Abstract
Sites for restriction endonuclease cleavage in double helical DNA are blocked from cleavage when the photoaffinity drug trimethylpsoralen is photobound at or near the site. In general, Hind III sites are about 15 fold more sensitive to inactivation than the other restriction sites which were tested, although sensitivity of different Hind III sites seems to vary somewhat depending on base sequences adjacent to the site. Hind III sites can be inactivated in two ways; one which completely blocks action of the specific restriction endonuclease and one permitting the introduction of a swivel which relaxes DNA supercoiling without producing a double strand break. Nucleosomes and perhaps other protein-DNA complexes can protect the underlying DNA sequence from trimethylpsoralen photobinding and thus protect restriction sites from inactivation. This property can be exploited to determine if specific sites are accessible to the psoralon probe in vivo and thus to establish if specific nucleotide sequences are nucleosome associated. Using this procedure evidence is obtained that nucleosomes on SV40 DNA in living infected cells are either distributed randomly or at many discrete alternate sites that approach a random distribution.
Full text
PDF




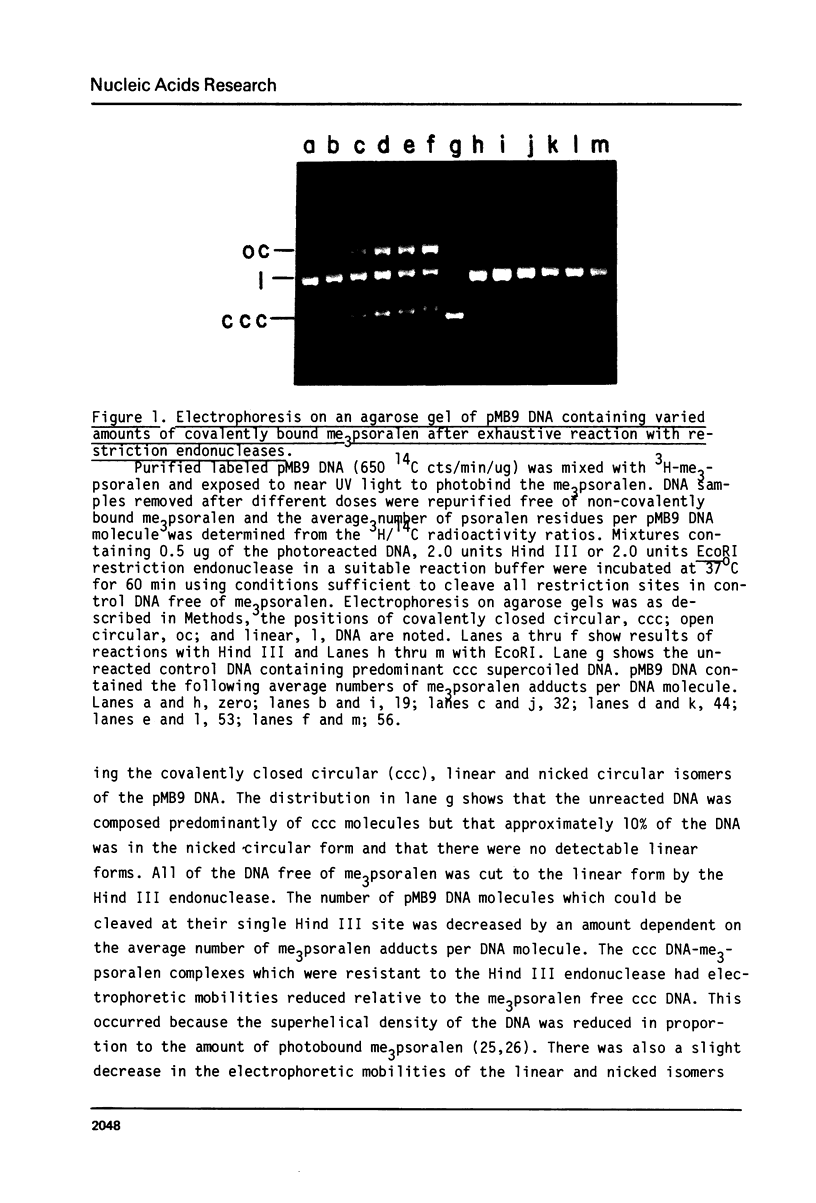

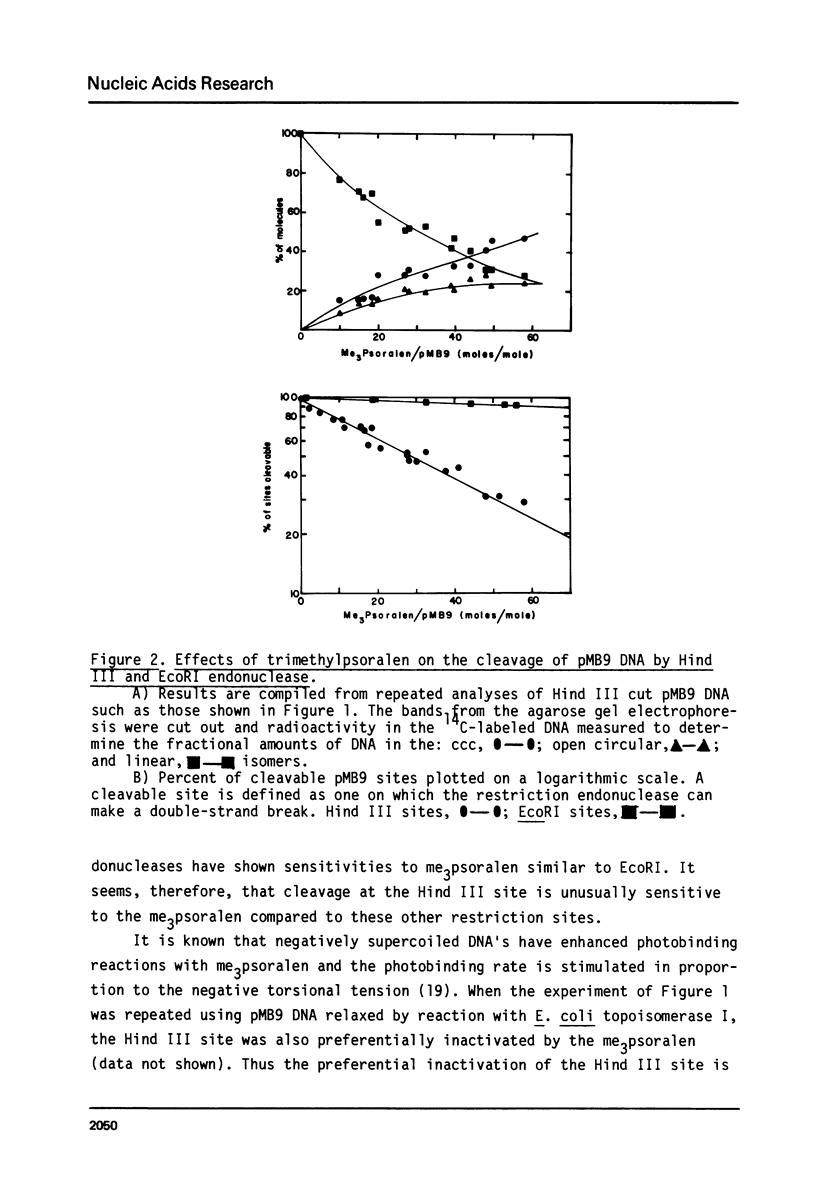









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Potter D., Pardue M. L. Chromatin structure in living cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):191–198. doi: 10.1101/sqb.1978.042.01.021. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Terbojevich M., Marciani S., Vedaldi D., Recher M. Investigation of the dark interaction between furocoumarins and DNA. Chem Biol Interact. 1978 Apr;21(1):103–115. doi: 10.1016/0009-2797(78)90071-6. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Lomonossoff G. P., Laskey R. A. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Bloomer L. S. Nonrandom alignment of nucleosomes on 5S RNA genes of X. laevis. Cell. 1980 Oct;21(3):751–760. doi: 10.1016/0092-8674(80)90438-9. [DOI] [PubMed] [Google Scholar]
- Hallick L. M., Yokota H. A., Bartholomew J. C., Hearst J. E. Photochemical addition of the cross-linking reagent 4,5', 8-trimethylpsoralen (trioxaslen) to intracellular and viral simian virus 40 DNA-histone complexes. J Virol. 1978 Jul;27(1):127–135. doi: 10.1128/jvi.27.1.127-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. V., Shen C. K., Hearst J. E. Cross-linking of DNA in situ as a probe for chromatin structure. Science. 1976 Jul 2;193(4247):62–64. doi: 10.1126/science.935855. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo-Kemenes T., Omori A., Zachau H. G. Non-random arrangement of nucleosomes in satellite I containing chromatin of rat liver. Nucleic Acids Res. 1980 Nov 25;8(22):5377–5390. doi: 10.1093/nar/8.22.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Noll M. Multiple phases of nucleosomes in the hsp 70 genes of Drosophila melanogaster. Nucleic Acids Res. 1980 Dec 20;8(24):6059–6068. doi: 10.1093/nar/8.24.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins G. L., English M., Goldstein D. A. Structural changes in simian virus 40 chromatin as probed by restriction endonucleases. J Virol. 1979 Sep;31(3):718–732. doi: 10.1128/jvi.31.3.718-732.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C., Schedl P., Samal B., Worcel A. Chromatin structure of the 5S RNA genes of D. melanogaster. Cell. 1980 Nov;22(2 Pt 2):387–392. doi: 10.1016/0092-8674(80)90349-9. [DOI] [PubMed] [Google Scholar]
- Polisky B., McCarthy B. Location of histones on simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2895–2899. doi: 10.1073/pnas.72.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Crawford L. V. The arrangement of nucleosomes in nucleoprotein complexes from polyoma virus and SV40. Cell. 1977 May;11(1):35–49. doi: 10.1016/0092-8674(77)90315-4. [DOI] [PubMed] [Google Scholar]
- Samal B., Worcel A., Louis C., Schedl P. Chromatin structure of the histone genes of D. melanogaster. Cell. 1981 Feb;23(2):401–409. doi: 10.1016/0092-8674(81)90135-5. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Wassarman P. M., DePamphilis M. L. Structure, spacing, and phasing of nucleosomes on isolated forms of mature simian virus 40 chromosomes. J Biol Chem. 1980 Jan 25;255(2):771–782. [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Chromatin structures of main-band and satellite DNAs in Drosophila melanogaster nuclei as probed by photochemical cross-linking of DNA with trioxsalen. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):179–189. doi: 10.1101/sqb.1978.042.01.020. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Streeck R. E., Zachau H. G. Nucleosome formation abolishes base-specific binding of histones. Nature. 1975 Dec 4;258(5534):447–450. doi: 10.1038/258447a0. [DOI] [PubMed] [Google Scholar]
- Tack L. C., Wassarman P. M., DePamphilis M. L. Chromatin assembly. Relationship of chromatin structure to DNA sequence during simian virus 40 replication. J Biol Chem. 1981 Aug 25;256(16):8821–8828. [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G., Hearst J. E. DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2703–2707. doi: 10.1073/pnas.75.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Wittig B., Wittig S. A phase relationship associates tRNA structural gene sequences with nucleosome cores. Cell. 1979 Dec;18(4):1173–1183. doi: 10.1016/0092-8674(79)90230-7. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Cross-linking and relaxation of supercoiled DNA by psoralen and light. Biochim Biophys Acta. 1978 Dec 21;521(2):529–546. doi: 10.1016/0005-2787(78)90295-2. [DOI] [PubMed] [Google Scholar]