Abstract
Context:
Activin A, myostatin, and follistatin have recently emerged as important regulatory molecules of reproduction and the musculoskeletal system. Little is known, however, about their day/night patterns of secretion and their physiological regulation by energy availability.
Objective:
The objective of the study was to explore day/night patterns of secretion and assess whether energy deprivation alters circulating levels of activin A, myostatin, follistatin, and cortisol and to examine whether leptin may mediate this effect.
Design, Setting and Patients, and Interventions:
Seven healthy lean men (aged 23.2 ± 3.7 yr, body mass index 23.6 ± 1.7 kg/m2) were studied for 72 h under three different conditions: on their baseline/isocaloric diet and in a complete fasting state with administration of either placebo or metreleptin. The two fasting studies were randomized and double blinded. Blood samples were obtained every 15 min from 0800 h on d 3 until 0800 h on d 4 and pooled hourly.
Main Outcome Measures:
Serum concentrations of activin A, myostatin, follistatin, cortisol, and leptin were measured.
Results:
In contrast to cortisol, we demonstrated no day/night pattern of activin A, myostatin, and follistatin secretion. Activin A concentrations decreased significantly in response to energy deprivation (P < 0.01). Follistatin and cortisol concentrations increased significantly (P < 0.01 and P < 0.01, respectively). Myostatin remained unaffected (P = 0.40). Leptin administration reversed cortisol response (P < 0.01) but failed to alter activin A, follistatin, or myostatin concentrations.
Conclusions:
Unlike cortisol, there is no day/night variation in the concentrations of activin A, myostatin, and follistatin in healthy young males. Although energy deprivation-induced cortisol changes are leptin mediated, the changes in follistatin and activin A concentrations occur through a leptin-independent pathway.
The TGF-β superfamily is a large family of secreted factors that controls a variety of biological processes by coordinating and regulating the differentiation, growth, and homeostasis of most cell types. The TGF-β family encompasses more than 40 ligands, including inhibins, activins, myostatin, TGF-β isoforms, and bone morphogenetic proteins (1). Activin A was isolated in 1980 in bovine follicular fluid and was initially thought to be exclusively a gonadal peptide that stimulates FSH secretion by the pituitary gonadotrophs (2, 3). It is now evident that activin A is a multifunctional protein with effects on a variety of cells and tissues in the periphery including skeletal muscle (4–7). Myostatin, also known as a growth/ differentiation factor-8, is produced by skeletal muscle cells and acts as a strong negative muscle regulator (8). Both molecules (myostatin and activin A) exert their function through the activin type II receptor A and activin type II receptor B receptor. They also share the same extracellular antagonist, i.e. follistatin, a structurally different TGF-β glycoprotein that binds these two molecules, rendering them biologically inactive (5, 7, 9–11). Evidence from recent in vitro and in vivo animal studies support the notion that activin A and follistatin not only participate in regulation of the reproductive system but also orchestrate muscle homeostasis along with myostatin (7, 8, 12–14).
Although there has been an explosion of information about these molecules' biochemistry and physiology, their physiological regulation has not yet been fully explored. Little is known about the secretion patterns and biological rhythms of these key molecules in humans. This knowledge is essential for interpreting any measured levels of these molecules. Foster et al. (15) reported a diurnal variability in activin A and follistatin secretion in girls in puberty. However, these data have yet to be confirmed with studies in healthy adults.
Clinical trials in humans have shown that both reproduction and muscle homeostasis respond to energy deprivation. In energy-deprived subjects, the levels of the reproductive hormones decrease in both genders (16, 17), and skeletal muscle works more efficiently to reduce total energy expenditure (18). However, evidence about the effect of energy deprivation on the regulatory molecules of activin A, myostatin and follistatin is limited. In the same context, it is not completely understood whether leptin, an adipose-secreted molecule that serves as a direct marker of energy stores and mediates neuroendocrine responses during starvation (16, 17, 19), may also mediate any alteration of circulating levels of these molecules in response to starvation.
Therefore, the aims of our study were the following: 1) to examine whether activin A, myostatin, and follistatin may have any day/night variation pattern, 2) to compare and contrast the above with the expected day/night variation pattern of cortisol, 3) to examine whether activin A, myostatin, follistatin, and cortisol levels are altered in acute energy deprivation states, and 4) if any response to energy deprivation indeed exists, to examine whether this may be mediated through changes in leptin levels. For this purpose, we measured activin A, myostatin, follistatin, cortisol, and leptin levels in seven lean normoleptinemic healthy males who were admitted to the General Clinical Research Center of the Beth Israel Deaconess Medical Center for 3 d under three different conditions: fed isocaloric state and two complete fasting states with administration of either placebo or replacement dose of meterleptin.
Subjects and Methods
Study design
Seven healthy lean men (aged 23.2 ± 3.7 yr, body mass index 23.6 ± 1.7 kg/m2), after a baseline screening visit, were studied for 72 h under three different conditions: isocaloric fed state and two complete fasting states with administration of either placebo or replacement dose of meterleptin (formerly known as r-metHuLeptin) (16). As previously described (16), only six subjects completed all three parts of the study. During the baseline fed state, subjects were on an isocaloric diet consisting of four standardized meals per day. During the fasting states, subjects were receiving only a standardized volume of calorie-free fluids as well as electrolytes and vitamin supplements. The replacement dose regimen of meterleptin was the following: 0.04 mg/kg daily on d 1 and 0.025 mg/kg four times per day on d 2 and 3. The subjects were assigned in a random and double-blind fashion to each arm of the study. Blood samples were drawn through an indwelling iv catheter, every 15 min from 0800 h on d 3 until 0800 h on d 4 and then pooled every hour to meet the assays' sample volume requirements. We managed to measure activin A, myostatin, and follistatin levels in six subjects in the fed state, seven in the fasting plus placebo state, and only five subjects in the fasting plus leptin state due to lack of serum volume. To examine the presence of the day/night pattern of secretion, we measured the serum levels of activin A (n = 6), myostatin (n = 6), follistatin (n = 6), and cortisol (n = 7) in the hourly pooled samples obtained during the third day of the isocaloric fed state. To examine the changes of these hormones in energy deprivation and to evaluate the role of leptin in this response, we compared the levels of these three molecules in hourly serum samples obtained from d 3 to d 4 of all three studies (isocaloric fed state: n = 6; fasting plus placebo state: n = 7; fasting plus leptin replacement state: n = 5).
The study was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center and took place in the General Clinical Research Center of the Beth Israel Deaconess Medical Center. All subjects gave written informed consent.
Sample analysis
The molecules of interest were measured with commercially available immunoassays. Activin A and follistatin levels were determined using ELISA assay from R&D Systems (Minneapolis, MN). Myostatin was tested with a commercially available competitive immunoassay (enzyme immunoassay; Immundiagnostik AG, Bensheim, Germany). Leptin levels were measured with a RIA (Linco Research, Inc., St. Charles, MO). Cortisol levels were measured with a RIA (Diagnostic Systems Laboratories, Inc., Webster, TX) in five subjects and with the Siemens Immulite 1000 automated platform immunometric assay (Siemens Healthcare Diagnostics, Deerfield, IL) in two subjects whose cortisol was determined after Diagnostic Systems Laboratories discontinued the production of their cortisol RIA. The two methods were strongly correlated with r = 0.997 (Immulite 1000 comparison data on file). All samples were analyzed in duplicate.
Statistical analysis
All analyses were performed with Stata, version 11.1 (Stata Corp., College Station, TX). For the circadian rhythm analysis, activin A, myostatin, and follistatin levels of the subjects in the fed state were plotted across time. The existence of possible underlying periodicity was examined in each individual with frequency spectral analysis through Stata pergram routine. Nonlinear four-parameter cosine ordinal least squares regression was performed to examine whether any day/night variability was present and to calculate the adjusted nonlinear coefficient of determination of the models (R2). For the comparison between the three studies, the trajectories of activin A, myostatin, and follistatin between the three conditions (fed, fasting with placebo administration, and fasting with the administration of replacement dose of meterleptin) were compared using a Hierarchical mixed-effects linear modeling approach through the Stata xtmixed routine. The level 1 model specification was each molecule to be a linear function of time, and in the level 2 specification, we introduced fixed effects of the study in both intercept and slope. All P values are two tailed and the criterion for significance was set at the α = 0.05 level.
Results
Day/night pattern of secretion of activin A, myostatin, follistatin, and cortisol in the fed isocaloric state (Fig. 1)
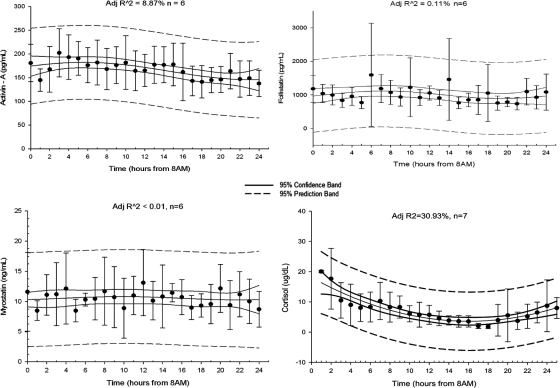
Fig. 1.
Average 24-h (0800–0800 h) activin A (picograms per milliliter), follistatin (picograms per milliliter), myostatin (nanograms per milliliter) and cortisol (micrograms per deciliter) levels on d 3 of baseline fed isocaloric state (adjusted R2 is displayed on top center of each panel).
Hourly measurements of activin A, myostatin, follistatin, and cortisol levels during the third day of the isocaloric fed state were used for the day/night pattern analysis. The spectral analysis did not show the presence of any underlying periodicity in activin A, myostatin, and follistatin secretion. The four-parameter cosine analysis yielded a nonlinear coefficient of determination of 8.87, less than 0.01, and 0.11% for each molecule accordingly. This allows us to report with great confidence that there is no day/night variation in the levels of activin A, myostatin, and follistatin. In contrast, cortisol levels exhibited day/night variability, as expected, with higher levels at 0700 h (R2 = 30.93%).
Effects of acute energy deprivation on activin A, myostatin, follistatin, and cortisol serum levels (Fig. 2)
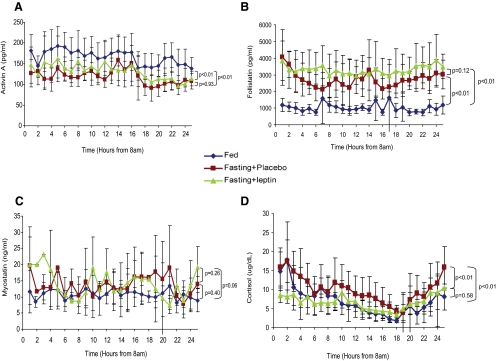
Fig. 2.
Activin A (in picograms per milliliter) (A), follistatin (in picograms per milliliter) (B), myostatin (in nanograms per milliliter) (C), and cortisol (in micrograms per milliliter) (D) serum levels on d 3 of three different studies (baseline isocaloric fed state, 72 h complete fasting with placebo treatment, and 72 h complete fasting with replacement dose meterleptin).
Prolonged 72-h fasting leads to significant leptin suppression (from 2.79 ± 0.55 ng/ml to 0.55 ± 0.28 ng/ml, P = 0.02) and decrease in body weight, which is disproportional to the change in fat mass. A 2.15 ± 0.75-kg weight loss (P < 0.01) was recorded on the third day of fasting, and this weight loss resulted from both fat mass and lean mass loss based on dual-energy x-ray absorptiometry measurements. In addition, the 3-d fasting resulted in an increase in serum cortisol levels by 29.94% (P < 0.01) (16). There was also a statistically significant increase of serum follistatin levels by 154.83% (P < 0.01) and a significant decrease of activin A levels by 31.96% (P < 0.01) during the fasting state compared with levels in the fed state, whereas myostatin serum levels did not exhibit any significant change between the fasting and the fed state (P = 0.40). The above response of the activin A-follistatin-myostatin axis in energy deprivation remained the same across all time points.
Effects of replacement dose of meterleptin administration during fasting on activin A, myostatin, follistatin, and cortisol serum levels (Fig. 2)
Administration of replacement dose of meterleptin in energy-deficit restores serum leptin levels to the physiological range (from 0.55 ± 0.28 to 9.09 ± 1.72 ng/ml, P < 0.01). The resultant leptin levels were higher than those in the fed state but remained within the normal range for lean men (from 2.79 ± 0.55 ng/ml in the fed state to 9.09 ± 1.72 ng/ml after meterleptin treatment, P = 0.01). Leptin administration during energy deprivation fully restored cortisol levels (fasting + placebo vs. fasting + leptin: P < 0.01). Conversely, leptin treatment during energy deprivation did not have any statistically significant effect on the circulating levels of the novel molecules studied herein. Follistatin levels remained increased compared with the fed state (P < 0.01) and did not differ significantly compared with levels in the fasting state with administration of placebo (P = 0.12). This unresponsiveness was also demonstrated in the case of activin A. Despite normalized leptin levels, serum activin A concentration remained low compared with the fed state (P < 0.01) and did not change significantly compared with the levels during the fasting state with administration of placebo (P = 0.93). Myostatin levels did not change significantly during the three different studies (fasting + leptin vs. fed state: P = 0.06 and fasting + placebo P = 0.26) (Fig. 2).
Discussion
We demonstrate herein that, in contrast to cortisol, activin A, myostatin, and follistatin secretions do not display a day/night variability pattern. We also assessed the response of their circulating levels to energy deprivation, and we found a significant decrease in activin A and a significant increase in follistatin and cortisol levels compared with the baseline fed state. Because prior studies have shown that leptin mediates a variety of neuroendocrine responses to energy deprivation, we then examined the effect of exogenous leptin administration on the response of these molecules to starvation (16, 17, 20, 21). Here we show that the levels of activin A, myostatin, and follistatin in states of energy deficit are not altered by restoring serum leptin levels. In contrast, we found that leptin treatment in replacement doses reverses the changes of cortisol levels in response to fasting and fully normalizes its circulating levels.
This is the first study to assess any potential day/night pattern in the secretion of activin A, myostatin, and follistatin in healthy male subjects. Our findings are in contrast to those by Foster et al. (15), who tested the diurnal changes in activin A, follistatin 288, and inhibin B concentrations during puberty in females and reported the presence of a day/night pattern of secretion in activin A, follistatin 288, and inhibin B in this subgroup of the general population. This discrepancy might be explained by the fact that we evaluated adult males in contrast to pubertal females. Importantly, the temporal resolution in our study was 1 h (in contrast to 3 h), increasing the confidence in the results of our study, which, in addition, replicated the day/night variation of cortisol, as expected.
The absence of diurnal variability allowed us to compare the fluctuation of levels of these three novel secreted molecules across the three states, modeling each hormone as a linear function of time. Activin A was initially considered to be solely a gonadal peptide that stimulates FSH secretion by the pituitary cells (3). Further studies have contributed to a better understanding of its physiology and have led to the consensus that activins are proteins with multiple functions (4, 5). Recently, using in vivo and in vitro studies on mstn−/− and fst+/− mice, Lee et al. (7) showed the potential role of activin A in regulating negatively muscle mass. We report a significant decrease of activin A concentration during starvation compared with the baseline levels, and this could be interpreted as a protective mechanism of the body that could function to eliminate any excessive loss of muscle mass when nutrient availability is limited. Examining this molecule through the reproductive prism, a decrease of activin A during starvation can ultimately influence negatively spermatogenesis by down-regulating the pituitary FSH secretion. The failure of exogenous leptin to alter this response driven by energy deficit implies that there is no cross talk between these two molecular entities. This finding is in agreement with data from studies in hypoleptinemic women in which leptin treatment had no effect on activin A levels (22).
Follistatin has been shown to be capable of binding both activin A and myostatin with different affinity. It can inhibit their action in vitro and in turn limit FSH secretion and promote muscle growth in vivo (11, 23–25). A number of studies in mice have demonstrated the significant role of follistatin in regulating skeletal muscle homeostasis with heterozygous loss of fst function to result in significant reduction of muscle mass (7). The increase in follistatin circulating levels during starvation is in concordance with the response of activin A levels to energy deprivation. This concurrent increase of follistatin and decrease of activin A could be interpreted as a dual protective mechanism used by the organism to eliminate the excessive muscle mass loss when nutrient availability is limited. At the same time, animal and human studies have shown that reproductive function is directly affected by energy availability (16, 17, 26, 27), and this response of activin A and follistatin seems to mediate this effect. In female subjects, this mechanism could serve as a natural protection from energy intensive processes such as reproduction and gestation when the energy sources are limited. Again, this response to energy deprivation occurred in a leptin-independent manner.
Myostatin's biological functions have been studied in animal models as well as in humans. Mice with homozygous deletion of the mstn gene exhibit dramatic increase in muscle mass (28, 29). Strong evidence for the important role of myostatin in muscle growth in humans has been provided by studying a case of a male child with myostatin mutation associated with marked muscle hypertrophy (30). In the present study, myostatin levels remained unaffected during the two fasting studies compared with the baseline fed state with exogenous leptin having no effect on its concentrations. These data come in sharp contrast with results from animal studies in which administration of meterleptin to hypoleptinemic ob/ob mice led to reduction of myostatin concentration and in turn to a significant increase in lean mass (31). Our findings, however, are consistent with the results of a recent study that examined young women with exercise induced hypothalamic amenorrhea (HA) and in which muscle mass and its regulators (activin A, myostatin, and follistatin) are not affected during treatment with leptin for 9 months (22).
Activation of the hypothalamic-pituitary-adrenal axis during starvation is an established physiological response in animals and humans. Leptin-induced CRH release has been demonstrated in vitro, whereas other studies have shown that ob/ob mice exhibit increased ACTH-induced adrenal stimulation (32, 33). In addition, leptin administration blunts the stress-mediated ACTH and cortisol increase in leptin deficient mice and/or normal mice after starvation (34, 35). Licinio et al. (36) have also reported an inverse relation between the circulating leptin and ACTH in healthy men. In humans in the context of an earlier open label study with leptin replacement in women with HA, a small but significant decrease in cortisol concentration was reported during leptin treatment (37). In a more recent double-blinded, randomized, placebo-controlled trial of leptin replacement for 9 months in women with HA, the leptin effect on the hypothalamic-pituitary-adrenal axis was more pronounced (38, 39). In a relatively smaller prior study on energy-deprived male subjects who were slightly different from the ones studied herein, we failed to show a statistically significant restoration of cortisol levels with leptin treatment (16). In this study, however, we used samples from a larger number of subjects, and we showed that the increased cortisol levels during energy deprivation can be normalized with leptin administered in replacement doses. This finding in normal subjects is in agreement with findings from both mice studies (35) and the above-mentioned randomized controlled trial in women with HA, in which the response of cortisol to energy deprivation occurred in a leptin-dependent manner (38).
As described in Subjects and Methods, this was a crossover interventional study including two double-blind and randomized fasting studies. The standardized design of the study, which controlled appropriately for confounders such as sleep, activity, and food intake, contributed significantly to the robustness of our results. The interval between the three admissions was no less than 7 wk to allow for recovery of the hematocrit and adequate washout and to ensure that subjects would return to their baseline weight. Another strength of our study is the high temporal resolution of our sampling intervals (every 15 min and then pooled every hour), which is much higher than the study examining similar patterns (15).
Although our study was performed in a relatively small number of subjects, we do demonstrate significant changes in cortisol levels. Also, studies with similar sample size examining other physiological responses in energy deprivation successfully detected significant changes in the concentrations of the molecules of interest (40, 41). In addition, we examined the circulating levels of activin A, follistatin, and myostatin in acute energy deficit and hypoleptinemia with administration of meterleptin in a certain dose as described in Subjects and Methods. Because the achieved serum leptin levels were within the normal range, one cannot exclude the possibility of different or more significant physiological responses, should higher dose of exogenous leptin be administered or the same hypothesis be tested in conditions of more severe and more prolonged energy deficit. These studies remain to be performed. Finally, despite prior findings linking cortisol with activin A expression (42), we failed to demonstrate changes in activin A in response to leptin administration despite contemporaneous normalization of cortisol levels after leptin treatment.
In summary, we examined the day/night pattern of secretion of activin A, myostatin, follistatin, and cortisol that are significant regulatory molecules involved in a variety of biological functions including reproduction, muscle homeostasis, and stress. Except for cortisol, we report the absence of periodicity in the secretion of the above-mentioned molecules in young healthy males. We then compared circulating levels of these molecules in the fed state with their levels in energy deficiency states as well as their response to treatment with exogenous leptin. We found that activin A concentrations decreased significantly in energy-deprived subjects and follistatin and cortisol levels changed significantly toward the opposite direction, whereas myostatin remained unaffected. This response of activin A and follistatin to energy deprivation was not altered with the administration of meterleptin in physiological replacement doses, whereas cortisol changed significantly. This implies that the physiological response of the concentration of activin A and follistatin to energy deficit is mediated through a leptin- and cortisol-independent pathway. Further studies need to be done to elucidate more clearly the role of longer and more severe energy deprivation in regulating the above-studied molecules and to investigate the potential cross talk of the activin A-follistatin-myostatin axis with other peripheral molecules.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This work was supported by National Institutes of Health-National Center for Research Resources Grant M01-RR-01032 (to the Harvard Clinical and Translational Science Center) and Grant UL1 RR025758. This work was also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 58785, 79929, 81913, and AG032030. Amylin Pharmaceuticals, Inc. supplied meterleptin for this study but had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- HA
- Hypothalamic amenorrhea.
References
- 1. Innis CA, Shi J, Blundell TL. 2000. Evolutionary trace analysis of TGF-β and related growth factors: implications for site-directed mutagenesis. Protein Eng 13:839–847 [DOI] [PubMed] [Google Scholar]
- 2. Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. 1986. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321:779–782 [DOI] [PubMed] [Google Scholar]
- 3. Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. 1986. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 321:776–779 [DOI] [PubMed] [Google Scholar]
- 4. Mathews LS, Vale WW. 1993. Molecular and functional characterization of activin receptors. Receptor 3:173–181 [PubMed] [Google Scholar]
- 5. Xia Y, Schneyer AL. 2009. The biology of activin: recent advances in structure, regulation and function. J Endocrinol 202:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. 2005. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 102:18117–18122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER. 2010. Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24:1998–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90 [DOI] [PubMed] [Google Scholar]
- 9. Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. 2009. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39:283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. 2002. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741 [DOI] [PubMed] [Google Scholar]
- 11. Welt C, Sidis Y, Keutmann H, Schneyer A. 2002. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227:724–752 [DOI] [PubMed] [Google Scholar]
- 12. McPherron AC. 2010. Metabolic functions of myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem 10:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McPherron AC, Lee SJ. 2002. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. 2009. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J 28:2662–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster CM, Olton PR, Padmanabhan V. 2005. Diurnal changes in FSH-regulatory peptides and their relationship to gonadotrophins in pubertal girls. Hum Reprod 20:543–548 [DOI] [PubMed] [Google Scholar]
- 16. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. 2003. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. 2006. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 103:8481–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, Rosenbaum M. 2010. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol 298:R79–R88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. 2005. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. 2010. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. 2002. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinkoetter M, Magkos F, Vamvini M, Mantzoros CS. 2011. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab 301:E99–E104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. 2002. Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- 24. Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. 2004. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30 [DOI] [PubMed] [Google Scholar]
- 26. Mantzoros CS. 2000. Role of leptin in reproduction. Ann NY Acad Sci 900:174–183 [DOI] [PubMed] [Google Scholar]
- 27. Mantzoros CS, Cramer DW, Liberman RF, Barbieri RL. 2000. Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Hum Reprod 15:539–544 [DOI] [PubMed] [Google Scholar]
- 28. Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. 2003. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 35:227–238 [DOI] [PubMed] [Google Scholar]
- 29. Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. 2007. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292:E985–E991 [DOI] [PubMed] [Google Scholar]
- 30. Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ. 2004. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688 [DOI] [PubMed] [Google Scholar]
- 31. Sáinz N, Rodríguez A, Catalán V, Becerril S, Ramírez B, Gómez-Ambrosi J, Frühbeck G. 2009. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1α in ob/ob mice. PLoS One 4:e6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costa A, Poma A, Martignoni E, Nappi G, Ur E, Grossman A. 1997. Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants. Neuroreport 8:1131–1134 [DOI] [PubMed] [Google Scholar]
- 33. Bray GA, York DA. 1979. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 59:719–809 [DOI] [PubMed] [Google Scholar]
- 34. Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. 1997. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138:3859–3863 [DOI] [PubMed] [Google Scholar]
- 35. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- 36. Licinio J, Mantzoros C, Negrão AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. 1997. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 3:575–579 [DOI] [PubMed] [Google Scholar]
- 37. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. 2004. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- 38. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. 2011. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA 108:6585–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. 2011. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 60:1211–1221 [DOI] [PubMed] [Google Scholar]
- 40. Chan JL, Williams CJ, Raciti P, Blakeman J, Kelesidis T, Kelesidis I, Johnson ML, Thorner MO, Mantzoros CS. 2008. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab 93:2819–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. 2007. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 66:49–57 [DOI] [PubMed] [Google Scholar]
- 42. Yu J, Shao LE, Frigon NL, Jr, Lofgren J, Schwall R. 1996. Induced expression of the new cytokine, activin A, in human monocytes: inhibition by glucocorticoids and retinoic acid. Immunology 88:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]