Abstract
Context:
Levothyroxine (l-T4) therapy is based on the assumption that the conversion of T4 into T3 provides adequate amounts of active hormone at target tissues. However, in rodents, l-T4 alone does not restore a euthyroid state in all tissues. Previous combination l-T4/liothyronine (l-T3) therapy trials focused on quality-of-life endpoints, and limited information is available on the effects on other measures of thyroid hormone action.
Objective:
Our objective was to evaluate the efficacy of thyroid hormone replacement with l-T4 or l-T3 at doses producing equivalent normalization of TSH.
Participants, Design, and Setting:
Fourteen hypothyroid patients participated in this randomized, double-blind, crossover intervention at the National Institutes of Health Clinical Center.
Interventions:
l-T3 or l-T4 were administered thrice daily to achieve a target TSH from 0.5–1.5 mU/liter. Volunteers were studied as inpatients after 6 wk on a stable dose and at the target TSH.
Main Outcome Measures:
Serum thyroid hormones, lipid parameters, and indices of glucose metabolism were evaluated.
Results:
No difference was observed in TSH between l-T3 and l-T4 treatments. l-T3 resulted in significant weight loss [l-T4, 70.6 ± 12.5, vs. l-T3, 68.5 ± 11.9 kg (P = 0.009)] and in a 10.9 ± 10.0% decrease in total cholesterol (P = 0.002), 13.3 ± 12.1% decrease in low-density lipoprotein-cholesterol (P = 0.002), and an 18.3 ± 28.6% decrease in apolipoprotein B (P = 0.018). No significant differences were observed in high-density lipoprotein-cholesterol, heart rate, blood pressure, exercise tolerance, or insulin sensitivity.
Conclusions:
The substitution of l-T3 for l-T4 at equivalent doses (relative to the pituitary) reduced body weight and resulted in greater thyroid hormone action on the lipid metabolism, without detected differences in cardiovascular function or insulin sensitivity.
Overt hypothyroidism has a prevalence of 0.3–2% in the general population (1, 2). Since 1891, hypothyroidism has been treated with replacement therapy, initially using desiccated thyroid gland (3), and since the 1930s with levothyroxine (l-T4) (4), the sodium salt of the natural thyroid hormone (TH) tetraiodothyronine (T4). Compared with desiccated gland, l-T4 is devoid of antigenicity, is chemically stable, and has uniform potency and prompt absorption allowing once-daily dosing. Therapy with l-T4 is the current standard of care (5–8), based on the assumption that conversion of inactive T4 into hormonally active T3 provides an adequate amount of TH at the target end-organs (9). Adequate replacement therapy is defined by a serum TSH within the normal range, indicating a state of euthyroidism at the hypothalamus-pituitary axis level (7, 8).
However, rodent data indicate that replacement therapy with l-T4 does not achieve adequate levels of T3 in all tissues, whereas a combination of l-T4 with liothyronine (l-T3, synthetic drug formulation of T3) does (10, 11). Furthermore, some patients treated with l-T4 experience hypothyroid symptoms despite a serum TSH concentration within the normal laboratory reference limits (12). These findings inspired clinical trials of l-T3/l-T4 combination therapy. Although combination therapy improved quality of life (QOL) and depression scale outcomes either in the study populations (13–15) or in subgroups (16–18), other studies failed to replicate these findings (19–23). In addition, little is known about the effects of combination therapy on other measures of TH action.
In this study, we evaluated multiple measures of hormone action in response to doses of l-T3 vs. l-T4 that produce equivalent steady-state baseline and TRH-stimulated TSH levels.
Materials and Methods
Study design
The study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases-National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board and conducted at the National Institutes of Health (NIH) Clinical Center. Informed consent was obtained from all study participants. Inclusion criteria were primary hypothyroidism on replacement therapy with a daily l-T4 dose of at least 1.6 μg/kg, age at least 18 yr, and body mass index of at least 20 kg/m2 and no higher than 30 kg/m2. Exclusion criteria were clinical indication for TH suppressive therapy, thyroid residual function greater than 5% as measured by a 24-h 123I thyroid uptake (in patients with thyroid gland or remnant greater than 1 cm3 by ultrasound) while on replacement therapy; hypertension or cardiovascular disease; pregnancy or use of hormonal contraception; diabetes; serum total cholesterol 240 mg/dl or higher; serum triglycerides 220 mg/dl or higher; chronic liver disease or alanine aminotransferase level greater than 2-fold the upper laboratory reference limit; glomerular filtration rate (24) less than 50 ml/min · 1.73m2; history or symptoms compatible with psychosis; major depression; use of antipsychotic medications; and use of drugs or dietary supplements known to influence thyroid status or TH pharmacokinetics. The details of this randomized, double blind, crossover intervention have been described elsewhere (25). After randomization, the study participants were assigned to a preprandial thrice-daily regimen of l-T3 or l-T4 with the therapeutic target of a serum TSH level within the range 0.5–1.5 mU/liter. Each capsule, with the exception of the placebo for sham adjustments, contained active drug (l-T3 2.5, 10, or 16 μg, l-T4 5, 10, or 33 μg). Adherence to the regimen was assessed by direct questioning during the follow-up visits and by pill counts. Patients returned to the clinic for biweekly follow-up visits and TSH sampling. Three unblinded investigators (M.Z., M.C.S., and F.P.) performed dosage adjustments. No dietary recommendations were given. Once the patients maintained a serum TSH level within the target range on a stable replacement dose for three consecutive follow-up visits, they were admitted for a 5-d hospital stay for metabolic testing. Upon discharge, the patients crossed over to the alternate treatment arm following the same scheme.
Study procedures
The technical references relative to the study procedures are reported in the Supplemental Material (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Blood collection
Fasting samples were collected on study d 1. TH samples were collected every 4 h for a 24-h period. All assays were performed by the Department of Laboratory Medicine, NIH Clinical Center.
Exercise test
A graded exercise test, with recording of anaerobic threshold, maximal oxygen consumption, maximal power (peak Watts), resting and maximal heart rate, and baseline and exercise-induced cardiac output was performed on study d 1. Gas exchange and electrocardiogram were measured by a metabolic cart (Ultima; Med Graphics Inc., Minneapolis, MN). Anaerobic threshold was determined using the V-slope method using an electromagnetically braked cycloergometer (Lode Corvival; Lode BV, Groningen, The Netherlands). Cardiac output was measured using impedance cardiography (Physioflow, Manatec Inc., Paris, France).
Array meal and equilibration diet
A standard buffet meal of 7566 kcal with 24 food items (46% carbohydrate, 14% protein, and 40% fat) was offered at lunchtime of study d 1. Study participants were instructed to eat to satiety and to indicate on a 10-cm visual-analog scale their level of hunger before and after the meal. The weight of each item was measured before and after the meal. After the array meal and throughout hospitalization, a 55% carbohydrate, 15% protein, and 30% fat caffeine-free, low-nitrate weight-maintenance diet was provided. Resting energy requirements were calculated using the Harris-Benedict equation with a standard activity factor of 1.5.
Physiology and body composition measurements
Body composition and bone mineral density were measured by dual-energy x-ray absorptiometry (DXA) with a QDR 4500A scanner (Hologic, Bedford, MA). Body weight was measured with a digital scale (Scale-Tronix 5702; Carol Stream, IL) the morning of study d 2. Resting energy expenditure (REE) and the respiratory quotient were measured the morning of study d 4 by indirect calorimetry after a 12-h fast using the ventilated hood technique (ParvoMedics, Sandy, UT).
Hyperinsulinemic-euglycemic clamp
The morning of study d 4, a hyperinsulinemic-euglycemic clamp was performed using an insulin infusion rate of 40 mU/m2 · min. Serum glucose was measured every 5 min by glucose analyzer (YSI, Yellow Springs, OH). After equilibration, glucose disposal rate was calculated over a 20-min period while serum glucose was maintained within the range 95–105 mg/dl.
Quality of life assessment
A psychological well-being metric (SF-36) and the hypothyroid-specific health-related QOL questionnaire were administered on study d 2. Questionnaires were evaluated by a clinical psychologist, blinded to the treatment assignments. At the completion of the second hospitalization, the study patients were asked which of the two treatments they preferred.
Cardiovascular function
Echocardiograms were obtained according to established guidelines. Endothelial function was assessed on study d 5 after a 12-h fast using an Acuson Sequoia C512 (Siemens, Malvern, PA) with a 15L8 transducer. Brachial artery flow-mediated vasodilation was assessed using established methods. Studies were acquired at baseline and during reactive hyperemia for measurement of endothelium-dependent vasodilation. Sublingual nitroglycerin (0.4 mg) was also given for measurement of nitrate-induced, endothelium-independent vasodilation.
Statistical analysis
A two-tailed paired t test was used to compare data between treatment arms. Nonparametric data were analyzed using Wilcoxon signed rank sum test. Results are expressed as mean ± sd; P < 0.05 was considered the threshold for statistical significance. Analyses were performed using Prism version 5 (GraphPad, La Jolla, CA).
Results
Study population characteristics
During the period August 2005 to July 2010, approximately 110 prospective volunteers contacted the NIH recruitment office in reference to this study. Most of the patients declined to participate because of the time commitment and/or the thrice-daily drug administration regimen. Twenty-nine patients (25 females and four males) were screened in the outpatient clinic for the participation in the study, and 18 (16 females and two males) of 20 eligible subjects agreed to enroll and were randomized. Four patients (three females and one male) withdrew from the study, one because of relocation and three because of poor compliance with the medication regimen. All of the dropouts were recorded during the first phase of the study (two while on l-T3 and two while on l-T4). Fourteen patients (seven initially allocated to l-T3 and seven to l-T4 treatment arm) completed the study. Patients' baseline characteristics are reported in Table 1. Drug dosage, serum TH levels, and TRH-stimulation test of the first 10 patients who completed the study have been reported elsewhere (25). No treatment sequence effect was observed for any of the parameters analyzed. No serious adverse events were recorded. One patient experienced an episode of generalized anxiety disorder while receiving l-T4 with a TSH of 0.27 mU/liter. The patient underwent cognitive behavior therapy with a clinical psychologist and completed the study without receiving anxiolytic therapy.
Table 1.
Baseline characteristics of the study population
| Mean ± sd (range)a | |
|---|---|
| Female/male (n) | 13/1 |
| Age (yr) | 49.3 ± 8.0 (41–72) |
| Ethnicity (n) | |
| Caucasian | 10 |
| Asian | 2 |
| Hispanic | 1 |
| African-American | 1 |
| Height (cm) | 162.8 ± 6.1 (151.5–173.9) |
| Weight (kg) | 69.7 ± 12.5 (51.3–89.9) |
| Body mass index (kg/m2) | 26.3 ± 4.3 (20.3–30.0) |
| Etiology of hypothyroidism (n) | |
| Multinodular goiter | 7 |
| Follicular adenoma | 1 |
| Papillary thyroid cancer | 2 |
| Follicular thyroid cancer | 1 |
| Medullary thyroid cancer | 1 |
| Hashimoto thyroiditis | 1 |
| 131I treatment for Graves' disease | 1 |
| Duration of hypothyroidism (yr) | 8.6 ± 13.1 (0.5–50) |
| TSH (mU/liter) | 1.1 ± 1.4 (0.04–4.52) |
| T3 (ng/dl) | 99.0 ± 24.2 (65–141) |
| Free T4 (ng/dl) | 1.76 ± 0.46 (1.1–2.5) |
| Time to therapeutic target l-T4 (d) | 153 ± 70.5 (60–298) |
| Time to therapeutic target l-T3 (d) | 132 ± 71.1 (53–275) |
| l-T4 dose [μg/d (μg/kg · d)] | 120.3 ± 39.8 (1.68 ± 0.33) |
| l-T3 dose [μg/d (μg/kg · d)] | 39.7 ± 10.2 (0.57 ± 0.08) |
Conversion factors for SI units are as follows: nanograms per deciliter × 0.0154 nanomoles per liter for T3; nanograms per deciliter × 12.87 picomoles per liter for free T4. The differences in time to therapeutic target were not significant (P = 0.335).
Unless indicated otherwise.
Replacement therapy and TH levels
The dose of l-T3 was 0.57 ± 0.08 μg/kg · d, whereas the l-T4 was 1.68 ± 0.33 μg/kg · d with a microgram/microgram l-T3 to l-T4 ratio of 0.34 ± 0.05 (Table 1). No difference was observed in the TSH values during the periods preceding the achievement of the therapeutic target (l-T3 2.07 ± 1.28 vs. l-T4 2.11 ± 1.56 mU/ml, P = 0.945). At the time of admission, there was no difference in the morning TSH values (l-T3 1.44 ± 0.79 vs. l-T4 1.30 ± 0.79 mU/ml, P = 0.674) or in the average TSH levels in the three follow-up visits preceding the admission (l-T3 0.92 ± 0.18 vs. l-T4 0.92 ± 0.25 mU/ml, P = 0.999). As expected, during l-T3 therapy, the morning fT4 levels were below the detection limits (l-T3 < 0.3 vs. l-T4 1.57 ± 0.30 ng/dl). l-T3 replacement resulted in higher serum morning T3 levels (l-T3 172.0 ± 88.25 vs. l-T4 92.86 ± 19.01 ng/dl, P = 0.003). The difference in serum T3 was evident throughout the 24-h [l-T3 area under the curve (AUC) 261,214 ± 85,309 vs. l-T4 AUC 126,531 ± 33,934 ng/dl · min, P < 0.0001]. No difference was observed in the 24-h profile of serum TSH (l-T3 AUC 2332 ± 1312 vs. l-T4 AUC 2495 ± 1786 mU/liter · min, P = 0.789) (Fig. 1). Although more variable than on l-T4, the average serum T3 levels remained within the normal range throughout the 24-h serial sampling period on l-T3 (Fig. 1).
Fig. 1.
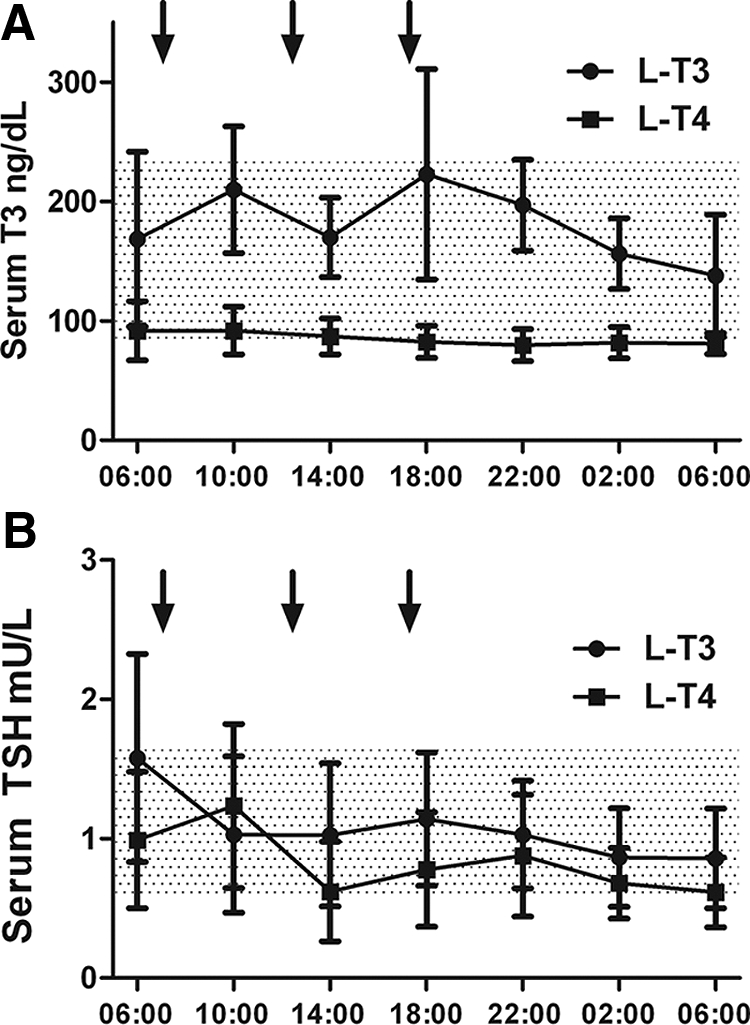
The 24-h profile of serum T3 (A), and TSH (B). The arrows indicate the administration of the study medication. For total T3, to convert to SI units, multiply nanograms per deciliter × 0.0154 to get nanomoles per liter.
Body weight and body composition data (Table 2)
Table 2.
Physiology and body composition data
| Parameter | l-T4 | l-T3 | P value (Δ %) |
|---|---|---|---|
| Physiology and body composition | |||
| Weight (kg) | 70.6 ± 12.5 | 68.5 ± 11.9 | 0.009 (−2.4 ± 2.6) |
| Fat mass (kg)a | 25.5 ± 6.9 | 24.1 ± 6.9 | 0.052 (−5.3 ± 10.0) |
| Heart rate (bpm) | 65.0 ± 8.1 | 68.2 ± 7.3 | 0.200 |
| Systolic blood pressure (mm Hg) | 113.4 ± 10.0 | 119.3 ± 10.6 | 0.122 (5.7 ± 11.6) |
| Diastolic blood pressure (mm Hg) | 68.2 ± 7.3 | 73.1 ± 10.0 | 0.078 (7.7 ± 14.3) |
| Pulse pressure (mm Hg) | 45.2 ± 9.0 | 46.2 ± 10.2 | 0.771 |
| L1–L5 bone densitometry (g/cm2) | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.707 |
| Lumbar spine Z-score | 0.55 ± 1.3 | 0.61 ± 1.3 | 0.312 |
| Resting energy expenditure (kcal/24 h) | 1201 ± 281.5 | 1177 ± 322.6 | 0.717 |
| Respiratory quotient | 0.86 ± 0.05 | 0.86 ± 0.05 | 0.841 |
| Laboratory data | |||
| Total cholesterol (mg/dl) | 195.9 ± 25.9 | 173.9 ± 27.7 | 0.002 (−10.9 ± 10.0) |
| Non-HDL cholesterol (mg/dl) | 132.9 ± 28.2 | 116.4 ± 31.6 | 0.008 (−12.1 ± 13.8) |
| LDL-cholesterol (mg/dl) | 122.6 ± 25.2 | 106.2 ± 27.7 | 0.002 (−13.3 ± 12.1) |
| HDL-cholesterol (mg/dl) | 63.0 ± 15.0 | 57.5 ± 11.7 | 0.067 (−7.0 ± 14.6) |
| Triglycerides (mg/dl) | 78.2 ± 30.8 | 78.8 ± 28.6 | 0.937 |
| Apolipoprotein A-I (mg/dl) | 151.6 ± 30.9 | 144.2 ± 16.0 | 0.230 (−3.1 ± 16.1) |
| Apolipoprotein B (mg/dl) | 86.6 ± 18.9 | 72.4 ± 14.8 | 0.018 (−18.3 ± 28.6) |
| Apolipoprotein B/apolipoprotein A-1 ratio | 0.59 ± 0.17 | 0.51 ± 0.13 | 0.021 (−9.0 ± 13.8) |
| SHBG (nmol/liter) | 49.9 ± 22.6 | 58.4 ± 26.8 | 0.038 (22.3 ± 27.0) |
| Fasting glucose (mg/dl) | 90.3 ± 9.8 | 90.3 ± 5.8 | 0.325 |
| Fasting insulin (μ U/ml) | 6.3 ± 4.4 | 7.5 ± 3.9 | 0.889 |
| HOMA-IR | 1.31 ± 1.1 | 1.42 ± 0.8 | 0.604 |
| Glucose disposal rate (mg/kg · min) | 7.26 ± 2.7 | 7.37 ± 4.4 | 0.889 |
| Echocardiogram | |||
| Left ventricle mass index (g/m2) | 71.27 ± 16.1 | 71.15 ± 18.5 | 0.973 |
| Left ventricle mass (g) | 127.0 ± 37.66 | 126.9 ± 42.38 | 0.980 |
| Left ventricle end-diastolic volume (ml) | 70.53 ± 22.72 | 76.61 ± 22.22 | 0.07 |
| Left ventricle end-diastolic volume/BSA (g/m2) | 39.4 ± 10.7 | 44.02 ± 10.0 | 0.07 |
| Left ventricle end-systolic volume (ml) | 22.46 ± 9.27 | 22.56 ± 7.17 | 0.672 |
| Left ventricle end- systolic volume/BSA (g/m2) | 11.6 ± 5.5 | 12.8 ± 3.9 | 0.546 |
| Isovolumetric relaxation time (msec) | 97.83 ± 14.83 | 89.19 ± 11.52 | 0.04 |
| E/A ratio | 1.19 ± 0.53 | 1.22 ± 0.30 | 0.626 |
| Deceleration time (msec) | 227.0 ± 58.1 | 230.0 ± 35.2 | 0.766 |
| Septal E/Ea ratio | 7.83 ± 2.5 | 7.20 ± 3.6 | 0.893 |
| Lateral E/Ea ratio | 6.38 ± 2.1 | 5.91 ± 2.8 | 0.732 |
| Flow-mediated dilation of the brachial artery | |||
| Flow-mediated vasodilatation (%) | 13.1 ± 7.1 | 12.7 ± 5.1 | 0.857 |
| Nitroglycerin-mediated vasodilatation (%) | 22.2 ± 8.7 | 21.7 ± 8.2 | 0.776 |
| Exercise tolerance test | |||
| Heart rate rest (bpm) | 81.2 ± 12.3 | 79.9 ± 10.0 | 0.622 |
| Heart rate maximal (bpm) | 162.8 ± 12.3 | 163.1 ± 18.3 | 0.385 |
| Stroke volume rest (ml) | 77.2 ± 14.6 | 74.5 ± 14.5 | 0.796 |
| Stroke volume maximal (ml) | 112.9 ± 19.5 | 117.2 ± 19.1 | 0.372 |
| VO2Max (ml/min) | 1669 ± 478.2 | 1660 ± 500.4 | 0.896 |
| Anaerobic threshold (ml/min) | 917.5 ± 311.9 | 925.0 ± 298.8 | 0.840 |
| Power (Watts) | 136.2 ± 49.3 | 137.6 ± 44.4 | 0.829 |
bpm, Beats per minute; BSA, body surface area; E/A ratio, early/late (atrial) ventricular filling velocity; E/Ea ratio, early diastolic transmitral velocity/early mitral annular diastolic velocity; HOMA-IR, homeostatic model assessment of insulin resistance; VO2Max, maximal oxygen consumption. Conversion factors for SI units are as follows: milligrams per deciliter × 0.0259 millimoles per liter for total cholesterol and cholesterol fractions; milligrams per deciliter × 0.01 grams per liter for apolipoproteins; milligrams per deciliter × 0.0555 millimoles per liter for glucose; and microunits per milliliter × 6.945 picomoles per liter for insulin.
Estimated by DXA scan.
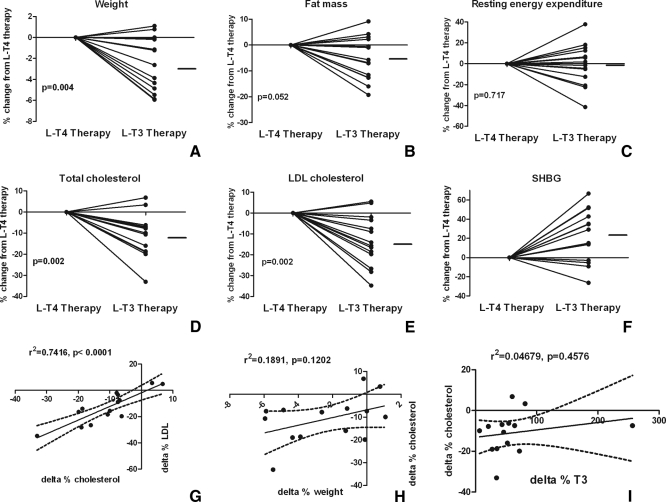
l-T3 treatment resulted in a significant decrease in body weight (l-T3 68.5 ± 11.9 vs. l-T4 70.6 ± 12.5 kg, P = 0.009) with an average individual weight loss of −1.8 ± 1.9 kg and a nonsignificant decrease in total fat mass (−5.3 ± 8.5%, P = 0.052). The individual changes in body weight and fat mass are reported in Fig. 2. No significant difference in fat-free mass, bone mineral density, or REE was observed between the treatment arms.
Fig. 2.
A–F, Individual percent change after substitution of l-T3 for l-T4. The data are presented as changes from l-T4 therapy: weight (A), fat mass (B), REE (C), total cholesterol (D), LDL-cholesterol (E), and SHBH (F). The horizontal line represents the average percent difference from l-T4 therapy. G–I, Intra-individual correlations of changes in various parameters between treatments: total vs. LDL-cholesterol (G), weight vs. total cholesterol (H), and serum T3 vs. total cholesterol (I). The dashed lines represent the 95% CI of the regression.
Lipid and other laboratory data (Table 2)
Compared with the l-T4 arm, l-T3 resulted in a 10.9 ± 10.0% decrease in total cholesterol (P = 0.002), a 13.3 ± 12.1% reduction in low-density lipoprotein (LDL)-cholesterol (P = 0.002), a 12.1 ± 13.8% reduction in non-high-density lipoprotein (non-HDL) cholesterol, (P = 0.008), and an 18.3 ± 28.6% decrease in apolipoprotein B (P = 0.018). A 7.0 ± 14.6% reduction in HDL-cholesterol with l-T3 treatment did not reach statistical significance (P = 0.067). Similarly, there was no significant change in apolipoprotein A-I levels. Individual changes and average point estimates are shown in Fig. 2.
l-T3 resulted in a 22.3 ± 27.0% increase in SHBG levels (P = 0.038). A significant correlation was observed among the changes in lipid parameters, whereas no significant correlation was observed among the changes in other parameters (Fig. 2). No significant differences were observed in fasting glucose, insulin, or insulin sensitivity as measured by the homeostatic model assessment index or hyperinsulinemic-euglycemic clamp.
QOL questionnaires and array meal
No difference between treatments was observed in the SF-36 (26), and no significant difference was observed in health-related QOL (19). The array meal showed no significant difference either in total calorie intake or in macronutrient preference. Similarly, no difference was observed in degree of hunger before and after the array meal test (data not shown). At the end of the study, four patients stated they had no preference in treatment, five preferred l-T4, and five preferred l-T3.
Cardiovascular function and exercise test (Table 2)
There were no statistically significant differences in resting heart rate or blood pressure between the two treatments, although a nonsignificant increase in diastolic blood pressure was observed during l-T3 treatment (l-T3 73.1 ± 10.0 vs. l-T4 68.2 ± 7.3 mm Hg, P = 0.078). No significant differences between treatments were observed either in chamber size or systolic function parameters. Although there was a significantly shorter isovolumic relaxation time in the l-T3 treatment arm, most diastolic function parameters showed no differences. No significant differences were observed in flow-mediated vasodilation or exercise tolerance test (Table 2). None of the subjects experienced anginal symptoms, and no ST-T wave changes were observed during exercise or during the recovery phase.
Discussion
l-T4 is the standard medication for the treatment of hypothyroidism (6), and the single daily dosing makes it an ideal drug for life-long management of this disease. The sensitivity of the pituitary to TH and accurate assays of TSH allow precise therapeutic titration, based on the assumption that a state of pituitary euthyroidism equates to euthyroidism throughout the target organs of the hormonal action. However, tissue euthyroidism is the net result of multiple steps including conversion of the prohormone T4 into its active metabolite T3, which is ultimately responsible for signaling at the end-organ target level. The circulating and intracellular pools of T3 of treated hypothyroid patients (i.e. devoid of endogenous TH production) depend entirely on the conversion of exogenous l-T4 into T3. This is assured by the deiodinases that regulate the circulating levels of T3 and its availability at the target tissue level, acting as a form of prereceptor, tissue-specific modulation of the TH action (27).
Replacement therapy with l-T4/l-T3 combinations has been suggested as a treatment for patients who do not achieve control of symptoms on l-T4 monotherapy (28). Several studies have compared l-T4/l-T3 vs. l-T4 monotherapy (13–23). However, these studies have focused on QOL and psychological endpoints, reporting only limited data on changes in other measures of TH action (16). Furthermore, therapy was administered as a once-daily regimen and/or using a fixed l-T3 dose, and the interventions were not tailored to a target TSH level (13–18, 20, 22, 23). A single study allowed dose titration, but the data reported were limited to QOL and psychological endpoints (21).
Recently, we demonstrated the feasibility of sustained replacement therapy with l-T3 alone on a thrice-daily regimen in a subgroup of patients presented in the current study (25). Aside from its potential use during TH withdrawal for management of thyroid cancer, the treatment with l-T3, bypassing the need for deiodination, allows the evaluation in vivo of the role of TH conversion. To this end, we systematically assessed the differences in the targets of the TH action, measured at a clamped, euthyroid state in the pituitary. The 0.34 ± 0.05 μg/μg ratio of l-T3/l-T4 daily dose is in agreement with previous estimates obtained by compartmental analysis (29). Although the mean serum T3 level remained within the normal range, l-T3 treatment resulted in significantly higher T3 serum concentrations. This is not surprising because type-2 deiodinase (30) is particularly active in the thyrotroph, and it is thought to play a major role in the hypothalamus-pituitary-thyroid axis (27, 31). Hence, this axis is probably more responsive to the inhibitory effects of T4 than T3.
The substitution of l-T3 for l-T4 caused a significant weight loss. TH is the major regulator of basal metabolic rate (32), so it is likely that this weight reduction is due to an increase in metabolic rate rather than a decrease in food intake. The lack of a detectable change in REE is likely due to the small magnitude of the effect, because a negative energy balance of only about 50 kcal/d is thought to be required to maintain the reduced body weight observed in our study (33). Given the sensitivity of indirect calorimetry using the hood technique (34) and the variation observed in this study, a 50 kcal/d change in resting metabolic rate is not expected to be detected. We cannot rule out that the effect could be secondary to changes in other components of the energy balance, such as the thermic effect of food or physical activity not captured by the hood indirect calorimetry technique.
The substitution of l-T3 for l-T4 caused a significant reduction in lipid parameters. These data suggest that the TH action is increased in the liver, and the SHBG increase supports this hypothesis. The changes in serum lipid metabolism parameters are similar to the effects observed with drugs approved for the treatment of dyslipidemia (35) or TH analogs (36). The increase in liver TH tone could be due to a first-pass effect because orally delivered l-T3 presumably produces higher concentrations in the liver than in other organs. This differential response appears to be limited to the lipid metabolism and SHBG, whereas no differences in indices of insulin resistance were detected. This is remarkable because hyperthyroid states are associated with an increase in hepatic gluconeogenesis (37), and overt thyrotoxicosis is a known cause of secondary diabetes.
Although the intra-individual changes in lipid parameters were strongly correlated, the changes among the other parameters were not. Due to the small number of observations, we cannot rule out the possibility of differential intra-individual tissue response to l-T3 therapy.
Despite the increase in serum T3, the l-T3 treatment did not cause major changes in cardiovascular or musculoskeletal function, as indicated by the echocardiographic and maximal exercise tolerance tests and DXA studies. Similarly, no significant differences were observed in blood pressure, heart rate, or endothelial vascular function. The shorter isovolumic relaxation time observed in the l-T3 treatment arm might suggest that the myocardium is more sensitive to this treatment, but the lack of significance in the other diastolic function indexes and the limited number of patients studied do not fully support this hypothesis. The presence of type-2 deiodinase in myocardium (38), skeletal muscle (38), and skeleton (39) could explain these findings. These tissues, like the pituitary, rely at least in part, on the intracellular conversion of T4 to maintain the cellular levels of T3 and thus are presumably less sensitive to the elevated serum T3 levels resulting from l-T3 therapy. This condition is opposite to the one observed in liver, whereby the tissue concentration of T3 depends almost entirely on the circulation levels of T3 (40).
Previous studies on l-T3/l-T4 combination therapy observed effects on QOL and/or behavior (13–23). Our lack of detected effects in these metrics could be due to the relatively small sample size. Furthermore, the duration of the intervention might have prevented the detection of significant changes in cardiovascular and musculoskeletal parameters.
The double-blind, crossover study design is robust and minimizes confounders due to inter-individual variability or comorbidity. Moreover, the sustained treatment on a stable dose of study drug with TSH levels within a narrow range minimizes carryover effects from previous treatment or unstable dosage. Finally, the stable serum T3 levels over the 24-h period, and the inpatient stay under a controlled diet, further reduced the variability of the data and improved the likelihood of detecting differences in the parameters analyzed. Nonetheless, it is possible that the observed changes do not represent the full effects of the l-T3 treatment because of the relatively short duration of the intervention while at a stable dose and target TSH. Alternatively, the nonsignificant differences in duration of the treatment might have affected the results of the study. This is unlikely because before reaching the therapeutic target, the patients spent similar time with TSH levels above or below the target range.
In conclusion, the results of this pharmacology, proof-of-concept study indicate that replacement therapy of hypothyroidism with l-T3, compared with l-T4 causes weight loss and favorable changes in the lipid profile without appreciable side effects. This intervention could be relevant for hypothyroid patients affected by comorbid conditions such as cardiovascular disease, diabetes, dyslipidemia, or obesity, where weight control and aggressive lowering of serum cholesterol are particularly important. However, presently, the prolonged use of l-T3 alone for the treatment of hypothyroidism cannot be advocated in a clinical setting, because thrice-daily dosing is not practical and may affect patients' adherence with treatment. Further studies are needed to characterize the long-term effects and the subset of patients that might benefit from l-T3 therapy, and the metabolic effects of l-T3/l-T4 combination therapy.
Acknowledgments
We gratefully acknowledge the help and professionalism of the nursing, laboratory, pharmacy (especially George Grimes and Judy Starling), and ancillary personnel of the NIH Clinical Center. This research could have not been accomplished without the selfless participation of the study volunteers. The comments of Marc L. Reitman, M.D., Ph.D., are gratefully acknowledged.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases program Z01-DK047057-02, and the Clinical Center, NIH.
Trial Registration: ClinicalTrials.gov identifier NCT00106119.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- DXA
- dual-energy x-ray absorptiometry
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- l-T3
- liothyronine
- l-T4
- levothyroxine
- QOL
- quality of life
- REE
- resting energy expenditure
- TH
- thyroid hormone.
References
- 1. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 3. Murray GR. 1891. Note on the treatment of myxoedema by hypodermic injections of an extract of the thyroid gland of a sheep. Br Med J 2:796–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harington CR, Barger G. 1927. Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem J 21:169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. 1987. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 316:764–770 [DOI] [PubMed] [Google Scholar]
- 6. Mandel SJ, Brent GA, Larsen PR. 1993. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med 119:492–502 [DOI] [PubMed] [Google Scholar]
- 7. Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G, Greenspan FS, McDougall IR, Nikolai TF. 1995. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. Standards of Care Committee, American Thyroid Association. JAMA 273:808–812 [PubMed] [Google Scholar]
- 8. Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR, Jr, Zeiger MA, Zini M. 2006. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 12:63–102 [DOI] [PubMed] [Google Scholar]
- 9. Jonklaas J, Davidson B, Bhagat S, Soldin SJ. 2008. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA 299:769–777 [DOI] [PubMed] [Google Scholar]
- 10. Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, Morreale de Escobar G. 1995. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest 96:2828–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. 1996. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137:2490–2502 [DOI] [PubMed] [Google Scholar]
- 12. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. 2002. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 57:577–585 [DOI] [PubMed] [Google Scholar]
- 13. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr 1999. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 340:424–429 [DOI] [PubMed] [Google Scholar]
- 14. Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. 2005. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab 90:2666–2674 [DOI] [PubMed] [Google Scholar]
- 15. Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ., Jr 2002. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves' disease. Endocrine 18:129–133 [DOI] [PubMed] [Google Scholar]
- 16. Slawik M, Klawitter B, Meiser E, Schories M, Zwermann O, Borm K, Peper M, Lubrich B, Hug MJ, Nauck M, Olschewski M, Beuschlein F, Reincke M. 2007. Thyroid hormone replacement for central hypothyroidism: a randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. J Clin Endocrinol Metab 92:4115–4122 [DOI] [PubMed] [Google Scholar]
- 17. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. 2005. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab 90:805–812 [DOI] [PubMed] [Google Scholar]
- 18. Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. 2009. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 94:1623–1629 [DOI] [PubMed] [Google Scholar]
- 19. Clyde PW, Harari AE, Getka EJ, Shakir KM. 2003. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 290:2952–2958 [DOI] [PubMed] [Google Scholar]
- 20. Escobar-Morreale HF, Botella-Carretero JI, Gomez-Bueno M, Galan JM, Barrios V, Sancho J. 2005. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing l-thyroxine plus liothyronine with l-thyroxine alone. Ann Intern Med 142:412–424 [DOI] [PubMed] [Google Scholar]
- 21. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. 2003. Does a combination regimen of thyroxine (T4) and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 88:4551–4555 [DOI] [PubMed] [Google Scholar]
- 22. Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Dabers T, Kirsch G, Sanger E, Engel G, Hamm AO, Nauck M, Meng W. 2004. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14:1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf) 60:750–757 [DOI] [PubMed] [Google Scholar]
- 23. Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, Dhaliwal SS, Chew GT, Bhagat MC, Cussons AJ. 2003. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 88:4543–4550 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 25. Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. 2010. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf) 72:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ware JE, Jr, Sherbourne CD. 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483 [PubMed] [Google Scholar]
- 27. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blanchard KR. 2004. Dosage recommendations for combination regimen of thyroxine and 3,5,3′-triiodothyronine. J Clin Endocrinol Metab 89:1486–1487; author reply 1487–1488 [DOI] [PubMed] [Google Scholar]
- 29. Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. 1990. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol 258:E715–E726 [DOI] [PubMed] [Google Scholar]
- 30. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 31. Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, Huang SA, Crescenzi A, Harney JW, Ridgway EC, Larsen PR, Lechan RM, Bianco AC. 2006. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology 147:1735–1743 [DOI] [PubMed] [Google Scholar]
- 32. Kim B. 2008. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 18:141–144 [DOI] [PubMed] [Google Scholar]
- 33. Hall KD, Jordan PN. 2008. Modeling weight-loss maintenance to help prevent body weight regain. Am J Clin Nutr 88:1495–1503 [DOI] [PubMed] [Google Scholar]
- 34. da Rocha EE, Alves VG, da Fonseca RB. 2006. Indirect calorimetry: methodology, instruments and clinical application. Curr Opin Clin Nutr Metab Care 9:247–256 [DOI] [PubMed] [Google Scholar]
- 35. Paras C, Hussain MM, Rosenson RS. 2010. Emerging drugs for hyperlipidemia. Expert opinion on emerging drugs 15:433–451 [DOI] [PubMed] [Google Scholar]
- 36. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362:906–916 [DOI] [PubMed] [Google Scholar]
- 37. Raboudi N, Arem R, Jones RH, Chap Z, Pena J, Chou J, Field JB. 1989. Fasting and postabsorptive hepatic glucose and insulin metabolism in hyperthyroidism. Am J Physiol 256:E159–E166 [DOI] [PubMed] [Google Scholar]
- 38. Salvatore D, Bartha T, Harney JW, Larsen PR. 1996. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3315 [DOI] [PubMed] [Google Scholar]
- 39. Gouveia CH, Christoffolete MA, Zaitune CR, Dora JM, Harney JW, Maia AL, Bianco AC. 2005. Type 2 iodothyronine selenodeiodinase is expressed throughout the mouse skeleton and in the MC3T3-E1 mouse osteoblastic cell line during differentiation. Endocrinology 146:195–200 [DOI] [PubMed] [Google Scholar]
- 40. Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. 2001. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev 22:451–476 [DOI] [PubMed] [Google Scholar]