. (1988) Left ventricular function in double inlet left ventricle before the Fontan operation: comparison with tricuspid atresia. Br. Heart J 60, 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaufman B. D., Desai M., Reddy S., Osorio J. C., Chen J. M., Mosca R. S., Ferrante A. W., Mital S. (2008) Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J. Card. Fail. 14, 760–767 [DOI] [PubMed] [Google Scholar]
- 31. Phillips D., Aponte A. M., Covian R., Neufeld E., Yu Z. X., Balaban R. S.Physiol Genomics. 2011. August 30 [Epub ahead of print] PMID:21878611 doi:10.1152/physiolgenomics.00121.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]